Abstract
Aims
The eye-blink response following sudden acoustic noise bursts is part of the startle reflex. The magnitude of the startle response can be attenuated by presentation of a weak stimulus before the ‘startle-eliciting’ stimulus (prepulse inhibition, PPI). PPI is a stable finding in awake humans but may be altered by anaesthetic drugs. We investigated whether the application of benzodiazepines altered the magnitude of PPI in healthy male volunteers.
Methods
In an open-label noncontrolled investigation, the effect of the benzodiazepine agonist midazolam on PPI was assessed in the absence and presence of the antagonist flumazenil. After an initial control period of 60 min three consecutive periods, each of 60 min, with progressively increasing concentrations of midazolam were studied (0.02, 0.06, 0.14 mg kg−1 h−1). A final 60 min period during the administration of flumazenil (0.004 mg kg−1 h−1) and while the agonist was still present was also studied. Drug was administered intravenuously as a combination of bolus, 50% of total dose and continuous infusion over the 60 min period. Electromyographic (EMG) response of the right orbicularis oculi muscle was used to assess the startle response to noise bursts of 50 ms duration (95 dB(A)). Noise bursts were randomly preceded by nonstartling prepulses (800 Hz sinus, 50 ms duration, 65 dB(A), prepulse to noise interval 120 ms). The magnitude of PPI was calculated by dividing the EMG response to nonprepulsed stimuli by the response to prepulsed stimuli for each individual and period. Eleven subjects participated in the study, two of them were excluded from the statistical analysis because startle responses could not be reliably elicited (final sample size n=9).
Results
The magnitude of PPI was inversely related to the concentration of midazolam. This relationship was described by a sigmoidal Emax model, giving an Emax of 0.65±0.13, an ED50 of 33.9±10.9 ng ml−1 and gamma of 3.5±1.0. During infusion of flumazenil and in the presence of midazolam, the magnitude of PPI increased by 0.11 (95% CI, 0–0.22, P≤0.04), which is consistent with its mode of action as a benzodiazepine antagonist.
Conclusions
In healthy male volunteers the magnitude of PPI varies according to agonism and antagonism of benzodiazepine receptors, suggesting that the assessment of PPI may be potentially useful to monitor the sedative effect of benzodiazepines in the clinical setting.
Keywords: acoustic startle reflex, benzodiazepine, flumazenil, midazolam, prepulse inhibition
Introduction
Benzodiazepine-receptor (BZR) agonists are among the most widely used drugs in intensive-care medicine [1–3]. They are well known for their potential to induce and maintain profound sedation in critically ill and mechanically ventilated patients. However, optimal dosing is difficult since individual dose requirements may vary considerably during progression of the underlying disease, due to alterations of drug metabolism, protein binding and drug distribution [4, 5]. Moreover, parent compound and active metabolites may accumulate during long-term administration of BZR agonists. This has been demonstrated not only for the ‘classical’ long-acting BZR agonists (e.g. diazepam) but even for short-acting agonists like midazolam [6]. Plasma levels of BZR agonists do not closely reflect their impact on the central nervous system (CNS) in individual patients [7] and therefore provide no help in establishing dosage regimens. Adjustment to estimates of actual CNS activity is mandatory. But so far no simple, objective and reliable bedside technique is available to assess benzodiazepine-induced CNS effects.
In searching for simple noninvasive indices with the potential of objective grading of sedation we decided to study the startle eye-blink response. Eye-blink responses can be elicited by delivery of sudden noise bursts. They are part of the polysynaptic startle reflex and occur involuntarily as fast as 20–150 ms after stimulus onset [8]. It is well established that the startle blink magnitude in awake humans and animals is attenuated when nonstartling acoustic signals precede the startling stimulus by a very short interval (i.e. 120 ms) [9]. This phenomenon has been named prepulse inhibition (PPI). It was found that the magnitude of PPI varies with the intensity of prepulses [10], but otherwise PPI is a rather stable and robust phenomenon. When determined as a ratio of nonprepulsed and prepulsed responses, the magnitude of PPI does not habituate during repetitive stimulation [11]. PPI was explained by active CNS processes which protect the prepulse signal from disruption during detailed cognitive analysis [12]. In accordance with this interpretation we had speculated that PPI may be suitable for monitoring higher cortical activity. Such an index would be particularly useful for quantifying the level of CNS activity during drug-induced sedation.
