Abstract
Aims
To examine individual use of hormone replacement therapy (HRT) in a defined population of Danish women during a 5-year period. HRT may reduce osteoporosis and cardiovascular disease in postmenopausal women, but may also have side-effects. Little is known about the use of HRT in most populations.
Methods
A Pharmacoepidemiological Prescription Database was used to identify all reimbursed prescriptions for HRT in the county during the period 1991 to 1995. The Danish retail pharmacies’ drug subsidy system made it possible to identify prescriptions by individual use.
Results
We examined 255 797 HRT prescriptions issued during the period in the County of North Jutland. Total sales reached 16.5 million defined daily doses (DDDs), purchased by 31 653 women, which corresponds to 26.9% of the female population above the age of 39 years. The annual prevalence proportion of current users rose from 10.4% to 14.8% during the study period, and the therapeutic intensity (DDD/1000 women/day) increased from 20.6 to 32.0. The mean DDD sum of systemic HRT per user was 73.4 in 1991; it and the proportion of users who received less than 90 DDD per year (83.4% in 1991) remained almost constant during the study period. The amount of oestrogen unopposed by progestin was high, 28.1% of all prescriptions.
Conclusions
Less than one-fifth of the study population used HRT for more than 3 months per year, and only 32.8% of the women who were new users of HRT in 1992 continued this therapy throughout the study period.
Keywords: drug use, hormone replacement therapy, oestrogen, prevalence, incidence, therapeutic intensity
Introduction
Many women suffer from postmenopausal symptoms, and osteoporosis is a major health problem in the elderly. About one quarter of ageing women experience a vertebral or hip fracture between the age of 60 to 90 years [1]. Hormone replacement therapy (HRT) is used to relieve symptoms and to prevent long-term sequelae of oestrogen deficiency. This approach is to some degree supported by results from observational studies [2–8] and by biochemical evidence of favourable effects on serum lipids [9], bone density [10], and on arterial tissue in animal studies [11, 12]. However HRT users seem to be healthier than nonusers, which makes selection bias a possible explanation for the beneficial results of HRT treatment [13–15]. In a recently published randomized study, no significant effect of HRT on the risk of cardiovascular death or nonfatal myocardial infarction was found [16]. Less is known about clinical endpoints such as fractures and cancer. Hulley et al. found that HRT increased the rate of thromboembolic events and gallbladder disease [16], and HRT may furthermore have side-effects such as increased risk of breast and endometrial cancer [1, 17–22]. A meta-analysis of more than 160 000 women found a small (1.35; 95% CI: 1.21–1.49) increased risk of breast cancer in women who had used HRT for 5 years or longer, but no increased mortality was found [23]. If oestrogen treatment is not opposed by progestin, the risk of endometrial cancer is approximately four fold greater (95% CI: 3.1–5.1) [17].
HRT may have a major impact on public health, but it is difficult to predict the net effect without valid and precise quantitative estimates of use, benefits, and risk. Existing utilization studies on HRT are almost exclusively based on drug sales figures, since individual data are usually not available [20, 24–26]. The aim of the present study was to examine individual use of HRT in a defined population of Danish women during a 5-year period.
Methods
Data sources
The study was conducted during the 5-year period 1991 to 1995 in the County of North Jutland, Denmark [27]. The population of North Jutland is about 487 000 inhabitants (approximately 10% of the Danish population).
We used a Pharmacoepidemiological Prescription Database to identify all reimbursed HRT prescriptions in the county during the period. All pharmacies are equipped with a computerized accounting system from which data are sent to the health insurance administration of the Danish National Health Service, which provides tax-supported health care for all inhabitants in Denmark. The National Health Service guarantees free access to general practitioners, hospitals, and public clinics, and refunds part of the costs of most drugs prescribed by doctors. If a drug is fully or partly refunded (which is the case with all the HRT drugs in this study), information about the customer’s personal identification number (which incorporates date of birth), the type of drug prescribed according to the Anatomical Therapeutical Chemical (ATC) classification system [28, 29], number of defined daily doses (DDD), and the date of purchase are entered by the pharmacies into the prescription database [27]. The use of a 10-digit personal identification number, which is assigned to all citizens at birth, ensures the establishment of a complete prescription history for each participant.
