Abstract
Aims
The aim of this study was to determine the potential influence of variables such as the cell content in the fluid, and serum levels, on the concentrations of ceftibuten, cefixime and azithromycin in the middle ear fluid of patients suffering from acute otitis media.
Methods
This randomized, open study compared the penetration of ceftibuten (9 mg kg−1, 18 patients), cefixime (8 mg kg−1, 16 patients) and azithromycin (10 mg kg−1, 16 patients) into the intracellular and extracellular compartments of middle ear fluid of 50 paediatric patients (aged 8–14 years) with acute otitis media. Middle ear fluid was extracted by tympanocentesis 4, 12 and 24 h after dosing and divided into two fractions: with cells (as collected) (C+) and cell-free (C−). Antibiotics were assayed in C+ and C− samples by h.p.l.c.
Results
Ceftibuten achieved greater penetration into middle ear fluid than cefixime and azithromycin. Higher concentrations of ceftibuten (CTB) and cefixime (CFX) were found in the C− fraction (CTB: 4 h 13.3 ±1.86; 12 h 4.7 ±1.18; 24 h 0.5 ±0.2. CFX: 4 h 3.2 ± 1.4; 12 h 1.5 ±0.5; 24 h > 0.1 mg l−1) than in the C+ fraction (CTB:4 h 8.4 ±4.3; 12 h 2.88 ± 1.19; 24 h 0.3 ± 0.27. CFX: 4 h 1.2 ± 0.6; 12 h 0.8 ± 0.2; 24 h >0.1 mg l−1) at the each time point, while the opposite was true for azithromycin (C−: 4 h 0.11 ± 0.04; 12 h 0.12 ± 0.08; 24 h 0.23 ± 0.12. C+: 4 h 0.38 ± 0.24; 12 h 0.9 ± 0.03; 24 h 1.05 ± 0.3 mg l−1).
Conclusions
This study demonstrates that the penetration of antibiotics into the middle ear fluid is influenced by its serum concentrations as well as by the cell content in the fluid. Ceftibuten achieved higher middle ear fluid concentrations than cefixime in C+ and C− fractions at all time points. Both ceftibuten and cefixime concentrations are negatively influenced by the cell content in the fluid. In contrast the concentration of azithromycin to the middle ear fluid is positively influenced by the cell content in the fluid.
Keywords: acute otitis media, azithromycin penetration, cefixime, ceftibuten, middle ear fluid
Introduction
Treatment of acute otitis media in children remains largely empirical, as diagnostic and microbiological culture techniques are often expensive and/or time-consuming. In a substantial percentage (8–15%) of children who receive antibiotic therapy for acute otitis media or effusive otitis media infection does not resolve clinically [1]. This clinical failure rate may be due to a variety of reasons including resistance of the major causative bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis), inadequate middle ear fluid penetration of the antibiotic, confounding viral infection or physiological or immunological dysfunction [2–4]. The potential clinical efficacy of antibiotics in the treatment of acute otitis media is best predicted by direct testing of middle ear fluid for concentrations of antibiotics which can then be compared with minimum inhibitory concentrations (MICs) and localized patterns of resistance [3, 4]. It has been suggested that clinical efficacy in acute otitis media is best determined by the bioavailability of the antibiotic within the aural compartment and whether this achieves MIC90s against the major causative pathogens [4].
Acute otitis media treatment is most often initiated with the first-line antibiotic amoxicillin, alone or in combination with clavulanate [5]. However, due to treatment failure rates, other newer drugs such as the broad spectrum cephalosporins, enhanced beta-lactams or newer macrolides are required [4]. Ceftibuten is a once daily oral third generation cephalosporin with a broad spectrum of activity in vitro and in vivo [6–8]. The bioavailability of ceftibuten (capsule or suspension formulation) is between 75 and 90% following oral administration, and the apparent volume of distribution after a single 400 mg dose was between 0.2 and 0.25 l kg−1. Ceftibuten penetrates cranio-facial tissue effectively. The drug is about 60–64% bound to plama protein. The elimination half-life is about 3 h [9–12]. Cefixime, like ceftibuten, is a third generation cephalosporin with activity against the major pathogens causing acute otitis media [13]. Cefixime has some activity against β-lactamase producing bacteria [7, 13] but little against penicillin-resistant S. pneumoniae. The bioavailability of cefixime (capsule or suspension formulation) is between 40 and 52% following oral administration, the apparent volume of distribution after a single intravenous dose was 0.67 l kg−1. Cefixime penetrates cranio-facial tissue effectively. The drug is about 70% bound to plasma protein. The elimination half-life is about 3 h. Azithromycin is a newer macrolide antibiotic that has improved activity, dosing, pharmacokinetics and less drug interactions than erythromycin and to some extent, clarithromycin [14–16]. The oral bioavailability of a single 500 mg dose of azithromycin in fasting healthy volunteers was 37% and the apparent volume of distribution was 23 l kg−1. The drug is distributed extensively in the tissues and slowly eliminated. The plasma half-life elimination was found to be 57 h. Serum protein binding of azithromycin is non linear over a range of serum concentrations and ranging from 50% at concentration of 0.02 mg l−1 to 37% at 0.7 mg l−1 [17].
