Abstract
Aims
Recent studies in patients with cardiovascular diseases suggest potential for the use of orally administered l-arginine, the precursor of nitric oxide, as a therapeutic agent. This crossover study was designed to examine the pharmacokinetics of single i.v. and oral doses of l-arginine in healthy volunteers (n = 10).
Methods
A preliminary control study (n = 12) was performed to assess the variation in plasma l-arginine concentrations when ingesting a normal diet. The observed variation was taken into account when interpreting the pharmacokinetic data obtained after exogenous administration.
Results
The mean baseline plasma concentration of l-arginine in the control study was 15.1±2.6 μg ml−1. After intravenous administration (30 g over 30 min), the plasma concentration reached 1390±596 μg ml−1. The disappearance of l-arginine appeared biphasic, with an initial rapid disappearance due to concentration-dependent renal clearance followed by a slower fall in plasma concentrations due to nonrenal elimination. The peak concentration after oral administration (10 g) was 50.0±13.4 μg ml−1, occurring 1 h after administration. Renal elimination was not observed after oral administration of this dose. The absolute bioavailability of a single oral 10 g dose of l-arginine is ≈20%.
Conclusions
This study provides basic knowledge of l-arginine pharmacokinetics in healthy humans. Intravenous and oral administrations show at minimum a biphasic pattern. Further studies will assess whether a similar profile is observed when the drug is administered to patients.
Keywords: l-arginine, pharmacokinetics, oral, intravenous
Introduction
l-arginine is considered a nutritionally dispensable (or non essential) amino acid in humans [1]. l-arginine is the substrate from which the ubiquitous mediator nitric oxide (NO) is synthesized [2]. There is now considerable interest in the potential therapeutic properties of l-arginine in modulating NO bioavailability in the vasculature. NO is a potent endogenous vasodilator and has antiplatelet and other properties that may inhibit atherogenesis. While impaired availability of NO in endothelium and platelets has been associated with cardiovascular risk factors and with ageing [3], experimental [4–9] and clinical studies [10–16] have shown that the attenuation of vascular and platelet NO activity can be reversed in some of these conditions by oral or intravenous administration of l-arginine. As a potential therapeutic agent, information about l-arginine kinetics in humans is needed. Due to various limitations related to the route of administration, assay sensitivity and methods of data analysis, none of the previously published studies [17, [18] provide definitive data on the pharmacokinetics of l-arginine in humans. Therefore, this study was designed to explore the pharmacokinetics of l-arginine after single oral and intravenous administration in healthy volunteers.
Methods
Subjects
Ten healthy volunteers (6M, 4F) aged 23–52-years old (mean±s.d.: 35±11) with normal body mass index (mean±s.d.: 25±3 kg m−2) were enrolled in this study. These subjects were nonsmokers, took no regular medication and had no significant past medical illnesses. They were screened by physical examination, blood tests (CBC, SMA-20), urinalysis and resting ECG. Written informed consent was obtained after full explanation of the study procedure. The protocol was approved by the Administrative Panel on Human Subjects in Medical Research at Stanford University.
Protocol
Pharmacokinetic study
No medication was permitted for at least 1 week prior to the study. Consumption of alcohol and caffeine was also restricted 24 h before the study. This study was designed as a crossover study. The subjects were admitted on two separate occasions (with at least a 1 week interval between them) for oral or intravenous administration of l-arginine. The sequence of administration for each volunteer was randomly selected. On the study day, volunteers were admitted in the morning in a fasting state. Meals without caffeine were provided 2 h after l-arginine administration. For the intravenous dose, 300 ml of a 10% l-arginine hydrochloride solution (30 g) (R-Gene10, Kabi Pharmacia, Clayton, NC, USA) was infused in one antecubital vein over a period of 30 min. For the oral dose, 100 ml of a 10% l-arginine solution (10 g) was swallowed in less than 2 min. This oral dose of l-arginine is similar to those used in previous clinical studies [11, [15, [19]. The intravenous dose is that used clinically for the investigation of growth hormone secretion, and it was chosen to provide sufficiently high concentrations for the determination of pharmacokinetic parameters. In both cases, the treatment was administered immediately after a baseline blood sample was obtained. Vital signs and possible adverse effects were monitored throughout the study.
