Abstract
Aims
The effect of the electrostatic charge in plastic spacers in vivo on drug delivery to the lung of hydrofluoroalkane (HFA) salbutamol spray was studied in children.
Methods
Five children, aged 7–12 years, were included in a 3-way crossover randomised single-blind trial. Salbutamol HFA spray was delivered on 3 different study days from plastic spacers with mouthpiece. Pre-treatment of the spacers differed between study days: (a) Non-electrostatic 350 ml Babyhaler (coated with benzalkonium chloride) (b) New 350 ml Babyhaler (rinsed in water), and (c) New 145 ml AeroChamber (rinsed in water). Plasma salbutamol was measured before and 5, 10, 15 and 20 min after inhalation of four single puffs of 100 μg salbutamol. Cmax and Cav (5–20min) were calculated as a reflection of lung dose.
Results
For Cmax: (A) Non-electrostatic Babyhaler 4.3 ng ml−1 (B) New Babyhaler 1.9 ng ml−1 (C) New AeroChamber 1.6 ng ml−1: AvsB (95% CI for difference 0.5–4.5 ng ml−1), A vs C (95% CI for difference 0.7–4.8 ng ml−1). The geometric mean ratio for A:B was 2.4 fold, and for A:C was 2.9 fold. The values for Cav were similar with ratios for A:B of 2.4 fold, and A:C of 4.1 fold. The nonelectrostatic Babyhaler delivered a significantly (P < 0.05) higher lung dose (for both Cmax and Cav) than either of the other two spacers.
Conclusions
The electrostatic charge in plastic spacers reduces lung dose in children by more than two-fold. This is clinically significant and the use of potentially electrostatically charged spacers should be avoided.
Keywords: children, electrostatic charge, lung delivery, salbutamol, hydrofluoroalkane
Introduction
The use of a plastic spacer device in conjunction with a pressurized metered dose inhaler (pMDI) is a simple and effective way of delivering inhaled drugs to asthmatic children [1]. Spacers for pressurized metered dose inhalers (pMDI) have the advantage of increasing respirable dose delivery, reducing oropharyngeal deposition as well as avoiding the need for co-ordination when using pMDIs on their own. In vitro studies have shown that the presence of electrostatic charge associated with plastic spacers may markedly reduce the dose of respirable drug particularly when multiple actuations are used or if there is a delay between inhalation and actuation [2–4].
The isolated effect of electrostatics in spacers on the lung dose in vivo has not previosuly been reported. The evaluation of drug delivery to the lung in vivo may be performed using either radiolabelling techniques or using pharmacokinetic techniques [5]. The pharmacokinetic evaluation of drug delivery in children is particularly attractive because of the concerns regarding the administration of radioisotopes in this age group.
The simplest and most direct pharmacokinetic method of evaluating lung dose is to measure the early absorption profile of plasma salbutamol in the first 20 min. The rationale behind this method is that gastrointestinal absorption contributes only 0.3% to the overall systemic absorption from the inhaled dose in the first 30 min after inhalation [6]. Using this technique it is therefore possible to calculate an index of relative lung dose as we have previously demonstrated [7–9]. The aim of the present study was to evaluate the in vivo effect of electrostatic charge in plastic spacers on the drug delivery to the lung with hydrofluoroalkane (HFA)-salbutamol aerosol administered to children.
Methods
Five children (four female) were recruited into the study: mean age 9.4 (s.d. 1.9) years (range 7–12 years), mean height 144 (s.d. 10) cm (range 134–162 cm), mean weight 34.4 (s.d. 9.7) kg (range 27–53 kg). Two of the subjects had mild asthma with normal spirometry and were on no regular treatment. A randomised single (investigator) blind crossover design was used with each child attending the laboratory on 3 separate days each separated by 1 week. At each laboratory visit a single 400 μg dose of HFA salbutamol pMDI (Sultanol 100 μg per actuation, Glaxo Wellcome, Denmark) was administered as single puffs with immediate inhalation from a plastic spacer with mouth piece.
The following spacers were prepared on each of the 3 study days: (a) a nonelectrostatic 350 ml Babyhaler, Glaxo Wellcome (UK) coated with benzalkonium chloride, (b) a new 350 ml Babyhaler rinsed in water, and (c) a new 145 ml AeroChamber (Trudell Medical, Canada) rinsed in water.
The children used a nose clip and were given detailed instruction on use of the spacer which involved tidal breathing for 30 s for each actuated puff of salbutamol. The Babyhaler was primed by immersing it three times in a solution of 0.05% benzalkonium chloride. Between immersions the spacer was allowed to air dry and the same spacer was used for all children. For each child a new AeroChamber or new Babyhaler was used having been rinsed in water and allowed to drip dry. Blood for salbutamol was taken off immediately prior to inhalation and again at 5, 10, 15 and 20 min after the end of inhalation. Venepuncture was performed using an EMLA plaster (Astra Draco, Sweden) to anaesthetize the skin following insertion of a plastic indwelling plastic venous cannula for blood sampling. The cannula dead space was removed prior to sampling at each time point.
