Abstract
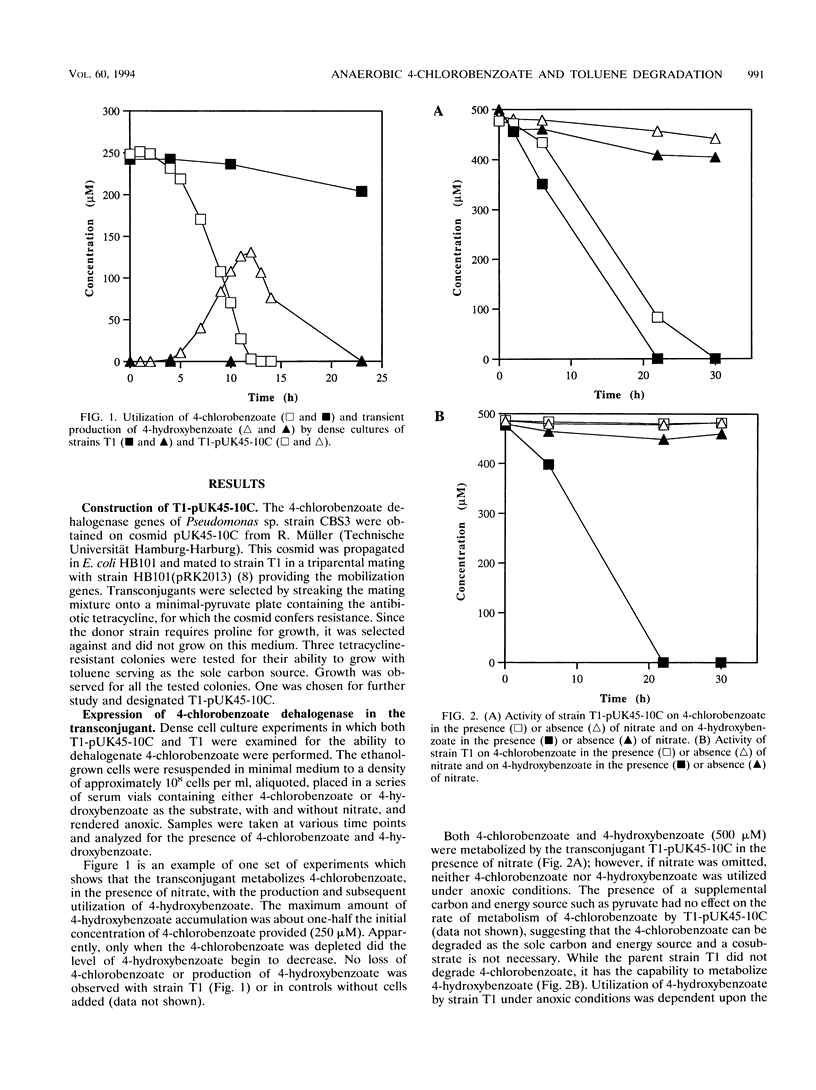
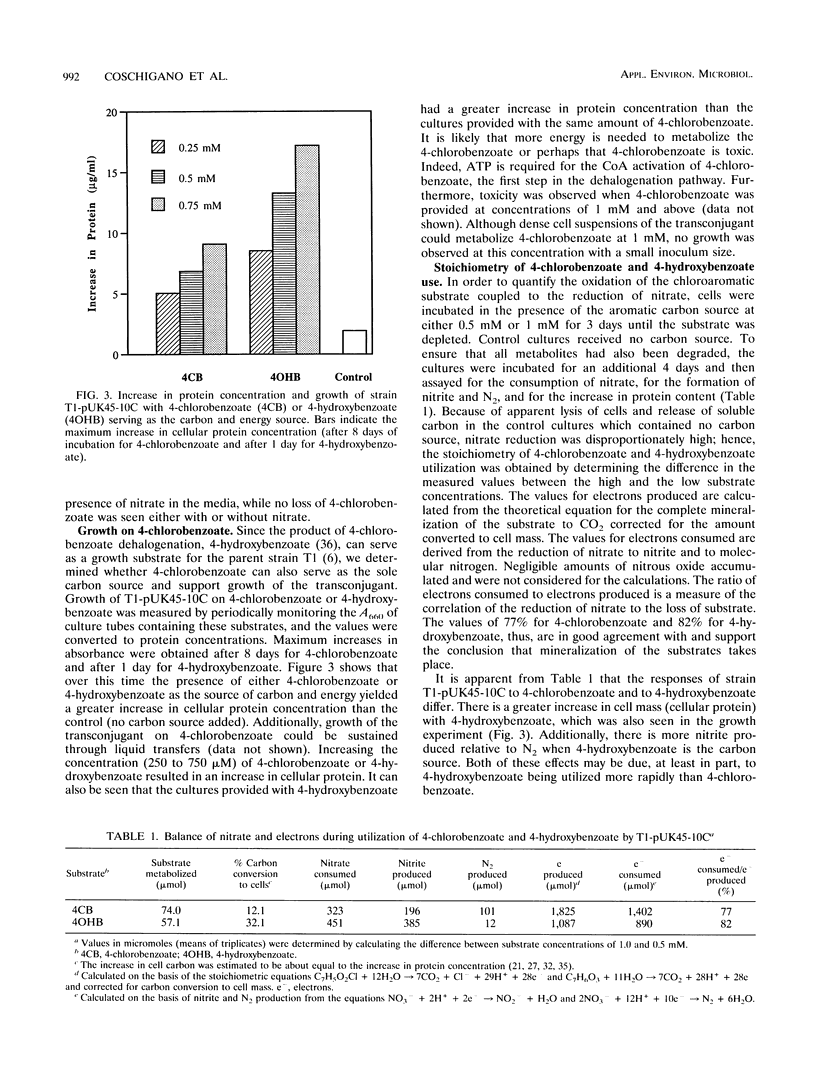
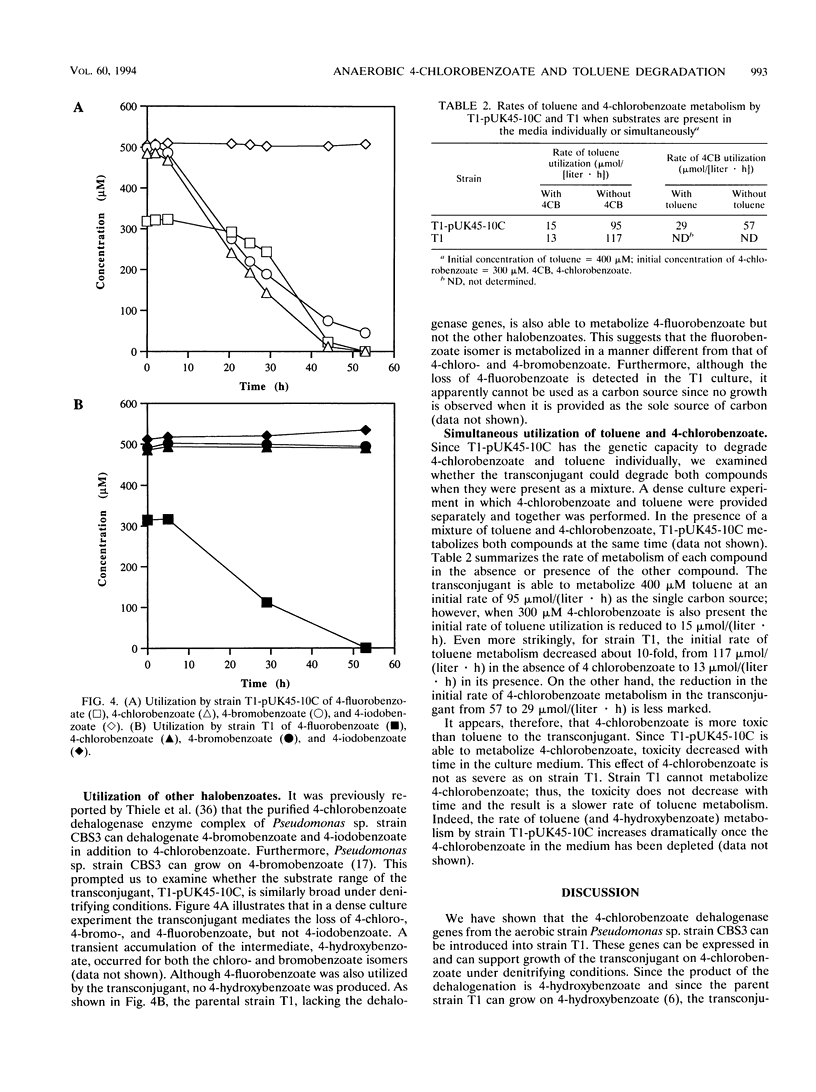
T1, a dentrifying bacterium originally isolated for its ability to grow on toluene, can also metabolize 4-hydroxybenzoate and other aromatic compounds under denitrifying conditions. A cosmid clone carrying the three genes that code for the 4-chlorobenzoate dehalogenase enzyme complex isolated from the aerobic bacterium Pseudomonas sp. strain CBS3 was successfully conjugated into strain T1. The cloned enzyme complex catalyzes the hydrolytic dechlorination of 4-chlorobenzoate to 4-hydroxybenzoate. Since molecular oxygen is not required for the dehalogenation reaction, the transconjugate strain of T1 (T1-pUK45-10C) was able to grow on 4-chlorobenzoate in the absence of O2 under denitrifying conditions. 4-Chlorobenzoate was dehalogenated to 4-hydroxybenzoate, which was then further metabolized by strain T1. The dehalogenation and metabolism of 4-chlorobenzoate were nitrate dependent and were coupled to the production of nitrite and nitrogen gas. 4-Bromobenzoate was also degraded by this strain, while 4-iodobenzoate was not. Additionally, when T1-pUK45-10C was presented with a mixture of 4-chlorobenzoate and toluene, simultaneous degradation of the compounds was observed. These results illustrate that dechlorination and degradation of aromatic xenobiotics can be mediated by a pure culture in the absence of oxygen. Furthermore, it is possible to expand the range of xenobiotic substrates degradable by an organism, and it is possible that concurrent metabolism of these substrates can occur.