Blood samples (5 ml) were drawn via an indwelling catheter in the antecubital vein (in the arm opposite that used for the i.v. administration) at intervals from 0 to 8 h after intravenous administration and from 0 to 8 h after oral administration. A few subjects had samples drawn at 24 h after the dose. The duration of sampling was determined on the basis of data obtained from a previous study of the pharmacokinetics of l-arginine following exogenous administration to humans [17]. Blood was collected into heparinized tubes and the plasma separated by centrifugation and stored frozen at −70° C until analysis. Urine samples were also collected at 4 h intervals during the study and stored at the same temperature.
Control study
In a separate protocol designed to evaluate the effects of a normal diet on plasma l-arginine concentrations, 12 healthy volunteers were admitted to the General Clinical Research Center (GCRC). Blood and urine samples were collected over an 8h period with a diet similar to that given during the pharmacokinetic studies containing normal amounts of proteins and l-arginine. Plasma samples were analysed for l-arginine concentrations and these data were used to interpret the variations observed after exogenous administration of l-arginine.
Assay procedures
Plasma l-arginine concentrations were determined by high performance liquid chromatography (h.p.l.c.) with fluorescence detection as described previously by Gopalakrishnan et al. [20]. Briefly, the assay involves precolumn derivatization of l-arginine with naphthalene-dicarboxaldehyde (NDA) and cyanide (CN) followed by h.p.l.c. with u.v. detection. The coefficient of variation for this assay is less than 2%. Some plasma and urine samples were analysed using a Beckman 6300 Amino Acid Analyser (Palo Alto, CA) which detects the coloured ninhydrin derivative of most amino acids at 570 nm.
Data analysis
Plasma concentrations of l-arginine were used to determine the area under the plasma concentration (AUC) vs time curve for 8 h after the administration of either an oral or iv dose of l-arginine. The AUC produced by exogenous (i.v. or oral) administration of l-arginine (AUCEXoral or AUCEXi.v.) was calculated by the linear trapezoidal method using the plasma concentration above the baseline value from each individual study and then adjusting the area for endogenous variation by subtracting the AUC derived from the control study. In some cases, the subject served as his/her own control, while in other cases an average control value was used. The bioavailability (F) of l-arginine was calculated as: [AUCEXoral]/[AUCEXi.v.] where the numerator and denominator were normalized to the oral or i.v. dose. Based on the urine concentration data, ≈5 g of l-arginine was excreted in urine after a 30 g intravenous infusion of l-arginine. Urinary excretion did not occur following oral administration of 10 g. To calculate the nonrenal clearance after intravenous administration, we corrected for the loss of drug due to renal excretion after the intravenous dose, which occurred rapidly and had little influence on AUCEX. Thus, the intravenous dose was normalized to a dose of 25 g and nonrenal clearance was obtained by dividing this dose by AUCEX i.v. The renal clearance of l-arginine was calculated in two subjects according to the equation U/AUCtotal where U is the urinary l-arginine concentration and AUCtotal is the total area under the plasma concentration vs time during the urine collection interval. Means±s.e.mean are shown for all parameters except where stated otherwise.
Results
Tolerability
Both intravenous and oral l-arginine administrations were well tolerated. No significant effect was detected on the vital signs recorded during the study (data not shown). No adverse reaction was observed.
Control study
The spontaneous variation of daytime plasma l-arginine concentrations over 8 h (09.00 h to 17.00 h) with a normal diet is shown in Figure 1. The average baseline plasma concentration of l-arginine was 15.1± 2.6 μg ml−1.
Figure 1.
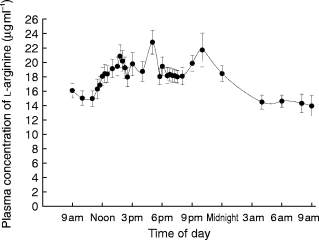
Average plasma concentrations of l-arginine in 12 subjects who did not receive l-arginine (control study) but ingested a normal hospital diet.