PPI of the startle eye-blink response can easily be assessed by electromyography (EMG). As amplifiers and technical instruments for stimulus presentation and data processing could possibly be contained in a small box, this methodology would, in principle, offer the properties of a future bedside technique.
We investigated whether the magnitude of PPI varies during BZR agonism and antagonism. Because of its widespread use in routine clinical practice, the BZR agonist midazolam was used. In a first step we decided to study healthy male volunteers by a simple dosing schedule with progressively increasing concentrations of midazolam and during infusion of the BZR antagonist flumazenil while the agonist was still present.
Methods
The research protocol was approved by the local ethics committee of the Department of Medicine, University Hospital of Basel.
Participants
Participants signed a declaration of informed consent. Eleven healthy, nonsmoking male volunteers (aged 23–42 years) participated in the study. However, startle responses could not be reliably elicited in two subjects so that their data were excluded from the final statistical analysis (final sample size: n=9). All volunteers had normal values in the physical examination, standard electrocardiography, routine blood chemistry, haematology and urine analysis and no evidence or history of drug abuse or use of sedatives.
Experimental protocol
Subjects entered the hospital at 08.00 h after a light breakfast without caffeine. The study took place in the intensive care unit ward to guarantee optimal monitoring. Two peripheral venous lines were inserted into antecubital veins. The subjects were in the supine position throughout the whole experiment. Arterial blood pressure was recorded intermittently every 5 min, using an automated sphygmomanometer (Dinamap, Criticon, Florida, USA). Single-lead ECG and pulse oximetry were monitored continuously for safety reasons. After attachment of all instruments the subjects were exposed to repetitive noise stimuli in order to familiarize them with the startle procedure.
Drug administration
After an initial control period (C) of 60 min three consecutive periods, each of 60 min, with progressively increasing concentrations of midazolam (M1, M2, M3) (0.02, 0.06, 0.14 mg kg−1 h−1) were studied. There was a final 60 min period of study, with administration of flumazenil (F) at a dose of 0.004 mg kg−1 h−1 and while the agonist was still present. Midazolam was administered intravenously as a combination of bolus (50% of total dose) and continuous infusion over the 60 min period in order to reach a stable plasma midazolam concentration during PPI testing.
Plasma samples were collected 30 and 60 min after the start of the period (the 60 min sample during flumazenil infusion was omitted), centrifuged and stored at −20° C for h.p.l.c. analysis of unconjugated midazolam [13] with a detection limit of 1 ng ml−1. Period averages of midazolam plasma concentration were calculated.
EMG registration
Solid gel Ag-AgCl electrodes (ARBO Co., Brunswick, Germany) were placed under the right eye. EMG was recorded bipolarly from the orbicularis oculi muscle, using a bioamplifier with 20–500 Hz bandpass and 50 Hz notch filter. The raw EMG signal was then rectified by true root mean square (RMS) conversion, integrated, and sampled on the PC by AD conversion (1000 Hz, 12 bit) for offline analysis.
Stimulus generation
Noise and prepulse generation were controlled by PC. Acoustic stimuli (white noise bursts, instantaneous rise, 50 ms duration, 95 dB(A)) were presented binaurally via head phones at varying (random) intervals between 10 and 20 s. Noise bursts (n=14 per period) were either preceded (n=7) or not preceded (n=7) by nonstartling prepulse stimuli (800 Hz sinus, 50 ms duration, 65 dB(A), prepulse-to-noise interval 120 ms) in random sequence. As PPI does not require the subject’s active awareness and co-operation [14], no specific instructions were given. PPI testing was started 40 min after infusion onset and lasted for 5 min.
Parameter calculation
EMG response amplitude was identified at EMG maximum up to 260 ms [15] after each noise onset by computer-assisted manual control. Response probability was calculated for each subject. If reponse probability was less than 50%, data were excluded from the statistical analysis. Average EMG response amplitude was calculated for prepulsed and nonprepulsed trials. The latencies from noise-stimulus onset to response offset and peak maximum were also determined. The magnitude of PPI was calculated as the PPI ratio, by dividing the average of nonprepulsed EMG response by the average of prepulsed responses for each individual and period, respectively. This was done because it has already been shown [11] that PPI ratio is not subject to habituation during repetitive stimulation.
Subjective sedation score
After each PPI trial, subjects were rated by two physicians according to a scale proposed by Ramsay [16]. The Ramsay sedation score is defined as follows. Awake levels (1): anxious and/or agitated (2), cooperative, orientated, and tranquil (3), responds to commands. Asleep levels (4): quiescent with brisk response to light glabelar tap or loud auditory stimulus (5), sluggish response to light glabelar tap or loud auditory stimulus (6), no response. The scores of the two physicians were averaged.