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenschmidt U., Oswald B., Fuchs G. Purification and characterization of benzoate-coenzyme A ligase and 2-aminobenzoate-coenzyme A ligases from a denitrifying Pseudomonas sp. J Bacteriol. 1991 Sep;173(17):5494–5501. doi: 10.1128/jb.173.17.5494-5501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburger G., Winter J. Purification and characterization of benzoyl-CoA ligase from a syntrophic, benzoate-degrading, anaerobic mixed culture. Appl Microbiol Biotechnol. 1992 Sep;37(6):789–795. doi: 10.1007/BF00174847. [DOI] [PubMed] [Google Scholar]
- Babbitt P. C., Kenyon G. L., Martin B. M., Charest H., Slyvestre M., Scholten J. D., Chang K. H., Liang P. H., Dunaway-Mariano D. Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl:adenyl ligases, enoyl-CoA hydratases/isomerases, and acyl-CoA thioesterases. Biochemistry. 1992 Jun 23;31(24):5594–5604. doi: 10.1021/bi00139a024. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Mang D. T., Kim K. S., Young L. Y. Anaerobic degradation of toluene by a denitrifying bacterium. Appl Environ Microbiol. 1991 Apr;57(4):1139–1145. doi: 10.1128/aem.57.4.1139-1145.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Mang D. T., Young L. Y. Degradation of toluene and m-xylene and transformation of o-xylene by denitrifying enrichment cultures. Appl Environ Microbiol. 1991 Feb;57(2):450–454. doi: 10.1128/aem.57.2.450-454.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Ling W., Young L. Y. Substrate induction and metabolite accumulation during anaerobic toluene utilization by the denitrifying strain T1. Appl Environ Microbiol. 