By means of the ATC classification index code, we extracted all recorded prescriptions for oestrogens (ATC codes G03C and L02A), progestins (G03D), and oestrogen/progestin combinations (G03F and G03H) prescribed for women 40 years of age and older. Each individual in the analysis was characterized by the following variables: age, sex, total drug purchase measured as DDD, ATC code, and date of each prescription [28]. The drug index DDD was developed by the WHO Drug Utilization Research Group [29], and the DDD was established by an expert panel as the assumed average maintenance dose for an adult when a drug is used for its main indication [29].
Epidemiological measures and analyses of drug use
A therapeutic intensity (TI) was calculated as the number of DDDs per 1000 women per day, using the female population in each age group as denominator. TI estimates the proportion of a population (per thousand) undergoing treatment with a particular drug on a given day (or period) of the year [29, 30].
We defined the one year period prevalence as the number of women who presented at least one prescription during the year, using the female background population in the age group as denominator.
The annual number of incident users was calculated as the number of women treated with HRT who had not received treatment during the preceding year. New users in 1991 could not be measured, since we did not have any information from 1990.
The one year cumulative incidence (Cum Inc) gives the probability of the use of HRT in individuals not using HRT during the preceding year, and was calculated as annual incident cases (I) divided by N-P, where N represent the number of persons in the age-specific background population, and P the prevalent cases. In order to evaluate the duration of the therapy we estimated repeat rates as the 1992 proportion of incident cases who received HRT therapy for either 1, 2, 3, or 4 years during the period. Furthermore, the number of prevalent users of systemic HRT receiving less than 90 DDDs/year was calculated in order to estimate short-term HRT.
Permission for this study was given by the local ethics committees in the Counties of North Jutland and Viborg.
Results
We identified 260 774 prescriptions with ATC codes for oestrogens, progestins, and oestrogen/progestin combinations issued to women in the county during the period. Prescriptions for non-residents were excluded (4977), leaving 255 797 prescriptions, for 31 653 women, for the final analysis. This corresponds to 29.9% of the female population above the age of 39 years.
The annual prevalence proportion (Figure 1) and therapeutic intensity (Table 1) increased throughout the period for all age groups, while the annual incidence remained almost stable (Table 1). The prevalence proportion, therapeutic intensity and annual incidence were highest in the age group 50–59 years; the prevalence proportion was 18% in 1991 and 25.5% in 1995. The relative increase in prevalence and therapeutic intensity was highest in the age group 70+; in this age group the prevalence proportion increased from 4.2% in 1991 to 8.2% in 1995.
Figure 1.
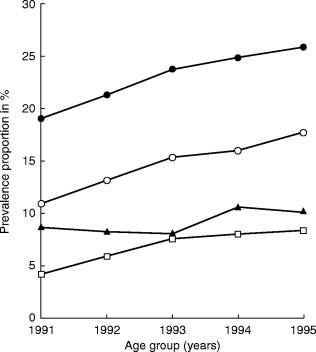
One-year prevalence proportion of hormone replacement therapy use in different age groups (▴ 40–49 years, •50–59 years, ○ 60–69 years, □ over 70 years).