Vigilance test
At the end of each infusion period (55 min after infusion onset), subjects had to perform a PC-based vigilance test using a complex reaction-time task to measure mental performance (BonnDet) [17]. During a 5 min period the volunteers had to respond to the presentation of coloured lights which appeared in random sequence, by pressing corresponding buttons as accurately and fast as possible. Using a PC-based control algorithm the interstimulus intervals were shortened or lengthened, thereby modifying task difficulty so that the subjects’ false response rate within a continuously moving window approached 50%. Average reaction time (RT) was calculated. As RT has to be readjusted when the average correct response rate (CR) is lower than 50%, the performance index (PI) was calculated according to the formula:
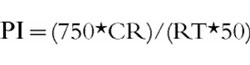 |
PI is adjusted to the mean complex reaction time (750 ms) of a previous reference group (healthy students, aged from 20–30 years) [17].
Statistical analysis
A multivariate design (manova with repeated measurements) was applied to test drug effects. Successive contrasts between infusion periods were constructed a priori. 95% confidence limits of differences were calculated. All statistical calculations were made using SAS software (release 6.11, SAS Institute Inc., Cary, NC, USA). Mean±s.e.mean are provided in text and figures. All testing was two-tailed. A P value <0.05 was considered significant. Unweighted nonlinear regression analysis of individual and mean concentration/effect relationships were performed with Origin software (Origin 5.0, Microcal Software Inc., Northampton, MA, USA, 1995) using the sigmoidal Emax model [18]. With this model it was possible to estimate the maximum effect (Emax), the concentration of half maximum effect (ED50) and the coefficient of sigmoidicity (γ).
Results
Midazolam and flumazenil were well tolerated in all subjects. No adverse events occurred during drug infusion and pulse oximetry did not reveal clinically significant desaturation.
Plasma midazolam was undetectable during the control period. The concentration was 11.5±6.7 ng ml−1 during the first midazolam infusion period M1, and rose in a dose-dependent manner (M2: 36.7±6.8 ng ml−1; M3: 92.4±9.6 ng ml−1), to decline again during the flumazenil period (62.9±13.3 ng ml−1).
Ramsay sedation scores increased with higher doses of midazolam (median, range at control period: 2, 1–2; M1: 3, 2–4; M2: 4, 4–5; M3: 5, 4–6) and decreased under flumazenil (3, 2–4).
The magnitude of PPI decreased with increasing plasma concentration and dose of midazolam (see Figure 1). This relationship could be described by a sigmoidal Emax model, revealing an Emax of 0.65±0.13, an ED50 of 33.9±10.9 ng ml−1 and sigmoidicity coefficient γ of 3.0±1.0 (data in Figure 1 represent exact fit of group means). As compared with the last midazolam infusion period, flumazenil increased the magnitude of PPI by 0.11 (95% confidence limit of difference: 0.0–0.22, P<0.04) to values above those expected with current plasma midazolam concentration (see Figure 1). The magnitude of PPI during flumazenil infusion still differed from the control level by 0.34 (95% confidence limit of difference: 0.04–0.64, P<0.01).
Figure 1.

The magnitude of prepulse inhibition (PPI-ratio) of the acoustic startle response decreases significantly during increasing doses of midazolam, in direct relation to the concentration. The relationship between plasma midazolam and effect could be evaluated (exact fit) by a sigmoidal Emax model, revealing an Emax of 0.5, an ED50 of 14.0 ng ml−1 and γ of 0.86. Plasma midazolam during flumazenil infusion (F) was not considered in the model and values are given for comparison. Data are means±s.e.mean.
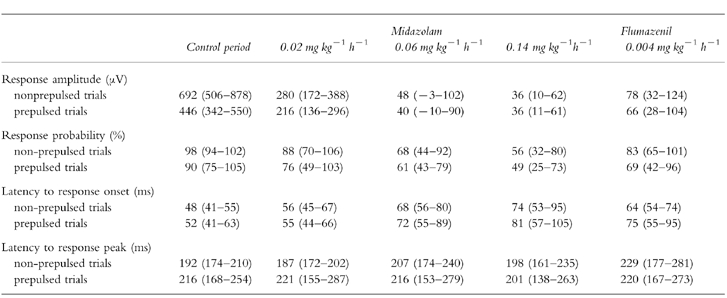
The effects of midazolam and flumazenil on startle amplitude, response probability and latency to response onset and peak are shown in Table 1. Startle amplitude decreased according to midazolam dose as did response probability. Latency to response onset increased. These effects were partially reversed by flumazenil. Latency to response peak did not show a clear dependence on midazolam or flumazenil infusion.
