Abstract
Aims
To determine the comparative efficacy of a new novel adenosine agonist (WAG 994) in postoperative pain after third molar surgery.
Methods
One hundred and twenty-two patients with postoperative pain after third molar surgery were randomised in a placebo double-blind trial with an active control group. In the early postoperative period patients received either a single dose of WAG 994 1 mg, ibuprofen 400 mg or matched placebos. Pain intensity score was recorded on serial visual analogue scales over a 6 h investigation period. Similarly, pain relief was completed on a 4 point categorical scale at each evaluation point. Patients had access to escape analgesic and if these were taken, the time and dosage were recorded. A sparse sampling technique was used to investigate the relationship between analgesic effects and plasma concentrations of WAG 994.
Results
All three treatment groups were matched for various demographic variables. For all efficacy measures, WAG 994 was not significantly different from placebo (P > 0.05), whereas ibuprofen 400 mg was significantly superior to placebo (P < 0.001). No significant relationships (P < 0.05) were found between WAG 994 pharmacokinetic variables and efficacy measures.
Conclusion
WAG 994, an adenosine agonist, did not show efficacy in the management of postoperative pain after third molar surgery. Although this pain responds well to nonsteroidal anti-inflammatory drugs, it appears to be resistant to compounds that interact with purinergic receptors.
Keywords: adenosine agonist, ibuprofen, postoperative pain, third molar surgery
Introduction
Adenosine is a naturally occurring nucleoside which, in addition to roles in intermediary metabolism, has widespread and potent extracellular actions on excitable membranes [1]. The pharmacological effects of adenosine are mediated via P1-purinergic receptors, of which four subtypes, designated A1, A2A, A2B and A3 have now been cloned [2, 3]. Adenosine and its agonists have been shown to have antinociceptive properties in animal pain models [4, 5–7]. Significant and long-lasting analgesic effects of adenosine, administered as an infusion, were also found in experimentally induced [8–10] and neuropathic pain in humans [11]. The antinociceptive effects of adenosine were demonstrated to be mediated via adenosine A1 receptors [12].
WAG 994 is a highly selective and potent agonist at adenosine A1 receptors. It was found to have antinociceptive effects in animal models of acute inflammatory and neuropathic pain, at doses of 30 μg kg−1 and higher. Evidence suggests that the antinociceptive effect of WAG 994 is mediated via A1 receptors mainly in the spinal cord.
Phase I studies evaluated single dose of WAG 994 from 0.1 to 5 mg. At 1 mg, the compound was well tolerated. However, at doses of 2 mg and above, dose-dependent adverse events occurred. These included headache, dizziness, fatigue, somnolence, dyspnoea and chest pain. In view of these adverse events it was decided to evaluate WAG 994 at a maximum dose of 1 mg in clinical trials.
Postoperative pain after removal of impacted third molars is an acute inflammatory pain of short duration, reaching its maximum intensity in the early postoperative period [13]. The use of the third molar pain model is used extensively to evaluate analgesic efficacy [14], especially in single dose studies. It is thus regarded as a well established model to assess analgesic efficacy and tolerability.
The aim of the present study was to determine the efficacy of a single oral dose of WAG 994, 1 mg in a placebo controlled, randomised, double-blind trial in patients with postoperative pain after removal of their impacted third molars, and to compare pain relief obtained after WAG 994 with that obtained after ibuprofen 400 mg. A secondary aim was to investigate the relationship, if any, between plasma concentrations of WAG 994 and analgesic variables using a sparse sampling technique.
Methods
One hundred and fifty patients who required removal of their impacted third molars were screened for the study. Informed written consent was obtained from each patient prior to their entry into the study, which had received ethical approval from the appropriate local health authority Ethical Committee. Patients enrolled into the study were fit and healthy and complied with the criteria of the American Society of Anaesthesiologist category 1 (ASA 1). All patients attended a prescreening clinic prior to their participation in the study. The prescreening was held some 2–3 weeks before surgery. All patients had to abstain from taking any analgesics or xanthine-containing food or beverages for 8 h prior to the study.