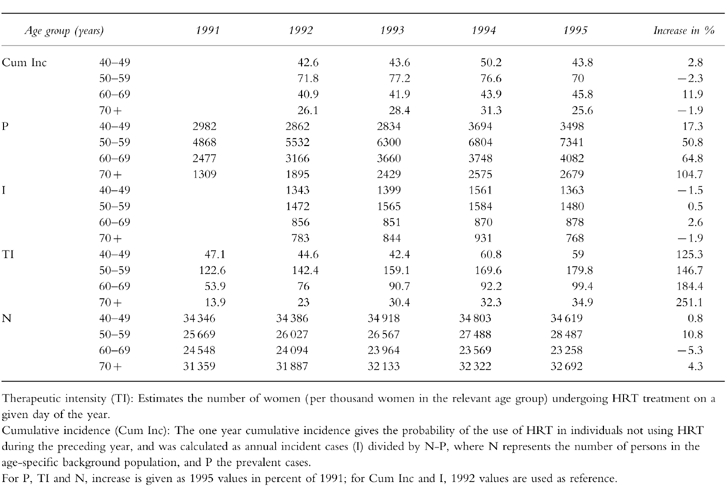
Table 1.
Cumulative incidence (Cum Inc) per 1000 women, number of prevalent cases (P), number of incident cases (I), Therapeutic intensity (TI) and female background population (N) by year of prescription in age groups.
The proportions of prescriptions for oestrogens, oestrogen/progestin combinations, and progestins were calculated from the total amount of HRT prescriptions for each year (Figure 2). Oestrogen unopposed by progestins, accounted for 60–70% of all prescriptions for systemic treatment. The prescribing pattern differed according to age. Among the total amount of prescriptions, the proportion of oestrogen unopposed by progestin, showed a slight increase with age, and women above the age of 69 years received oestrogen almost exclusively.
Figure 2.
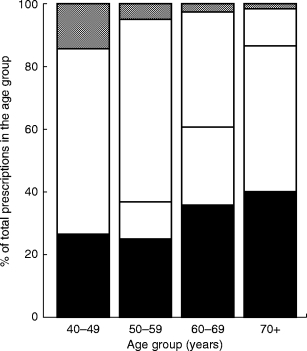
Distribution of HRT prescriptions in age groups. (▪ oestrogen systemic, □ oestrogen local, □ oestrogen/progestin,  progestin.
progestin.
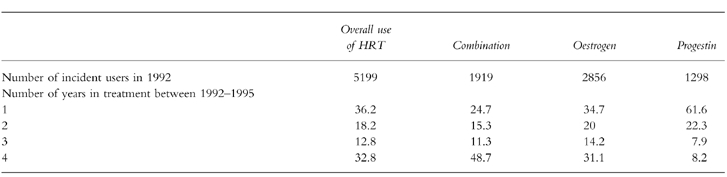
Table 2 shows repeat proportions of 1992 incident cases. The overall 4 year proportion for 1992 incident cases was 36.2%. However, the repeat proportions differed among the drug groups. Thus 1992 incident users of the combination therapy had the highest 4 year repeat proportion (48.7%), and incident users of progestins had the lowest 4 year repeat rate (8.2%). The proportion of women who were incident users in 1992 and only received therapy for 1 year during the study period showed a trend in the opposite direction. Thus 61.6% of women who were incident users of progestin in 1992 received progestin therapy for only 1 year, and 24.7% of incident users of combination therapy in 1992 received combination therapy for only 1 year.
Table 2.
Percentage of incident users in 1992 who received treatment for 1, 2, 3 or 4 years during the study period, within drug groups.
When studying ATC specific 4 year repeat proportions stratified by age (data not shown), the highest proportions were in the 50–59 year-old (60.7%) and 60–69 year-old (57.4%) women who were incident users of combination therapy in 1992. The mean numbers of DDDs per user remained almost constant throughout the study period (71.7–75.0 DDD), as did the number of prescriptions per user (3.2–3.4 per year). The proportion of prevalent users of systemic HRT who received less than 90 DDDs per year was high throughout the study period (80.9–85.3%).