Pharmacokinetics
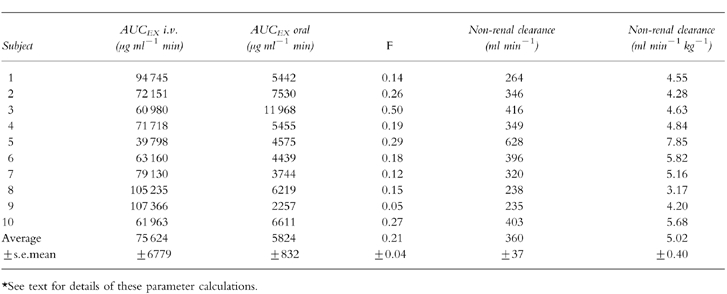
After the 30 min intravenous infusion, l-arginine plasma concentrations reached a maximum of 1390±596 μg ml−1 and then declined rapidly (Figure 2). Approximately 5 g l-arginine was excreted into urine after the 30 g intravenous infusion. Substantial urinary excretion of l-arginine occurred only within the first 90 min, during which the renal threshold for reabsorption was presumably exceeded. After oral administration (10 g), the peak plasma concentration of l-arginine was 50.0±13.4 μg ml−1, occurring 1 h after dosing (Figure 2). There was no significant renal clearance after oral administration of 10 g. When plotted vs time on a semilogarithmic scale, the difference between plasma concentrations of l-arginine (after oral or i.v. administration) and the plasma concentrations of l-arginine at the corresponding times was not log-linear (Figure 2) indicating that the disposition of the amino acid is at a minimum biphasic in its pattern. Average bioavailability was 21±4% and the average nonrenal clearance was 360 ml min−1 (Table 1). The maximum renal clearance of intravenous l-arginine observed during the first 90 min in two volunteers was 1.16 and 1.18 ml min−1 kg−1.
Figure 2.
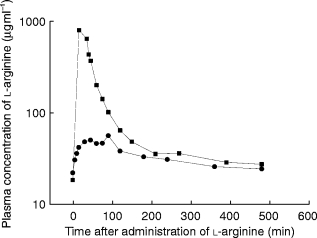
Plasma concentrations of l-arginine in a representative subject after receiving 10 g l-arginine orally (circles) or 30 g by intravenous infusion over 30 min (squares).
Table 1.
Summary of pharmacokinetic data in 10 normal volunteers*. For the intravenous study, 30 g l-arginine hydrochloride was infused over 30 min. For the oral study, 10 g l-arginine solution was swallowed in less than 2 min.
Discussion
This pharmacokinetic study carried out in healthy subjects shows a biphasic elimination pattern after both oral and intravenous l-arginine administration, and concentrations of l-arginine have not returned to baseline after 8 h of sampling. Moreover, plasma concentration data obtained from the control study over 8 h with a normal diet clearly show that there is a substantial variation in plasma concentrations of l-arginine. The variation in the daytime concentrations was taken into account in the analysis of these data in order to better estimate values for clearance and bioavailability following oral and intravenous exogenous l-arginine administration.
A review of data in the literature shows that the pharmacokinetics of l-arginine in humans has not been extensively studied and is not well understood. In one study, using an enzymatic assay for l-arginine [17], the pharmacokinetics of l-arginine was examined in eight volunteers using four different doses from 6 to 44 g given as an intravenous infusion over 30 min. The pharmacokinetic calculations were performed based on the assumption that steady state had been reached at the end of the infusion and clearance and half-life were calculated from only two data points (at the end of the infusion and 15 min later). The steady-state assumption is incorrect, and therefore the pharmacokinetic parameters obtained from this study are unreliable. In a second study [18], 30 g l-arginine was given by intravenous infusion over 30 min. Plasma concentration data points were available for only 60 min after the end of the infusion due to low assay sensitivity in that study. These authors observed a log-linear decline after the infusion and used it to estimate half-lives. We have shown that renal clearance is significant and occurs after infusion of high doses, when plasma l-arginine concentrations exceed the renal threshold. This phenomenon was neglected in both studies when analysing pharmacokinetic parameters. Neither of these two studies [17, [18] provides definitive data on the disposition of l-arginine or its bioavailability after oral dosing.
During the control study we found that the mean value for the baseline plasma concentration of l-arginine when ingesting a normal diet over 8 h is 15.1± 2.6 μg ml−1. This level is consistent with a previous observation in hypercholesterolaemic subjects [11]. The variability in plasma concentrations of l-arginine observed from 09.00 h to 17.00 h in this control study may theoretically be influenced by dietary intake and modifications in l-arginine synthesis and/or clearance. Changes in the baseline due to dietary intake, changes in l-arginine production or in clearance, or differences in subject diet between the control and the study day could all be reasons why concentrations in plasma after exogenous administration failed to return to baseline. Further research will be needed to determine the precise mechanism responsible for this observation, and would best be carried out using a radiolabled or stable isotopic form of l-arginine. From a practical point of view, an important consequence of our design is that we were unable to determine a half-life for l-arginine.