Patients underwent the removal of their impacted third molars under general anaesthesia. The anaesthetic regimen was standardized for each patient and included induction with intravenous propofol (2–2.5 mg kg−1 body weight), and muscle relaxation with pancuronium (50–100 μg kg−1 body weight) or atracurium (300–600 μg kg−1 body weight). Anaesthesia was maintained with enflurane, nitrous oxide and oxygen. Peri-operative analgesia was provided by a single dose of fentanyl (1 μg kg−1 body weight).
Impacted third molars were removed following a standard technique. The operating time (from first incision to completion of last suture) was recorded for each patient. On completion of the surgical procedure, time was allowed for the patients to recover fully from the effects of the anaesthetic. They were then returned to the ward where they were monitored by the study nurse and their pain intensity assessed on 100 mm visual analogue scales (VAS). The boundaries of the scale were marked ‘no pain’ and ‘worst pain imaginable’. When patients pain intensity reached a level >30 mm on the VAS (equivalent to moderate pain) they were entered into the study and randomised to receive either single doses of ibuprofen 400 mg, WAG 994 1 mg or placebo. If patients did not reach this level of pain within 1 h, they did not participate in the study. Matched placebos were prepared for both medications. To ensure true double-blind conditions, the double-dummy technique was used. Thus, each patient received both preparations which were swallowed with sips of water.
Pain assessment
The following 5 measures were used:
Pain intensity was assessed on 100 mm VAS at 0, 15, 30, 45, 60, 120, 180, 240, 300 and 360 min after dosage. The pain intensity difference score (PIDS) at each post dosage evaluation point was calculated as the pain intensity score at baseline minus the pain intensity score at each evaluation. The PIDS at each evaluation was summed to give an aggregate sum of the pain intensity difference score (SPIDS).
Pain relief scores were evaluated at each postdose evaluation point on a 5-point categorical scale where 0 = none, 1 = a little, 2 = some, 3 = a lot, 4 = complete. The pain relief scores at each evaluation were summed to give an aggregate total pain relief score (TOTPARS).
Time to achieve 50% pain relief: At each evaluation time point, patients were asked whether their baseline pain was a least half gone.
Time to remedication (escape analgesia): In the event of poor pain control, patients were allowed access to alternative analgesia (cocodamol). Patients requiring remedication in the first hour were excluded from the study. For those taking additional analgesics after 60 min, the time was recorded and their previous VAS pain intensity score was extrapolated over the remaining time points [15–26].
Global assessment: At the end of the 6 h investigation period, patients were asked to provide an overall assessment of their medication on a 5-point scale. The categories were 1 = very poor; 2 = poor; 3 = moderate; 4 = good; 5 = very good.
At the end of the 6 h observation period patients were discharged home. They were asked to complete additional pain recordings (pain intensity, relief and additional analgesic consumption) at 8, 12 and 24 h postdosing.
Throughout the 6 h investigation period, a study nurse was responsible for monitoring the patient and recording any adverse events.
Blood sampling
Just prior to dosage, an indwelling catheter was placed in a convenient forearm vein, and a 5 ml venous blood sample was withdrawn at 0, 0.5, 1, and 2 h after dosing. The blood samples were placed in lithium-heparin tubes kept in ice. Plasma was separated by centrifugation and stored at −20° C before analysis in duplicate for WAG 994.
Analysis
Efficacy analyses were carried out on all randomised subjects who received medication, provided baseline pain measurements and had at least one postdose assessment of efficacy. The primary comparison of interest was defined as WAG 994 vs placebo. Therefore adjustment of α-level for pair wise comparisons was not required. All hypothesis tests were two-tailed with α = 0.05.
The following information was available for each patient.
PIDS/SPIDS: treatment comparisons for PIDS at each time point were analysed by analysis of covariance (ANOCOVA), with the baseline pain severity score as a covariate and treatment group, sex and centre as factors. All pairwise comparisons were based on Student’s t-test using the pooled error term from the model. The PIDS at each evaluation were summed to give an aggregate SPIDS using appropriate weights according to the time interval between successive evaluations (e.g. the ratings at 15, 30, 45 and 60 min were given a weight of 0.25 and hourly evaluations were given a weight of 1). SPIDS were also analysed by ANOCOVA.
PARS at each evaluation were summed to give TOTPARS in the same manner. Although pain relief scores (analgesia) represent ordinal rather than interval data, parametric procedures have proved to be an adequate approach in analgesic studies [27]. PARS and TOTPARS were also analysed using the same method as for PIDS & SPIDS.