1993 Sep;59(9):3157–3160. doi: 10.1128/aem.59.9.3157-3160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen P. E., Driessen A. J., Konings W. N., de Bont J. A. Energy-dependent uptake of 4-chlorobenzoate in the coryneform bacterium NTB-1. J Bacteriol. 1990 Jan;172(1):419–423. doi: 10.1128/jb.172.1.419-423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A., Suflita J. M., Tiedje J. M. Reductive dehalogenations of halobenzoates by anaerobic lake sediment microorganisms. Appl Environ Microbiol. 1983 May;45(5):1459–1465. doi: 10.1128/aem.45.5.1459-1465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol Rev. 1992 Sep;9(1):29–71. doi: 10.1111/j.1574-6968.1992.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Häggblom M. M., Rivera M. D., Young L. Y. Influence of alternative electron acceptors on the anaerobic biodegradability of chlorinated phenols and benzoic acids. Appl Environ Microbiol. 1993 Apr;59(4):1162–1167. doi: 10.1128/aem.59.4.1162-1167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londry K. L., Fedorak P. M. Benzoic acid intermediates in the anaerobic biodegradation of phenols. Can J Microbiol. 1992 Jan;38(1):1–11. doi: 10.1139/m92-001. [DOI] [PubMed] [Google Scholar]
- Löffler F., Müller R., Lingens F. Dehalogenation of 4-chlorobenzoate by 4-chlorobenzoate dehalogenase from pseudomonas sp. CBS3: an ATP/coenzyme A dependent reaction. Biochem Biophys Res Commun. 1991 May 15;176(3):1106–1111. doi: 10.1016/0006-291x(91)90398-q. [DOI] [PubMed] [Google Scholar]