Plasma salbutamol was assayed by high performance liquid chromatography (h.p.l.c.) and the extraction process using silica adsorption with chromatography followed by reverse phase ion pair h.p.l.c. and electrochemical detection. The analytical imprecision in the plasma salbutamol was 7.8% and 6.7% for within and between assay. The detection limit for the assay was 0.2 ng ml−1.
The results were analysed using a Statgraphics statistical software package (STSC Software Publishing Group, Rockville, USA). Salbutamol concentrations were calculated as maximal (Cmax) an average over 5–20 min (Cav). Comparisons were made by an overall multifactorial variance (manova) followed by Bonferroni multiple range testing to establish where the differences were significant. 95% confidence intervals were calculated for mean differences between the three spacers. In addition the geometric mean ratios for the differences between the three spacers were calculated. A probability value P < 0.05 (two-tailed) was considered as being of statistical significance.
Results
Values for Cmax showed an overall significant (P < 0.01) difference between the three spacers: (A) Non-electrostatic Babyhaler 4.3 ng ml−1 (B) New Babyhaler 1.9 ng ml−1 (C) New AeroChamber 1.6 ng ml−1: A vs B (95% CI for difference 0.5–4.5 ng ml−1), A vs C (95% CI for difference 0.7–4.8 ng ml−1) (Table 1). The geometric mean ratio for A:B was 2.4-fold, and for A:C was 2.9-fold. Corresponding values for Cav also showed a significant (P < 0.05) overall difference between the three spacers: (A) 3.5 ng ml−1 (B) 1.5 ng ml−1 (C) 1.2 ng ml−1: A vs B (95% CI for difference 0.1–4.0 ng ml−1), A vs C (95% CI for difference 0.3–4.2 ng ml−1). The ratio for A:B was 2.4-fold and for A:C was 4.1-fold. The nonelectrostatic Babyhaler delivered a significantly P < 0.05 higher lung dose (as Cmax and Cav) in comparison with either of the other two spacers. There was no significant difference between the new Babyhaler or the new AeroChamber for either parameter. The pharmacokinetic time profile for the three spacers is depicted in Figure 1.
Table 1.
Summary of pharmacokinetic parameters.
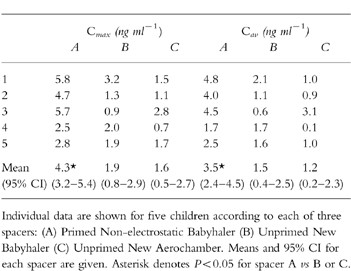
Figure 1.
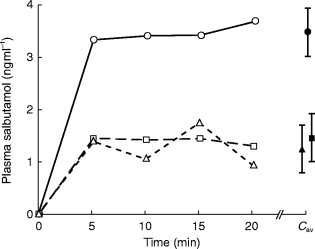
Pharmacokinetic time profiles depicting plasma salbutamol absorption in the first 20 min after inhalation of a single 400 μg dose of HFA-salbutamol aerosol from three plastic spacers (• primed non-electrostatic Babyhaler, ▪ unprimed Babyhaler, ▴ unprimed Aerochamber). The mean and standard error is shown for the average concentration over the 20 min period (Cav). Values for Cmax and Cav were significantly higher with the nonelectrostatic Babyhaler compared with the new Babyhaler or the new AeroChamber.
Discussion
The results of our study showed that the lung dose from a nonelectrostatic-primed Babyhaler was significantly higher than either a new Babyhaler or new AeroChamber, amounting to more than a two-fold difference.
It is important to appreciate that the Babyhaler is intended for use in young children while the children in this study were of school age. The differences in lung doses achieved would be expected to be greater in young children. Due to the lower minute volume of the younger children lung dose would be more affected by the rapid fall-out of aerosol in electrostatically charged spacers as compared with a slower fall-out rate in electrostatically neutral spacers [10].
It should also be pointed out that each drug/device combination must be considered as a unique entity [1], and the present data cannot be extrapolated to what happens with other aerosols and spacer combinations. We used an HFA formulation of salbutamol pMDI, which has previously been shown to afford a greater lung dose than a CFC salbutamol pMDI when used on its own [7]. The interaction between HFA driven pMDI and electrostatically charged spacers may not be comparable to the interaction between CFC driven pMDI and electrostatically charged spacers.
Despite the small sample size we were able to show significant differences between the three spacers, although there was a relatively large degree of intraindividual variance as indicated by the confidence intervals for between treatment differences. Nonetheless the difference in lung dose of a factor of 2 or more is clinically relevant. Dose titrations of inhaled drug from pDMI and spacers often operate in the range of two-fold dose changes. The present data show that the change of spacer brand or the alteration of the electrostatic charge of the spacer could have an effect of a similar magnitude to that of a normal change in the clinical prescription.