Table 1.
EMG response amplitude, response probability and latency to response onset are shown under control conditions, under midazolam and under flumazenil infusion. The data represent mean values (95% confidence limits). Response amplitude and response probability substantially decrease during midazolam infusion. Latency to response onset increases during midazolam infusion. All effects are partially reversed by flumazenil. Latencies to response peak were not associated with midazolam dose.
PC-based vigilance testing revealed a decreasing performance level PI (P<0.0001) at higher doses of midazolam (control: 104[94–117]; M2: 79[55–103]; M3: 38[13–63]; mean [95% confidence limits]) and increasing PI after administration of flumazenil (96[84–108]). Successive differences were statistically significant for M1-M2 (difference: −27, 95% confidence limit of differences: −10–−44, P<0.004), M2–M3 (difference: −43, 95% confidence limit of differences: −17.5–−68.5, P<0.002) and M3-F (difference: +59, 95% confidence limit of differences: 34–84, P<0.0001). During the last midazolam infusion period, M3, there was substantial intra and interindividual variation in mental performance. Mental performance was not impaired during the first midazolam infusion period, M1, when compared with the control period. Flumazenil restored mental performance so that no significant difference could be detected as compared with the control period (difference: −8, 95% confidence limit of differences: −18–+2, not significant).
Discussion
The current investigation was designed to study the effect of increasing doses of the BZR agonist midazolam and its specific antagonist flumazenil on PPI of the acoustic startle response in healthy humans. Based on the theory that PPI reflects higher cortical functioning (i.e. active processes protecting the prepulse signal from disruption during cognitive analysis) we have speculated that this index might be useful for the assessment of benzodiazepine effects in vivo and ultimately provide a tool to monitor benzodiazepine-induced CNS effects in clinical settings. By using a simple midazolam dose regimen, progressively increasing plasma concentration of midazolam and increasing sedative effect (assessed by vigilance testing and by the Ramsay sedation score) could be achieved. The benzodiazepine antagonist flumazenil, given in the presence of the agonist, caused a reversal of the sedative effects of midazolam in all subjects.
The main result of the present investigation was that the magnitude of PPI decreased under increasing plasma concentrations of midazolam and increased after antagonism with flumazenil. However, flumazenil infusion did not cause a complete reversal of the magnitude of PPI. This may be explained by an intrinsic BZR agonistic property of flumazenil [19], or more likely, by deficient dosing of flumazenil in the present protocol. The present work is limited by some other factors. We have chosen to study the effects of flumazenil after midazolam infusion, but decided to prove the presence of midazolam by plasma measurements. Plasma levels during flumazenil infusion were sufficient to induce and maintain sedative effects.
We tested PPI in a simple paradigm without modifying stimulus and/or prepulse intensity. We would like to emphasize that other stimulus or prepulse characteristics and modalities may even be more appropriate. Frequently, some low level background noise was delivered continuously to guarantee that subjects are not distracted by novel acoustic inputs. However, as it was found that response amplitude and latency remained unaffected by continuous background noise [20] our data are comparable with other studies.
This is, to our knowledge, the first human study demonstrating that PPI of the acoustic eye-blink reflex decreases in a dose-dependent manner during midazolam infusion and increases after antagonization with flumazenil.
Our data are in contrast to those of Abduljawad and colleagues [21]. They have recently investigated startle responses after oral diazepam and found that there was no impact on the magnitude of prepulse inhibition [21]. This discrepancy may be explained by a different sedation level achieved. In their study 10 mg of oral diazepam failed to induce changes in the critical flicker fusion frequency, a test otherwise very sensitive to the sedative effects of benzodiazepines [22–24]. However, in our study effective sedation occurred as indicated by vigilance testing and the Ramsay sedation score. Furthermore, a methodological difference exists concerning the calculation of PPI: in the study of Abduljawad PPI was quantified as percentage inhibition—in our study PPI was quantified as a PPI ratio.