Oral administration of 10 g l-arginine, a dose within the range of those proposed for therapeutic purposes [11, [15], was associated with a three-fold increase in plasma concentration of the amino acid. The mean absolute bioavailability for this oral dose was modest (20%) but a wide range was observed among subjects (5%-50%). Further studies will be needed to determine the precise mechanism responsible for this intersubject variability and the relationship between plasma concentrations of l-arginine and NO-mediated functions such as vascular relaxation and platelet aggregation. As with other amino acids, the intestinal absorption of l-arginine occurs in the jejunum, where there is a specific transport system, known as system Y+ [21]. After its absorption by the brush-border membrane, l-arginine is extensively catabolized by enterocytes [21].
Studies in humans have also demonstrated that total body arginine homeostasis is related to the rate of degradation by hepatic arginase [22]. The activity of this enzyme is directly related to the concentration of arginine substrate [23]. Excess arginine intake in animals is also associated with increased urinary excretion of the amino acid [24] as the renal tubular reabsorption of arginine in the distal loop of Henle exhibits a transport maximum [25, [26]. The 90-fold increase in l-arginine plasma concentration observed after i.v. infusion in our study is consistent with a measurement obtained in a pharmacodynamic study using the same dose in healthy volunteers [19]. Interestingly, this increase was associated with the transient presence of renal excretion (1.17 ml min−1 kg−1) which was not observed after oral administration. After this initial urinary loss, plasma l-arginine declined slowly by nonrenal clearance (5 ml min−1 kg−1). This pattern is consistent with the concept that high concentrations of the amino acid exceeds the renal threshold for reabsorption in humans.
We did not evaluate the effects of exogenous l-arginine on in vivo indices of NO production. However, available data suggest that the intravenous dose, but not the oral dose, used in our study is likely to be associated with an increase in NO generation. In aqueous solution, NO is rapidly oxidized to nitrite (NO−2) and nitrate (NO−3); both compounds are present in plasma and subsequently excreted into urine, mainly as NO−3 [27]. Under standardized dietary intake of NO−3, the quantification of NO−2 and NO−3 has been suggested as a suitable noninvasive method to determine NO activity in vivo [28]. A single intravenous administration of l-arginine in healthy subjects at a dose similar to that used in our study (30 g over 30 min) has been shown to be associated with an 80% increase in urinary excretion of NO−3 [19]. Another study showed that supplementation with l-arginine in the diet (40 g day−1) during 6 days did not alter the total daily rate of urinary NO−3 excretion nor the plasma concentration of NO−3 [29].
This study, by integrating the variation of endogenous plasma l-arginine concentrations, provides important new information on l-arginine pharmacokinetics in healthy subjects. Further studies are needed in patients, particularly in hypercholesterolaemic subjects, to determine if the same pattern is observed.
Acknowledgments
This work was supported by National Institute of Health Grants AG-05627 and RR-00070. Dr Oranee Tangphao was supported by Ananthamahidol Foundation, Bangkok, Thailand. Dr Stephan Chalon was supported by Merck Sharp and Dohme International Fellowships in Clinical Pharmacology. We gratefully acknowledge the help of the nurses and staff of the Geriatric Research Education and Clinical Center of the Palo Alto Veterans Affairs Hospital and the General Clinical Research Center at Stanford Hospital. Tina Moy and Patricia Burton provided excellent technical assistance.
References
- 1.Visek WJ. Arginine needs, physiological state and usual diets. A Reevaluation. J Nutr. 1986;116:36–46. doi: 10.1093/jn/116.1.36. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 4.Hutchison S, Reitz M, Sudhir K, et al. Chronic dietary l-arginine prevents endothelial dysfunction secondary to environmental tobacco smoke in normocholesterolemic rabbits. Hypertension. 1997;29:1186–1191. doi: 10.1161/01.hyp.29.5.1186. [DOI] [PubMed] [Google Scholar]
- 5.Cooke JP, Andon NA, Girerd XJ, Hirsch AT, Creager MA. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation. 1991;83:1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- 6.Tsao PS, Theilmeier G, Singer AH, Leung LLK, Cooke JP. l-arginine attenuates platelet reactivity in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:1529–1533. doi: 10.1161/01.atv.14.10.1529. [DOI] [PubMed] [Google Scholar]