An inadvertent change in lung dose by a factor of 2 or more may affect safety of treatment [11], cost-effectivity [12] as well as clinical control. Therefore, it is mandatory to avoid electrostatics in spacer devices. This can be achieved by the use of inherently nonelectrostatic materials for the spacer such as metal [2, 10]. Using the same in vivo pharmacokinetic technique we have shown that HFA salbutamol delivered via a 250 ml metal spacer (NebuChamber, Astra Draco, Sweden) produces a seven-fold greater lung dose compared with a Sidestream nebuliser [8] and a two-fold greater lung delivery compared with the same nominal dose from a reservoir dry powder inhaler [13]. However, there are as yet no published data in vivo comparing an optimally primed plastic spacer with a metal spacer.
Plastic spacers can be coated to reduce the electrostatic charge. We have chosen a simple coating using benzalkonium chloride. Alternatives are antistatic paints [3], or repeated uses of pMDIs the additives of which will coat the plastic [14], or submersion of the plastic in detergents [15]. The optimal chemical priming method needs to be defined and its possible interaction with the drug delivered should be studied. The stability of the chemical priming and the user compliance to such priming procedures warrants further evaluation before any recommendations on antistatic pretreatment of plastic spacers can be made.
In conclusion we have found that the electrostatic charge in plastic spacers reduces the lung dose in children by at least two fold. This effect is clinically important to safety, cost-effectivity and disease control, and the use of electrostatically active spacers should be avoided in future treatment plans.
Acknowledgments
This study was jointly sponsored by the Universities of Dundee and Copenhagen and received no pharmaceutical support.
References
- 1.Bisgaard H. Delivery of inhaled medication to children. J Asthma. 1997;34:443–468. doi: 10.3109/02770909709055389. [DOI] [PubMed] [Google Scholar]
- 2.Bisgaard H. A metal aerosol holding chamber devised for young children with asthma. Eur Respir J. 1995;8:856–860. [PubMed] [Google Scholar]
- 3.Barry PW, O’Callaghan C. The effect of delay, multiple actuations and spacer static charge on the in vitro delivery of budesonide from the nebuhaler. Br J Clin Pharmacol. 1995;40:76–78. doi: 10.1111/j.1365-2125.1995.tb04538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildhaber J, Devadason SG, Eber E, et al. Effect of electrostatic charge, flow, delay and multiple actuation on the in vitro delivery of salbutamol from different small volume spacers for infants. Thorax. 1996;5:985–988. doi: 10.1136/thx.51.10.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrystyn H, Corlett Silkstone V. Lung bioavailability of generic and innovator salbutamol metered dose inhalers. Thorax. 1996;51:658. doi: 10.1136/thx.51.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark DJ, Lipworth BJ. Lung bioavailability of chlorofluorocarbon free dry-powder and chlorofluorocarbon containing formulations of salbutamol. Br J Clin Pharmacol. 1996;41:247–249. doi: 10.1111/j.1365-2125.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Lipworth BJ, Clark DJ. Lung delivery of non-CFC salbutamol via a small volume metal spacer and large volume plastic spacer devices compared with an open vent nebuliser. Br J Clin Pharmacol. 1998;45:160–163. doi: 10.1046/j.1365-2125.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DJ, Lipworth BJ. Effect of multiple actuations, delay in inhalation and antistatic treatment of the lung bioavailability of salbutamol via a spacer device. Thorax. 1996;51:981–984. doi: 10.1136/thx.51.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisgaard H, Amhøj J, Klug B, Burg E. A non-electrostatic spacer for aerosol delivery. Arch Dis Child. 1995;73:226–230. doi: 10.1136/adc.73.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipworth BJ. New perspectives on inhaled drug delivery and systemic bioactivity. (Editorial) Thorax. 1995;50:105–110. doi: 10.1136/thx.50.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisgaard H. Drug delivery from inhaler devices (Editorial) Br med J. 1996;313:895–896. doi: 10.1136/bmj.313.7062.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipworth BJ, Clark DJ. Lung bioavailability of salbutamol given by dry powder inhaler (Turbuhaler) on small volume antistatic metal spacer (Airomir CFC-free MDI plus NebuChamber) Eur Respir J. 1997;10:1820–1823. doi: 10.1183/09031936.97.10081820. [DOI] [PubMed] [Google Scholar]
- 14.Berg E, Bisgaard H, Madsen J. In vitro performance of three combinations of spacers and pressurised metered dose inhalers for treatment in children. Eur Respir J. 1998;12:472–476. doi: 10.1183/09031936.98.12020472. [DOI] [PubMed] [Google Scholar]
- 15.Wildhaber JH, Devadason SG, Hayden MJ, et al. Electrostatic charge on a plastic spacer device influences the delivery of salbutamol. Eur Respir J. 1996;9:1943–1946. doi: 10.1183/09031936.96.09091943. [DOI] [PubMed] [Google Scholar]