Previous work has suggested that BZR agonists and other sedatives reduce the startle effect [25] to acoustic noise. Effects on PPI are less clear. However, diazepam has been found to reduce PPI in animal research [26]. Other sedatives, such as ethanol, did not impact PPI [27]. Surprisingly, partial inverse agonists not given in the presence of an BZR agonist were found to reduce the startle response and increase response latencies [27]. However, PPI, when expressed as percentage inhibition, was not affected.
It is particularly interesting that PPI of the acoustic startle response does not depend on the subject’s active co-operation and motivation but occurs involuntarily [14], although there is evidence that PPI can be modulated by voluntary attention [10, 28, 29]. It is very promising that emotional factors do not substantially influence PPI. This is in contrast to the magnitude of the startle reflex itself which is known to be less reproducible and greatly affected by states of fear [30, 31], administration of anxiolytic drugs [32] and habituation during repetitive stimulation [11, 33]. Another advantage is that PPI is rather insensitive to technical artefacts.
In conclusion, this investigation in healthy young volunteers suggests that assessment of PPI may be useful for monitoring the level of benzodiazepine-induced CNS effects. Further studies are needed to replicate the effect with other benzodiazepines, to induce complete reversal of the effect by sufficient antagonism, to clarify whether the effect of benzodiazepines on PPI is subject to habituation, and to determine the optimal stimulus characteristics. Finally, studies in patients are needed to confirm these findings in normal clinical settings.
Acknowledgments
We are indebted to PD Dr Walter Haefeli, PD Dr Jürgen Drewe (Department of Internal Medicine, University of Basel, Switzerland) and Prof. Dr Terry D. Blumenthal (Department of Psychology, Wake Forest University, NC, USA) for their recommendations and critical review of the manuscript.
References
- 1.Hobbs WR, Rall TW, Verdoorn TA. Hypnotics and sedatives; Ethanol. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, editors. The Pharmacological Basis of Therapeutics, Goodman and Gilman’s, Ninth. New York: McGraw-Hill; 1996. pp. 361–396. [Google Scholar]
- 2.Burns AM, Shelly MP, Park GR. The use of sedative agents in critically ill patients. Drugs. 1992;43:507–515. doi: 10.2165/00003495-199243040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ritz R. Benzodiazepine sedation in adult ICU patients. Intensive Care Med. 1991;17(Suppl 1):11–14. doi: 10.1007/BF01731148. [DOI] [PubMed] [Google Scholar]
- 4.Vree TB, Shimoda M, Driessen JJ, et al. Decreased plasma albumin concentration results in increased volume of distribution and decreased elimination of midazolam in intensive care patients. Clin Pharmacol Ther. 1989;46:537–544. doi: 10.1038/clpt.1989.182. [DOI] [PubMed] [Google Scholar]
- 5.Malacrida R, Fritz ME, Suter PM, Crevoisier C. Pharmacokinetics of midazolam administered by continuous intravenous infusion to intensive care patients. Critical Care Med. 1992;20:1123–1126. doi: 10.1097/00003246-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bauer TM, Ritz R, Haberthür C, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–147. doi: 10.1016/s0140-6736(95)91209-6. [DOI] [PubMed] [Google Scholar]
- 7.Levy G, Ebling WF, Forrest A. Concentration- or effect-controlled clinical trials with sparse data. Clin Pharmacol Ther. 1994;56:1–8. doi: 10.1038/clpt.1994.93. [DOI] [PubMed] [Google Scholar]
- 8.Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;3:377–395. [PubMed] [Google Scholar]
- 9.Blumenthal TD. Prepulse inhibition of the startle eyeblink as an indicator of temporal summation. Percept Psychophysics. 1995;57:487–494. doi: 10.3758/bf03213074. [DOI] [PubMed] [Google Scholar]
- 10.Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: a tool for investigating early and late attentional processes. Biol Psychol. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal TD. Prepulse inhibition decreases as startle reactivity habituates. Psychophysiology. 1997;34:446–450. doi: 10.1111/j.1469-8986.1997.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham FK. Control of reflex blink excitability. In: Thompson RF, Hicks LH, Shvyrkov VB, editors. Neural mechanisms of goal directed behavior and learning. New York: Academic Press; 1980. pp. 511–519. [Google Scholar]