The method of assay of middle ear fluid has also been reported to influence study results [9]. Homogenization of a tissue sample, centrifugation and subsequent assay of the supernatant have been used to predict the activity of antibiotic concentrations. This method assumes that the tissue is homogeneous and antibiotics as well as bacteria are evenly distributed distributed through it. Tissues, however, are not homogeneous and antibiotics as well as bacteria are not evenly distributed [18–20]. Within infective tissue, an antibiotic may be concentrated in cells and/or interstitial fluid, depending upon its physico-chemical properties [21]. This study aimed to provide data in both sequential middle ear fluid samples from children with acute otitis media and the penetration of two third generation oral cephalosoporins: ceftibuten that is well absorbed and exhibits high blood levels and cefixime that is poorly absorbed and exhibits relative low blood levels, azithromycin a new macrolide antibiotic that exhibits high intracellular penetration. These data, correlated with MIC information and resistance rates, could form the basis of a guideline for the comparative efficacies of these three antibiotics in acute otitis media. In turn, such data could help with clinical decision making for acute otitis media patients.
Methods
This was a randomised, open-label, study in 50 paediatric patients (22 male and 28 female aged 8–14 years) diagnosed with acute otitis media. The clinical protocol (and any revisions) were approved by the Institutional Review Board, Ear Nose and Throat Hospital, Vimercate, Milan, Italy. Written, informed consent was obtained from the patients’ parents or guardians prior to study enrolment or the initiation of any study procedure. Patients were enrolled if they exhibited the clinical signs and symptoms of acute otitis media and had no other antimicrobial drug for 2 weeks prior to the study entry, or any serious infection or disease which might affect antibiotic absorption. Patients were randomised to receive a single oral dose of either ceftibuten 9 mg kg−1 (18 patients), cefixime 8 mg kg−1 (16 patients) or azithromycin 10 mg kg−1 (16 patients). Middle ear fluid was extracted by tympanocentesis at 4, 12 and 24 h postdose.
Each, blood-free, sample was divided into two fractions for antibiotic assay. One fraction (C+) was sonicated to lyse cells, homogenized by using a Ultra-Turrax blade homogenizer (3 burst, 6 s each) under constant cooling, centrifuged and supernatant was assayed; supernatant included the content of the cells (most cells were inflammatory i.e. granulocytes, macrophages and lymphocytes a lesser extend were cells from the surrounding tissue i.e. epithelial cells). The second fraction (C−) was cleared from the cells and the fluid was assayed for antibiotics. The cell-free fraction was obtained via centrifugation in a refrigerated centrifuge at 13000 rev min−1 for 2 min.
Samples were all assayed in parallel, for each of the antibiotics. Cephalosporins were assayed by h.p.l.c. as previously described [22]. Briefly, duplicate samples (400 μl) of C− and C+ were combined with 20 μl of internal standard (acyclovir 100 μg ml−1) and 600 μl acetonitrile (Bracco, Italy). The mixtures were then vortexed for 20 s and subsequently centrifuged for 2 min at 13 000 rev min−1. The supernatant was removed and 3 ml dichloromethane (Bracco, Italy) added. The mixtures were vortexed for 30 s and subsequently centrifuged at 2500 rev min−1 for 15 min (with a 95% estimated recovery). A 20 μl aliquot of each ceftibuten-and cefixime-containing sample was analysed by h.p.l.c. in isocratic conditions using as mobile phase ammonium acetate 0.05 m (Meerck,Darmstadt Germany) and acetonitrile (985/15 v/v, Merck) at a flow rate of 1 ml min−1. Standard solutions were made up in drug free human serum and phosphate buffer with concentrations ranging from 0, 1 to 50 μg ml−1. The h.p.l.c. system consisted of a Shimadzu Liquid Chromatograph equipped with a variable wavelength u.v. detector SPD-6 A, LC-9 A pumps, SIL-9 A Autosampler and reverse-phase column of C18 Spherisorb. The method was sensitive (detection limits 0.1–1 μg ml−1) and reproducible (coefficient of variation 2.9–4.5%) and gave high recovery rates (95% estimated recovery). Azithromycin-containing samples were prepared and assayed by h.p.l.c. with electrochemical detector as described previously [17]. Briefly: drug was isolated from supernatants and from fluid homogenates by solvent extraction with methyl-t-buthyl ether under alkaline conditions-back-extraction into a citric acid solution and reextraction with methyl-t-buthyl ether under alkaline conditions. The ether layer was evaporated to dryness and the residue taken up in an acetonitrile:water (1:1) mixture and washed with hexane. Aliquots were injected using acetonitrile:potassium phosphate (3:7) adjusted to pH 11 as mobile phase at rate of 1 ml min−1. The h.p.l.c. system was equipped with an electtrochemical detector (ESA-5100 A-Coulochem), the control potential E1 was set at+0.7 V and the oxidating potential was set at +0.9 V; a Bondapak C18 (300 ±3.9 mm) column was used. The method was sensitive (detection limits 0.1–2 μg ml−1) and reproducible (coefficient of variation 3.1–5.2%) and gave high recovery rates (92%) estimated recovery).