- 7.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of l-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böger RH, Bode-Böger SM, Mugge A, et al. Supplementation of hypercholesterolemic rabbits with l-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995;117:273–284. doi: 10.1016/0021-9150(95)05582-h. [DOI] [PubMed] [Google Scholar]
- 9.Wang BY, Candipan RC, Arjomandi M, Hsiun PTC, Tsao PS, Cooke JP. Arginine restores nitric oxide activity and inhibits monocyte accumulation after vascular injury in hypercholesterolemic rabbits. J Am Coll Cardiol. 1996;28:1573–1579. doi: 10.1016/s0735-1097(96)00337-3. [DOI] [PubMed] [Google Scholar]
- 10.Tsao PS, McEvoy LM, Drexler H, Bucther EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by l-arginine. Circulation. 1994;89:2176–2182. doi: 10.1161/01.cir.89.5.2176. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson P, Adams MR, Powe AJ, et al. Oral l-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97:1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois-Rande JL, Zelinsky R, Chabrier PE, Castaigne A, Geschwind H, Adnot S. l-arginine improves endothelium-dependent relaxation of conductance and resistance coronary arteries in coronary artery disease. J Cardiovasc Pharmacol. 1992;20:S211–S213. doi: 10.1097/00005344-199204002-00060. [DOI] [PubMed] [Google Scholar]
- 13.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolemic patients by l-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 14.Creager MA, Gallagner SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. l-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf A, Zalpour C, Theilmeier G, et al. Dietary l-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol. 1997;29:479–485. doi: 10.1016/s0735-1097(97)00523-8. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan A, More R, Mullins P, Taylor G, Petch C, Schofield P. Aging-associated endothelial dysfunction in humans is reversed by l-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 17.Van Haeften TW, Konings CH. Arginine pharmacokinetics in humans assessed with an enzymatic assay adapted to a centrifugal analyzer. Clin Chem. 1989;35:1024–1026. [PubMed] [Google Scholar]
- 18.Roberts I, Smith IM. A simple method for the measurement of arginine in serum. Ann Clin Biochem. 1984;21:515–518. doi: 10.1177/000456328402100614. [DOI] [PubMed] [Google Scholar]
- 19.Bode-Boer SM, Boer RH, Creutzig A, et al. l-arginine infusion decreases peripheral arterial resistance and inhibits platelet aggregation in healthy subjects. Clin Sci. 1994;87:303–310. doi: 10.1042/cs0870303. [DOI] [PubMed] [Google Scholar]
- 20.Gopalakrishnan V, Burton PJ, Blaschke TF. High-performance liquid chromatographic assay for the quantitation of l-arginine in human plasma. Anal Chem. 1996;68:3520–3523. doi: 10.1021/ac960613n. [DOI] [PubMed] [Google Scholar]
- 21.Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. Am J Physiol. 1994;267:F331–F346. doi: 10.1152/ajprenal.1994.267.3.F331. [DOI] [PubMed] [Google Scholar]
- 22.Castillo L, Sanchez M, Chapman TE, Ajami AM, Burke JF, Young VR. The plasma flux and oxidation rate of ornithine adaptively decline with restricted l-arginine intake. Proc Natl Acad Sci USA. 1994;91:6393–6397. doi: 10.1073/pnas.91.14.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris SM. Regulation of enzymes of urea and arginine synthesis. Ann Rev Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- 24.Southern LL, Baker DH. Performance and concentration of amino acids in plasma and urine of young pigs fed diets with excesses of either arginine or lysine. J Animal Sci. 1982;55:857–866. doi: 10.2527/jas1982.554857x. [DOI] [PubMed] [Google Scholar]
- 25.Young JA, Freedman SB. Renal tubular transport of amino acids. Clin Chem. 1971;17:245–266. [PubMed] [Google Scholar]
- 26.Dantzler WH, Silbernagl S. Basic amino acid transport in renal papilla: microinfusion of Henle’s loops and vasa recta. Am J Physiol. 1993;265:F830–F838. doi: 10.1152/ajprenal.1993.265.6.F830. [DOI] [PubMed] [Google Scholar]
- 27.Wennmalm A, Benthin G, Petersson AS. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br J Pharmacol. 1992;106:507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki H, Ikenaga H, Hishikawa K, Nakaki T, Kato R, Saruta T. Increases in NO2−/NO3− excretion in the urine as an indicator of endothelium-derived relaxing factor during elevation of blood pressure. Clin Sci. 1992;82:631–634. doi: 10.1042/cs0820631. [DOI] [PubMed] [Google Scholar]
- 29.Beaumier L, Castillo L, Ajami AM, Young VR. Urea cycle intermediate kinetics and nitrate excretion at normal and ‘therapeutic’ intakes of arginine in humans. Am J Physiol. 1995;269:E884–E896. doi: 10.1152/ajpendo.1995.269.5.E884. [DOI] [PubMed] [Google Scholar]