Proportion of patients showing at least 50% relief: treatment comparisons were analysed using linear logistic regression with baseline pain intensity score as a covariate and centre, sex and treatment as blocking factors.
The proportion of subjects taking escape medication was again analysed using logistic regression analysis. The time to re-medication with escape analgesic was analysed using the Cox proportional hazard logistic model with baseline pain score, sex and centre included in the model [16]. If no escape medication was taken during the 24 h, then the time to re-medication was recorded as 24.
Pairwise comparisons between treatment groups for patients overall assessment of their medication were determined using Wilcoxon’s rank sum test, and the number of patients taking escape medication using Fisher’s Exact Test.
Results
One hundred and fifty patients were screened for the study and 127 underwent surgery. Five patients failed to reach a sufficient level of baseline pain in the immediate postoperative period. The remaining 122 patients were medicated and their demographic details, together with the operating times and mean baseline pain scores are shown in Table 1; it can be seen that the treatment groups were well balanced with no significant differences between the groups. There was no significant differences between groups (P > 0.05) for all the parameters listed in Table 1.
Table 1.
Demographic details of patients who participated in the study [where appropriate results are expressed as means and standard deviations (s.d.)].
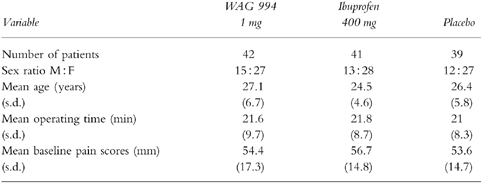
Efficacy variables for SPIDS, TOTPARS, numbers of patients indicating a 50% reduction in their pain, and details of escape medication are shown in Table 2. On all of the measures, WAG 994 showed no difference in efficacy from placebo (P > 0.05). By contrast, ibuprofen was significantly superior to the placebo and WAG 994.
Table 2.
Efficacy parameters recorded in the investigation. Results are expressed as means (s.d.), with differences showing 95% confidence intervals.
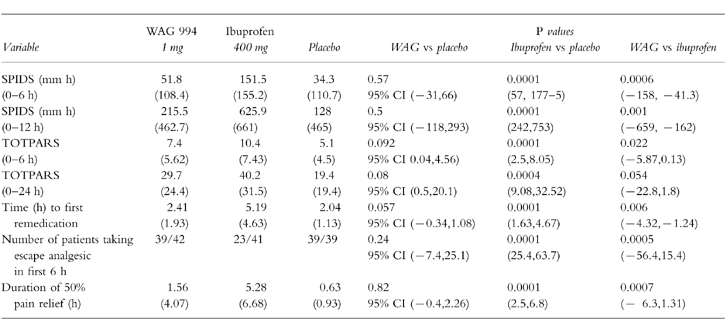
Patients overall tolerability of their medication is reported in Table 3. The distribution of scores shows that there was no significant difference (P > 0.05) between the three treatment groups.
Table 3.
Patients’ overall global assessment of their medication.
The plasma concentrations of WAG 994 after the 1 mg dose, together with other pharmacokinetic variables, are shown in Table 4. There was no significant relationship between any of these measures and the efficacy variables.
Table 4.
Pharmacokinetic parameters assessed from a random sampling technique following administration of WAG 994 1 mg. Results are expressed as means±s.d.

There were no serious adverse events, and most of the adverse events recorded in this study were directly related to the surgical procedure and anaesthetic agents.
Discussion
The findings from this study have shown unequivocally that 1 mg WAG 994, an adenosine agonist, has poor analgesic properties in postoperative pain after third molar surgery. The results have also confirmed the established efficacy of ibuprofen in this pain model. The lack of efficacy of the adenosine agonist may be due to an inappropriate dose or limited involvement of A1 receptors in the pathogenesis of nociception after this type of surgery. Increasing the dose of WAG 994 is not an option in this pain model since at doses >1 mg there is a significant increase in the incidence of unwanted effects.
The efficacy of adenosine in certain pain conditions seems to be attributable to low doses of the compound (50–80 μg kg−1 min−1) given by infusion [6, 17]. Two further small studies have confirmed that low dose adenosine infusions are of value in the control of neuropathic pain [11, 18]. Similarly, in experimental pain, adenosine infusions provided a modest, but selective increase of cutaneous heat pain threshold [19] and reduced experimentally induced ischaemic muscle pain [9]. It is suggested that adenosine reduces c-fibre transmission and low doses appear to be selective for prejunctional modulation of nociceptive transmission. Such transmission involves the A1 subclass of the P1 purinoceptors [20].