Levels of the three antibiotics in the samples were recorded and their standard deviations derived.
Results
The concentrations of once daily oral ceftibuten, cefixime and azithromycin in the fluid containing cells (C+) and in the free cell fluid (C−) fractions of the middle ear fluid are shown in Table 1. The cephalosporins and the macrolide show opposite behaviour. Ceftibuten and cefixime concentrations are higher in the C− fractions than in the C+ fractions while azithromycin is higher in the C+ fractions than in the C− (Figures 1 and 2). Peak concentration of ceftibuten and cefixime occurred rapidly after dosing (4 h) in both the C+ and C− fractions. Peak ceftibuten concentrations were 4–8 fold greater than those of cefixime and 20–100 fold greater than those of azithromycin (Table 1). Ceftibuten and cefixime concentrations declined between 4 and 24 h postdosing (Figure 2). Concentrations of azithromycin in the C− fractions were low throughout the 24 h duration of the study (Figure 2). 24 h postdosing the concentration of ceftibuten in the C− fraction was ≈2-fold that of azithromycin while, at the same time, cefixime was unmeasurable. The concentrations of ceftibuten and cefixime in the C+ fraction peaked at 4 h postdosing and declined thereafter. Conversely, azithromycin slowly increased into the C+ fraction of middle ear fluid and the maximum concentration was found 24 h postdosing when it was ≈3-fold that of ceftibuten while cefixime was unmeasurable (Table 1; Figure 1).
Table 1.
Mean (±s.d.) middle ear fluid ceftibuten, cefixime and azithromycin concentrations (μg ml−1) in the total (C+) and cell-free (C−) fraction h postdose.
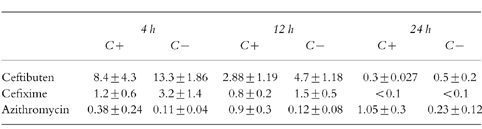
Figure 1.
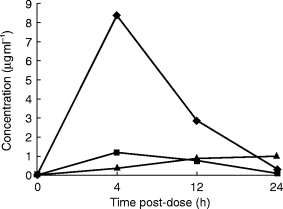
Time course of ceftibuten, cefixime and azithromycin in the total (C+) fraction of the middle ear fluid.
Figure 2.
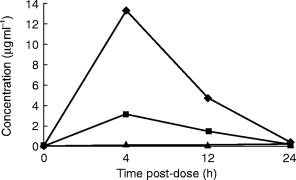
Time course of ceftibuten, cefixime and azithromycin in the cell-free fraction of the middle ear fluid.
Discussion
The choice of an antimicrobial agent depends not only on the drug activity against the suspected pathogens, but also on its ability to reach and maintain effective concentrations at sites of infections. Antibiotic concentrations within the middle ear fluid are governed by the same factors determining serum and tissue concentrations in other parts of the body. As far as the concentration at the site of infection is concerned the distribution is the most important parameter to be considered. The total blood supply, the lipid content of the tissue, the lipophilic or lipophobic character of the drug will affect the rapidity of the drug delivery at the tissue site.
The total inflammatory fluid concentrations as well as the total serum concentrations may have no direct correlation with the concentration at the target site. Inflammatory fluid is composed of fluid and the cells themselves. Since antibiotics may penetrate into the cells, the cell content in the fluid may influence the total fluid concentration.