Discussion
The observed prevalence of HRT users increased in all age groups during the study period. The incidence was almost constant, suggesting a tendency towards increased duration of use. However, a persistently large proportion of users received HRT for less than 3 months per year; thus HRT was probably used to relieve climacteric symptoms and not to prevent osteoporosis or cardiovascular diseases. Other studies have also indicated that HRT is mainly used to relieve climacteric complaints, and hardly ever used for prevention of osteoporosis and cardiovascular diseases [13–15, 31–[34]. Systemic oestrogen unopposed to progestin, was used to a high degree especially in the older age groups.
The observed prevalence was in accordance with the findings of Oddens & Boulet (18.4%) who carried out a cross-sectional national survey in Denmark in 1994 among 1,015 women aged 45–65 years [32]. Other Danish studies have reported a prevalence of 16% in women aged 40–59 [33], and 22% in 51-year-old women living in Copenhagen [34].
We found that only 32.8% of the women who were incident users of HRT in 1992, also used it during the following 3 years. Furthermore, HRT treatment was almost exclusively used as intermittent, short-term therapy, which probably has limited or no effect on osteoporosis or cardiovascular diseases. For instance, in the Nurses Health Study in the USA, the beneficial effect of oestrogens on the risk of coronary diseases was lost fairly soon following cessation of HRT [35]. This could also explain why the use of HRT by elderly women was low, because these women no longer had postmenopausal symptoms [25]. In a recent analysis Col et al. concluded that HRT use increased life expectancy for nearly all postmenopausal women, and that the benefits of HRT in reducing the risk of cardiovascular diseases outweighed the risk of breast cancer [36]. However Hulley et al. did not find any protective effect of HRT on the risk of cardiovascular death or myocardial infarction [16]; this study included postmenopausal women with established coronary disease. A high degree of HRT use may be beneficial to women’s health if the treatment is cost-effective, but the long-term health outcome of HRT utilization patterns, as reported here in Danish women, are impossible to predict. One can only speculate how intermittent, short-term HRT will affect the risk of cancer.
The high proportion of preparations for systemic oestrogen unopposed by progestin, and its increase with age, was not in agreement with other findings. A study in the USA reported that 69% used oestrogen alone [20], and in a recent prescription study in Scotland, 46% of HRT prescribed to 19 500 users were unopposed preparations [37]. Combined oestrogen and progestin therapy has been recommended to reduce the risk of endometrial cancer during HRT, and oestrogen without progestin treatment might be for women whose uterus has been removed. However, we found that very few women above the age of 70 years received combination therapy and the reason for this is probably the strong dislike of induced bleeding after the natural menopause [32].
The advantages, i.e. the population-based design, and limitations of our study should be noted. Oestrogen/progestin drugs prescribed for birth control were not included in our database since these drugs are not subsidised by the National Health Service, and we may thus underestimate the use of HRT. A 1990 survey on sales statistics found that 6% of Danish women between 40 and 44 years were treated with oral contraceptives [38]. Furthermore, we did not have the clinical indications for HRT use, and there might be discrepancies between prescribed and defined daily doses [30, 39].
In conclusion, our data demonstrate that the overall use of HRT is slowly increasing, but many women still tend to use HRT as intermittent short-term therapy. The public health benefits of this pattern of utilization are probably very limited with regard to prevention of osteoporosis and cardiovascular disease, and the consequences for the risk of cancer are unknown. Some doctors and patients may find the current evidence from epidemiological studies on risks and benefits of HRT too unreliable to decide whether to use HRT, and a more widespread acceptance of HRT for preventive purposes may await further ongoing randomized trials.
Acknowledgments
This study was supported by the Danish National Research Foundation, the Danish Medical Research Council (grant no. 9700677), Helsefonden (grant no. 11/121–95), and the Aarhus University Research Foundation (grant no. F-1996-SUN-1–77).