By contrast, higher doses of adenosine induce pain when injected intravascularly and can stimulate the pain of angina and peptic ulceration [21–23]. Adenosine can also produce cutaneous pain when applied to blister base preparations [24] and when injected intradermally [25]. This study also showed that the local infiltration of adenosine produced hyperalgesia to mechanical and heat stimuli at the injection site. Adenosine-induced pain and hyperalgesia was blocked by bamiphylline, an adenosine antagonist. This suggests that algogenic properties of adenosine in cutaneous tissue are mediated by the A1 receptor.
Thus, the role of adenosine in nociception and pain expression is equivocal and appears to be related to dose and the activation of the various purinergic receptors. Our findings that 1 mg of an orally administered adenosine agonist was no better than placebo in the control of postoperative pain after third molar surgery may also suggest that the various purinergic receptors have little or no role in the pathogenesis of this type of pain. Postoperative dental pain is an acute inflammatory pain that responds well to nonsteroidal anti-inflammatory drugs [14]. This type of pain appears to be resistant to opioids [26]. The evidence from the present study suggests that this type of pain is also resistant to compounds that interact with purinergic receptors.
Figure 1.
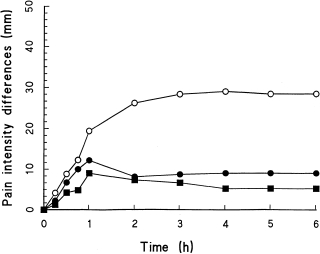
Mean pain intensity difference scores throughout the 6-h investigation period for (^—^ = ibuprofen 400 mg (•—• = SDZ WAG 994 1 mg, and ▪mdash;▪ = placebo).
Figure 2.
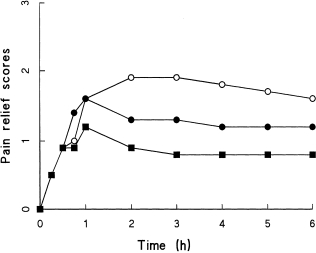
Mean pain relief scores throughout the 6-h investigation period (key as in Figure 1).
Acknowledgments
The authors are grateful to Novartis Pharma AG (ex Sandoz Pharma AG) for supplying WAG 994.
References
- 1.Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Ann Rev Pharmacol Toxicol. 1987;27:315–435. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- 2.Stiles GL. Adenosine receptors. J Biol Chem. 1992;267:6451–6454. [PubMed] [Google Scholar]
- 3.Zhou QY, Li C, Olah ME, et al. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Nat Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlson R, Gordh T, Hartvig P, Claeo P. Effects of intrathecal injection of the adenosine receptor agonist R-phenylisopropyl-adenosine and N-ethylcarboxamide-adenosine on nociception and motor function in the rat. Anesth Analg. 1990;71:60–64. doi: 10.1213/00000539-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sawynok J, Sweeney MI. The role of purines in nociception. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- 6.Sollevi A. Adenosine infusion during isoflurane-nitrous oxide anaesthesia: indicators of perioperative analgesic effect. Acta Anaesth Scand. 1992;36:595–599. doi: 10.1111/j.1399-6576.1992.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 7.Segerdahl M, Ekblom A, Sandelin K, et al. Perioperative adenosine infusion reduces the requirements for isoflurane and postoperative analgesics. Anaesth Analg. 1995;80:1143–1149. doi: 10.1097/00000539-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Segerdahl M, Ekblom A, Sollevi A. The influence of adenosine, ketamine and morphine on experimentally-induced ischaemic pain in healthy volunteers. Anesth Analg. 1994;79:787–791. doi: 10.1213/00000539-199410000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Segerdahl M, Ekblom A, Sjolund KF, et al. Systemic adenosine atenuates touch-evoked allodynia induced by mustard oils in humans. Neurol Report. 1995;6:753–756. doi: 10.1097/00001756-199503270-00012. [DOI] [PubMed] [Google Scholar]