- 13.Ha HR, Rentsch KM, Kneer J, Vonderschmitt DJ. Determination of midazolam and its alpha-hydroxy metabolite in human plasma and urine by high-performance liquid chromatography. Ther Drug Monitor. 1993;15:338–343. doi: 10.1097/00007691-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein LD, Graham FK, Calloway JM. Preconditioning and excitability of the human orbicularis oculi reflex as a function of state. Electrenceph Clin Neurophysiol. 1980;48:406–417. doi: 10.1016/0013-4694(80)90133-9. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxalone-alphadalone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langewitz W, Bieling H, Stephan JA, Otten H. A new self adjusting reaction time device (BonnDet) with high test-retest reliability. J Psychophysiology. 1987;1:67–77. [Google Scholar]
- 18.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application of d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 19.Brogden RN, Goa KL. Flumazenil. A reappraisal of its pharmacologic properties and therapeutic efficacy as a benzodiazepine antagonist. Drugs. 1991;42:1061–1089. doi: 10.2165/00003495-199142060-00010. [DOI] [PubMed] [Google Scholar]
- 20.Lane SJ, Ornitz EM, Guthrie D. Modulatory influence of continuous tone, tone offset, and tone onset on the human acoustic startle response. Psychophysiology. 1991;28:579–587. doi: 10.1111/j.1469-8986.1991.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 21.Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of clonidine and diazepam on the acoustic startle response and on its inhibition by prepulses in man. J Psychopharmacol. 1997;11:29–34. doi: 10.1177/026988119701100110. [DOI] [PubMed] [Google Scholar]
- 22.Claffey L, Plourde G, Morris J, Trahan M, Dean DM. Sedation with midazolam during regional anaesthesia: is there a role for flumazenil? Can J Anaesth. 1994;41:1084–1090. doi: 10.1007/BF03015659. [DOI] [PubMed] [Google Scholar]
- 23.Heine PR, Weyer G, Breuel HP, Muck W, Schmage N, Kuhlmann J. Lack of interaction between diazepam and nimodipine during chronic oral administration to healthy elderly subjects. Br J Clin Pharmacol. 1994;38:39–43. doi: 10.1111/j.1365-2125.1994.tb04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short TG, Young KK, Tan P, Tam YH, Gin T, Oh TE. Midazolam and flumazenil pharmacokinetics and pharmaco-dynamics following simultaneous administration to human volunteers. Acta Anaesthesiol Scand. 1994;38:350–356. doi: 10.1111/j.1399-6576.1994.tb03906.x. [DOI] [PubMed] [Google Scholar]
- 25.Davis M. The mammalian startle response. In: Eaton RC, editor. Neural mechanisms of startle behavior. New York: Plenum Press; 1984. pp. 287–351. [Google Scholar]
- 26.Depoortere R, Perrault G, Sanger DJ. Potentiation of prepulse inhibition of the startle reflex in rats: pharmacological evaluation of the procedure as a model for detecting antipsychotic activity. Psychopharmacology. 1997;132:366–374. doi: 10.1007/s002130050357. [DOI] [PubMed] [Google Scholar]
- 27.Grillon C, Sinha R, O’Malley SS. Effects of ethanol on the acoustic startle reflex in humans. Psychopharmacology. 1994;114:167–171. doi: 10.1007/BF02245459. [DOI] [PubMed] [Google Scholar]
- 28.Berntson GG, Hart S, Sarter M. The cardiovascular startle response: anxiety and the benzodiazepine receptor complex. Psychophysiology. 1997;34:348–357. doi: 10.1111/j.1469-8986.1997.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 29.DelPezzo EM, Hoffman HS. Attention factors in the inhibition of a reflex by a visual stimulus. Science. 1980;210:673–674. doi: 10.1126/science.7433993. [DOI] [PubMed] [Google Scholar]
- 30.Hazlett EA, Buchsbaum MS, Haznedar MM, et al. Prefrontal cortex glucose metabolism and startle eye-blink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- 31.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 32.Davidson RJ, Sutton SK. Affective neuroscience: the emergence of a discipline. Curr Opinion Neurobiol. 1995;5:217–224. doi: 10.1016/0959-4388(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 33.Kumari V, Cotter P, Corr PJ, Gray JA, Checkley SA. Effect of clonidine on the human acoustic startle reflex. Psychopharmacology. 1996;123:353–360. doi: 10.1007/BF02246646. [DOI] [PubMed] [Google Scholar]
- 34.Ornitz EM, Guthrie D. Long-term habituation and sensitization of the acoustic startle response in the normal adult human. Psychophysiology. 1989;26:166–173. doi: 10.1111/j.1469-8986.1989.tb03149.x. [DOI] [PubMed] [Google Scholar]