References
- 1.Ernster VL, Bush TL, Huggins GR, Hulka BS, Kelsey JL, Schottenfeld D. Benefits and risks of menopausal estrogen and/or progestin hormone use. Prev Med. 1988;17:201–223. doi: 10.1016/0091-7435(88)90064-3. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Paganini Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–78. [PubMed] [Google Scholar]
- 3.Walsh LJ, Wong CA, Pringle M, Tattersfield AE. Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross sectional study. Br Med J. 1996;313:344–346. doi: 10.1136/bmj.313.7053.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukert BP, Johnson BE, Robinson RG. Estrogen and progesterone replacement therapy reduces glucocorticoid-induced bone loss. J Bone Mineral Res. 1992;7:1063–1069. doi: 10.1002/jbmr.5650070909. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg L, Palmer JR, Shapiro S. A case-control study of myocardial infarction in relation to use of estrogen supplements. Am J Epidemiol. 1993;137:54–63. doi: 10.1093/oxfordjournals.aje.a116602. [DOI] [PubMed] [Google Scholar]
- 6.Ross RK, Paganini Hill A, Mack TM, Henderson BE. Cardiovascular benefits of estrogen replacement therapy. Am J Obstet Gynecol. 1989;160:1301–1306. doi: 10.1016/s0002-9378(89)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki E, McPherson K. Impact of postmenopausal hormone therapy on cardiovascular events and cancer: pooled data from clinical trials. Br Med J. 1997;315:149–153. doi: 10.1136/bmj.315.7101.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tikkanen MJ, Nikkila EA, Vartiainen E. Natural oestrogen as an effective treatment for type-II hyperlipoproteinaemia in postmenopausal women. Lancet. 1978;ii:490–491. doi: 10.1016/s0140-6736(78)92216-x. [DOI] [PubMed] [Google Scholar]
- 10.Bilizikian JP, Silverberg SJ. Osteoporosis: a practical approach to the perimenopausal woman. J Women’s Research. 1992;1:21–27. [Google Scholar]
- 11.Beldekas JC, Smith B, Gerstenfeld LC, Sonenshein GE, Franzblau C. Effects of 17 beta-estradiol on the biosynthesis of collagen in cultured bovine aortic smooth muscle cells. Biochemistry. 1981;20:2162–2167. doi: 10.1021/bi00511a014. [DOI] [PubMed] [Google Scholar]
- 12.Fischer GM. In vivo effects of estradiol on collagen and elastin dynamics in rat aorta. Endocrinology. 1972;91:1227–1232. doi: 10.1210/endo-91-5-1227. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Hemminki E, McPherson K, et al. Re: ‘Prior to use of estrogen replacement therapy, are users healthier than nonusers?’. Am J Epidemiol. 1997;146:283. doi: 10.1093/oxfordjournals.aje.a009264. [DOI] [PubMed] [Google Scholar]
- 14.Grodstein F. Invited commentary: can selection bias explain the cardiovascular benefits of estrogen replacement therapy? Am J Epidemiol. 1996;143:979–982. doi: 10.1093/oxfordjournals.aje.a008679. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 16.Hulley S, Grady D, Bush T, Furberg C, Herrington D, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 17.Beresford SAA, Weiss SN, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet. 1997;349:458–461. doi: 10.1016/S0140-6736(96)07365-5. [DOI] [PubMed] [Google Scholar]
- 18.Willis DB, Calle EE, McMahill HL, Heath CW. Estrogen replacement therapy and risk of fatal breast cancer in a prospective cohort of postmenopausal women in the United States. Cancer Causes Control. 1996;7:449–457. doi: 10.1007/BF00052671. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Mervis CA, Thun MJ, Rodriguez C, Wingo PA, Heath CW, Jr Jr. Diethylstilbestrol and risk of fatal breast cancer in a prospective cohort of US women. Am J Epidemiol. 1996;144:645–652. doi: 10.1093/oxfordjournals.aje.a008976. [DOI] [PubMed] [Google Scholar]