- 10.Karlsten R, Gordh T. An A1-selective adenosine agonist abolishes allodynia elicited by vibration and touch after intrathecal injection. Anesth Analg. 1995;80:844–847. doi: 10.1097/00000539-199504000-00037. [DOI] [PubMed] [Google Scholar]
- 11.Sollevi A, Belfrage M, Landeberg T, et al. Systemic adenosine infusion: a new treatment modality to alleviate neuropathic pain. Pain. 1995;61:155–158. doi: 10.1016/0304-3959(94)00187-J. [DOI] [PubMed] [Google Scholar]
- 12.Sawynok J, Sweeney MI, White TD. Classification of adenosine receptors mediating antinociception in the rat spinal cord. Br J Pharmacol. 1986;88:923–930. doi: 10.1111/j.1476-5381.1986.tb16267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour RA, Meechan JG, Blair GS. An investigation into postoperative pain after third molar surgery under local anaesthesia. Br J Oral Maxillofac Surg. 1985;23:410–418. doi: 10.1016/0266-4356(85)90025-7. [DOI] [PubMed] [Google Scholar]
- 14.Meechan JG, Seymour RA. The use of third molar surgery in clinical pharmacology. Br J Oral Maxillofac Surg. 1993;31:360–365. doi: 10.1016/0266-4356(93)90191-x. [DOI] [PubMed] [Google Scholar]
- 15.Lasagna L. Analgesic methodology: a brief history and commentary. J Clin Pharmacol. 1980;20:373–400. [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables (with discussion) J Roy Statist Soc. 1972;34:187–220. [Google Scholar]
- 17.Segerdahl M, Person E, Ekblom A, et al. Perioperative adenosine infusion reduces isuflurane concentrations during general anaesthesia for shoulder surgery. Acta Anaesthiol Scand. 1996;40:792–797. doi: 10.1111/j.1399-6576.1996.tb04534.x. [DOI] [PubMed] [Google Scholar]
- 18.Belfrage M, Sollevi A, Segerdahl M, et al. Systemic adenosine infusion alleviates spontaneous and stimulus evoked pain in patients with peripheral neuropathic pain. Anaesth Analg. 1995;81:713–717. doi: 10.1097/00000539-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ekblom A, Segerdahl M, Sollevi A. Adenosine increases the cutaneous heat pain threshold in healthy volunteers. Acta Anaesthesiol Scand. 1995;39:717–722. doi: 10.1111/j.1399-6576.1995.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 20.Reeve AJ, Dickenson AH. The roles of spinal adenosine receptors in the control of acute and more persistent nociceptive responses of dorsal horn neurones in the anaesthetised rat. Br J Pharmacol. 1995;116:2221–2228. doi: 10.1111/j.1476-5381.1995.tb15057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sylven C, Jonzon BN, Fredholm BB, et al. Adenosine injection into the brachial artery produces ischaemia like pain or discomfort in the forearm. Cardiovasc Res. 1988;22:674–678. doi: 10.1093/cvr/22.9.674. [DOI] [PubMed] [Google Scholar]
- 22.Watt AH, Lewis DJM, Horne JJ, et al. Reproduction of epigastric pain of duodenal ulceration by adenosine. Br Med J. 1987;294:10–12. doi: 10.1136/bmj.294.6563.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagergvist BO, Sylven C, Beermann B, et al. Intracoronary adenosine causes angina pectoris-like pain—an inquiry into the nature of visceral pain. Cardiovasc Res. 1990;8:609–613. doi: 10.1093/cvr/24.8.609. [DOI] [PubMed] [Google Scholar]
- 24.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 25.Pappagallo M, Gaspardone A, Tomai F, et al. Analgesic effect of bamphylline on pain induced by intradermal injection of adenosine. Pain. 1993;53:199–204. doi: 10.1016/0304-3959(93)90081-Y. [DOI] [PubMed] [Google Scholar]
- 26.Seymour RA, Walton JG. Pain control after third molar surgery. Int J Oral Surg. 1984;13:457–485. doi: 10.1016/s0300-9785(84)80017-4. [DOI] [PubMed] [Google Scholar]
- 27.Group for Analgesic Drugs, US Food & Drug Administration. Guidelines for the Clinical Evaluation of Analgesic Drugs. 1992. p. 12. Document Number 91D—0425,