Beta-lactam antibiotics such as cephalosporins and penicillins are unevenly distributed in tissue, with a tissue: serum ratio <1:1 for most sites. They are distributed mostly in the blood and extracellular fluid, that represents about 20% of the total body mass. Conversely macrolides have high tissue:serum ratios (>2:1) and are found predominantly inside cells. Concentrations of these drugs are therefore lower extracellularly while concentrations of beta-lactams are higher. Ceftibuten and cefixime are distributed mainly in the extracellular fluids, and are underestimated inside cells while azithromycin extensively penetrates the intracellular space and is overestimated in traditional methods of estimation using homogenised samples.
The majority of common bacterial pathogens are confined to the blood and interstitial tissue fluid, which is precisely where aminoglycosides and beta-lactam antibiotics are found. Since the concentration of an antibiotic in interstitial tissue fluid often equates with its concentration in serum, estimation of the latter may be adequate to predict efficacy despite beta-lactams having low and macrolides having high tissue: serum ratios. Apart from straight-forward errors in the methods of processing the assay of drug concentrations in tissue, the concentrations in extracellular fluid (site of bacterial growth) of beta-lactams are grossly understimated while macrolide concentrations are overestimated [18–20].
The current study shows that the tested cephalosporins rapidly and extensively penetrate into the middle ear fluid of paediatric patients with acute otitis media. It has been recently postulated that macrolides achieve higher middle ear fluid concentrations than cephalosporins or penicillins [4]. However, in this study, the concentrations of azithromycin were mainly lower than ceftibuten and cefixime, and were only higher at the 24 h postdosing point in the C+ fraction. Comparison of the penetration of the antibiotics into the middle ear fluid in this study with that in a recent review [4], reveals a similar level of penetration of cefixime but a lower level of penetration of azithromycin. This difference in the macrolide penetration could be due to a number of factors, not least the use of pooled data in the Harrison study [4], a different patient population, multiple antibiotic dosing or sample collection and assay conditions.
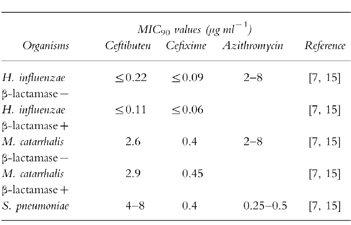
Comparison of the middle ear fluid concentrations of the antibiotics with published MIC90s can provide a guide to the potential for bacterial eradication and also clinical success in acute otitis media [4]. Analysis of published MIC90s for ceftibuten [7], cefixime [15] and azithromycin [14] (Table 2), and the middle ear fluid concentrations from this study, allows a comparison to be made of the potential antibacterial capacity of the antibiotics in the middle ear fluid. Ceftibuten and cefixime achieved MIC90 concentrations for H. influenzae (including β-lactamase producing strains), M. catarrhalis (including β-lactamase producing strains) and S. pneumoniae in both the C+ and C− compartments at 0–12 h postdosing. At 24 h postdosing, ceftibuten still achieved MIC90 for H. influenzae (including β-lactamase producing strains) in both the C− and C+ fractions. The potential antibacterial coverage of azithromycin is represented by its concentration-time profile. In the C− fraction, azithromycin did not achieve MIC90 concentrations for any of the major acute otitis media pathogens until 12 h postdosing. Only by 24 h postdosing did azithromycin concentrations reach levels sufficient to provide coverage against H. influenzae, M. catarrhalis and S. pneumoniae. Considering the long half-life of this drug these concentrations should be maintained during prolonged treatment. These data suggest that once daily ceftibuten can achieve rapid middle ear fluid concentrations capable of antibacterial activity against the major acute otitis media pathogens. Cefixime has similar penetrative power but the azithromycin data seems to confirm recent speculation [15] that achieving concentrations for antibacterial activity against H. influenzae in the extracellular fluid may be problematic. Comparison of these data with a similar analysis in the recent review by Harrison [4] yields broadly similar results, but the middle ear fluid antibacterial capacity of azithromycin in the current study appears lower.
Table 2.
In vitro activity of ceftibuten, cefixime, and azithromycin against the main pathogens; acute otitis media.
In conclusion the interpretation of the concentrations of antibiotics in the middle ear fluid must take account the cell content in the fluid, that represents a ‘dead space’ for beta-lactam antibiotics while representing a reservoir for macrolides. This type of study and these data may be useful in clinical decision-making for the treatment of acute otitis media.
Acknowledgments
This work was supported in part by a public grant (MURST 60%) and by an educational grant from Schering Plough.