- Marks T. S., Smith A. R., Quirk A. V. Degradation of 4-Chlorobenzoic Acid by Arthrobacter sp. Appl Environ Microbiol. 1984 Nov;48(5):1020–1025. doi: 10.1128/aem.48.5.1020-1025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn W. W., Tiedje J. M. Microbial reductive dehalogenation. Microbiol Rev. 1992 Sep;56(3):482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Thiele J., Klages U., Lingens F. Incorporation of [18O]water into 4-hydroxybenzoic acid in the reaction of 4-chlorobenzoate dehalogenase from pseudomonas spec. CBS 3. Biochem Biophys Res Commun. 1984 Oct 15;124(1):178–182. doi: 10.1016/0006-291x(84)90933-1. [DOI] [PubMed] [Google Scholar]
- Ruisinger S., Klages U., Lingens F. Abbau der 4-chlorbenzoesäure durch eine Arthrobacter-Species. Arch Microbiol. 1976 Nov 2;110(23):253–256. doi: 10.1007/BF00690235. [DOI] [PubMed] [Google Scholar]
- Savard P., Péloquin L., Sylvestre M. Cloning of Pseudomonas sp. strain CBS3 genes specifying dehalogenation of 4-chlorobenzoate. J Bacteriol. 1986 Oct;168(1):81–85. doi: 10.1128/jb.168.1.81-85.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schocher R. J., Seyfried B., Vazquez F., Zeyer J. Anaerobic degradation of toluene by pure cultures of denitrifying bacteria. Arch Microbiol. 1991;157(1):7–12. doi: 10.1007/BF00245327. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- Zaitsev G. M., Karasevich Iu N. Utilizatsiia 4-khlorbenzoinoi kisloty Arthrobacter globiformis. Mikrobiologiia. 1981 Jan-Feb;50(1):35–40. [PubMed] [Google Scholar]
- van der Meer J. R., de Vos W. M., Harayama S., Zehnder A. J. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol Rev. 1992 Dec;56(4):677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