- 20.Brett MK, Madams HJ. Use of postmenopausal hormone replacement therapy: estimates from a nationally representative cohort study. Am J Epidemiol. 1997;145:536–545. doi: 10.1093/oxfordjournals.aje.a009142. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines for counseling postmenopausal women about preventive hormone therapy. American College of Physicians. Ann Intern Med. 1992;117:1038–1041. doi: 10.7326/0003-4819-117-12-1038. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw JM, Warlow CP, Counsell C. Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet. 1997;350:607–614. doi: 10.1016/s0140-6736(97)03022-5. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52705 women with breast cancer and 108411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 24.Griffiths F, Jones K. The use of hormone replacement therapy; results of a community survey. Fam Pract. 1995;12:163–165. doi: 10.1093/fampra/12.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Handa VL, Landerman R, Hanlon JT, Harris T, Cohen HJ. Do older women use estrogen replacement? Data from the Duke Established Populations for Epidemiologic Studies of the Elderly (EPESE) J Am Geriatr Soc. 1996;44:1–6. doi: 10.1111/j.1532-5415.1996.tb05630.x. [DOI] [PubMed] [Google Scholar]
- 26.Jolleys JV, Olesen F. A comparative study of prescribing of hormone replacement therapy in USA and Europe. Maturitas. 1996;23:47–53. doi: 10.1016/0378-5122(95)00952-3. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen GL, Sørensen HT, Weijin Z, Steffensen FH, Olsen J. The Pharmacoepidemiological Prescription Database of North Jutland. Int J Risk & Safety in Med. 1997;10:203–205. doi: 10.3233/JRS-1997-10309. [DOI] [PubMed] [Google Scholar]
- 28.Guidelines for ATC and DDD. World Health Organization Collaborative centre for Drug Statistics Methodology. 1996 [Google Scholar]
- 29.Methods and uses. Introduction. WHO Regional Publications; 1993. Drug utilization studies. European Series, No.45. [PubMed] [Google Scholar]
- 30.Bergman U, Sjoqvist F. Measurement of drug utilization in Sweden: methodological and clinical implications. Acta Med Scand Suppl. 1984;683:15–22. doi: 10.1111/j.0954-6820.1984.tb08709.x. [DOI] [PubMed] [Google Scholar]
- 31.Barrett Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med. 1991;115:455–456. doi: 10.7326/0003-4819-115-6-455. [DOI] [PubMed] [Google Scholar]
- 32.Oddens BJ, Boulet MJ. Hormone replacement therapy among Danish women aged 45-65 years: prevalence, determinants, and compliance. Obstet Gynecol. 1997;90:269–277. doi: 10.1016/S0029-7844(97)00264-0. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen SH, Jeune B. Prevalence of hormone replacement therapy in a sample of middle aged women. Maturitas. 1988;9:339–345. doi: 10.1016/0378-5122(88)90099-0. [DOI] [PubMed] [Google Scholar]
- 34.Köster A. Hormone replacement therapy: Use patterns in 51-year-old Danish women. Maturitas. 1990;12:345–356. doi: 10.1016/0378-5122(90)90014-w. [DOI] [PubMed] [Google Scholar]
- 35.Stampfer MJ, Colditz GA, Willett WC. Postmenopausal estrogen therapy and cardiovascular disease. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 36.Col NF, Eckman MH, Karas RH, et al. Patient-specific decisions about hormone replacement therapy in postmenopausal women. JAMA. 1997;277:1140–1147. [PubMed] [Google Scholar]
- 37.Evans JMM, Orr C, Duncan DI, MacDonald TM. Use of hormone replacement therapy in the community: could this be improved? Pharmacoepidemiol Drug Safe. 1997;6:81. [Google Scholar]
- 38.Lidegaard O. Use of oral contraceptives in Denmark 1980–1990 and smoking habits among fertile women in 1990. Ugeskr Laeger. 1993;155:3550–3558. [PubMed] [Google Scholar]
- 39.Studies in drug utilization. Methods and applications. WHO Regional Publications; 1979. European Series, No.8. [Google Scholar]