References
- 1.Harrison CJ, Marks MI, Welch DF. Microbiology of recently treated acute otitis media compared with previously untreated acute otitis media. Pediatr Infect Dis J. 1985;4:641–646. doi: 10.1097/00006454-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Barza M. Principles of tissue penetration of antibiotics. J Antimicrobial Chemotherapy. 1981;8(Suppl C):7–28. doi: 10.1093/jac/8.suppl_c.7. [DOI] [PubMed] [Google Scholar]
- 3.Craig WA. Interreationship between pharmacokinetics and pharmacodynamics in determining dosage regimes for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 4.Harrison CJ. Using antibiotic concentrations in middle ear fluid to predict potential clinical efficacy. Pediatr Infect Dis J. 1997;16:S12–S16. doi: 10.1097/00006454-199702001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. Pediatr Infect Dis J. 1997;16:S9–S11. doi: 10.1097/00006454-199702001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Barry A, Jones R The Collaborative Antimicrobial Susceptibility Testing Group. Interpretative criteria and quality control limits for ceftibuten disc-susceptibility tests. J Clin Microbiol. 1990;28:605–607. doi: 10.1128/jcm.28.3.605-607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiseman L, Balfour J, Ceftibuten Ceftibuten. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1994;47:784–808. doi: 10.2165/00003495-199447050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Jones R. Ceftibuten: a review of the antimicrobial activity, spectrum and other microbiological features. Pediatr Infect Dis J. 1995;14(Suppl 1):S83–S83. [PubMed] [Google Scholar]
- 9.Barr W, Affrime M, Lin C, Batka V. Pharmacokinetics of ceftibuten in children. Pediatr Infect Dis J. 1993;12:55–63. doi: 10.1097/00006454-199507001-00005. [DOI] [PubMed] [Google Scholar]
- 10.Blumer J, McLinn S, De Abate CA, et al. Multinational multicenter controlled trial comparing ceftibuten with cefaclor for the treatment of acute otitis media. Pediatr Infect Dis J. 1995;14(Suppl 1):S115–S120. doi: 10.1097/00006454-199507001-00008. [DOI] [PubMed] [Google Scholar]
- 11.McLinn SE, McCarty JM, Perrotta R, et al. Multicenter controlled trial comparing ceftibuten with amoxicillin/clavulanic acid in the empiric treatment of acute otitis media. Pediatr Infect Dis J. 1995;14(Suppl 1):S108–S114. doi: 10.1097/00006454-199507001-00007. [DOI] [PubMed] [Google Scholar]
- 12.Scaglione F, Pintucci JP, Demartini G, Dugnani S. Ceftibuten concentration in human tonsillar tissue. Eur J Clin Microbiol Infect Dis. 1996;15:940–943. doi: 10.1007/BF01690513. [DOI] [PubMed] [Google Scholar]
- 13.Markham A, Brogden RN. Cefixime: a review of its therapeutic efficacy in lower respiratory tract infections. Drugs. 1995;49:1001–1022. doi: 10.2165/00003495-199549060-00010. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. Azithromycin concentrations at the site of pulmonary infection. Eur Resp J. 1990;3:886–890. [PubMed] [Google Scholar]
- 15.Guay DRP. Macrolide antibiotics in paediatric infectious diseases. Drugs. 1996;51:515–536. doi: 10.2165/00003495-199651040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pascual A, Rodriguez-Bano J, Ballesta S, Garcia I, Perea EJ. Azithromycin uptake by tissue cultured epithelial cells. J Antimicrob Chemother. 1997;39:293–295. doi: 10.1093/jac/39.2.293. [DOI] [PubMed] [Google Scholar]
- 17.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 18.Schentag JJ. Clinical significance of antibiotic tissue penetration. Clin Pharmacokinet. 1989;16(Suppl 1):25–31. doi: 10.2165/00003088-198900161-00005. [DOI] [PubMed] [Google Scholar]
- 19.Scaglione F, Demartini G, Dugnani S, Fraschini F. A new model examining intracellular and extracellular activity of amoxicillin, azithromycin, and clarithromycin in infected cells. Chemotherapy. 1990;34:1056–1060. doi: 10.1159/000238987. [DOI] [PubMed] [Google Scholar]
- 20.Scaglione FG, Demartini MM, Arcidiacono JP, Pintucci Pintucci. Optimum treatment of streptococcal pharyngitis. Drugs. 1997;53:86–97. doi: 10.2165/00003495-199753010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ryan DM, Cars O, Hoffstedt B. The use of antibiotic serum levels to predict concentrations in tissues. Scand J Infect Dis. 1986;18:381–388. doi: 10.3109/00365548609032352. [DOI] [PubMed] [Google Scholar]
- 22.Chan CY, Chan K, French GL. Rapid high performance liquid chromatographic assay of cefalosporins in biological fluids. J Antimicrob Chemother. 1986;18:537–545. doi: 10.1093/jac/18.4.537. [DOI] [PubMed] [Google Scholar]


