Abstract
Aims
Ursodeoxycholic acid (UDCA) has cholesterol lowering and anti-inflammatory effects and bile acids are reported to exert vasodilator effects; all of these properties might be considered desirable in a drug used in the treatment of patients with coronary heart disease. We investigated a hypothesis that UDCA may dilate arteries and the mechanism of action.
Methods
We evaluated effects of a 6-week treatment with UDCA in 11 coronary heart disease patients on endothelium-dependent (acetylcholine-induced) and -independent (nitroprusside-induced) vasodilatations in forearm vasculature by strain-gauge plethysmography. Healthy individuals (n = 14) served as baseline controls.
Results
The percentage increase by acetylcholine in the flow of the infused arm relative to the non-infused arm of coronary heart disease patients during the trial remained unaltered, but vasodilatation to NG-monomethyl-l-arginine+acetylcholine was improved by 161±27% with UDCA vs 83±22% with placebo (mean difference 91% [95% CI 35%, 147%], P = 0.016).
Conclusions
Six weeks’ UDCA therapy improved endothelium-dependent nitric oxide-independent vasodilatation, which might maintain arterial flow in coronary heart disease patients under conditions of impaired nitric oxide production.
Keywords: ursodeoxycholic acid, coronary heart disease, endothelium
Introduction
Nitric oxide (NO) is the most potent vascular endothelium derived vasodilator, but in atherosclerosis its formation is decreased. In addition the endothelium produces other vasoactive agents: prostacyclin, endothelium-derived hyperpolarizing factor, thromboxane A2, prostaglandin H2 and superoxide anions [1], of which the first two maintain endothelial vasodilator function when NO production is impaired [2]. Because there is an inflammatory component in atherosclerosis [3], a reduction in inflammation might improve endothelium-dependent vasodilatation [4,5].
Ursodeoxycholic acid (UDCA) is an effective medication for gallstones [6], with anti-inflammatory and chemoprotective properties in animal models [7]. It stabilises cell membranes and mimics the effect of cholesterol [8]. It may alter the ability of endothelium to hyperpolarize. Moreover, UDCA lowers cholesterol levels [9], and bile acids have vasodilatory properties [10]. Based on these observations, we tested a hypothesis that a treatment with UDCA could affect endothelial function in patients with coronary heart disease.
Methods
Subjects
Subjects (<65 years) with clinical signs of ischaemia and angiographically verified coronary artery stenosis (≥60%) were included. Patients with any systemic disease other than atherosclerosis were excluded. Acetylsalicylic acid ≤250 mg day−1 (11 patients), a β-adrenoceptor-blocker without peripheral vasodilatory effect (10 patients) or long acting nitrates (two patients) were the only medications allowed. Medical history, clinical examination, abdominal ultrasound and haematological and biochemical profiles were undertaken. Control studies for plethysmography were carried out in 14 age and weight matched, non-smoking, healthy male subjects.
Written informed consent was obtained from all participants. The study protocol was approved by The Ethics Committee of The Helsinki University Central Hospital and The Finnish National Agency for Medicines.
Design outline of the study
The study was designed to compare the effects of UDCA (Adursal®, Leiras Pharmaceuticals, Turku, Finland) twice daily (13 to 19 mg kg−1) and placebo on forearm endothelial function according to a randomised, double-blind, placebo-controlled crossover protocol. Of 25 coronary heart disease patients (51±2.5 years), 12 (11 male, 1 female) had clear endothelial dysfunction based on the results of previous studies (acetylcholine-induced flow below the 50th percentile = poor acetylcholine responder), and were selected for study; six received UDCA and six placebo. After 6 weeks, forearm vascular function and laboratory measurements were repeated. After crossover, subjects were restudied 6 weeks later. Compliance was assessed by pill count.
Fasting blood samples were drawn from the antecubital vein between 08.00 and 09.00 h for screening laboratory measurements. Plasma thromboxane B2 was measured by r.i.a. [11].
Vascular function tests
Subjects were studied between 09.00 and 12.00 h at a stable 24±1° C temperature in a quiet room as described earlier [12]. Acetylsalicylic acid was discontinued for 72 h and all other medication except trial tablets for 24 h before the study. Flow measurements were performed simultaneously in both arms by plethysmography with subjects in the supine position [13] by automatic electrical calibration (EC 4 Strain Gauge Plethysmograph, D.E.Hokanson, WA, USA). An analogue-to-digital converter (McLab/4e, AD Instruments, Hastings, UK) served to record blood flow.
A fine cannula (27 SWG) was inserted into the right brachial artery, and basal measurements were obtained after 12 min of infusion of 0.9% saline (1 ml min−1). Forearm blood flow was measured after the infusion of nitroprusside (an endothelium-independent vasodilator) [14], acetylcholine (endothelium-dependent vasodilator) [2,15] and NG-monomethyl-l-arginine (l-NMMA). Acetylcholine and nitroprusside doses were chosen based on dose-response curves previously recorded in these patients (unpublished data). l-NMMA which competitively antagonises the synthesis of NO provides a tool for the investigation of the role of endothelial NO production in vasodilator response. A dose of 1 mg min−1 l-NMMA blunts in vivo synthesis of NO and reduces the vasodilatory effect of acetylcholine in human forearm [16]. The drugs were infused with an infusion pump (Braun AG, Melsungen, Germany) at the same rate (1 ml min−1) in the following sequence: 1) nitroprusside (Nipride®, F. Hoffman-La Roche Ltd, Basel, Switzerland; 3 μg min−1 and 10 μg min−1) 2) acetylcholine (Miochol®,Ciba Vision A/S, Roschilde, Denmark; 7.5 μg min−1 [low dose] and 15 μg min−1 [high dose]); 3a) l-NMMA (Clinalfa AG, Läufelfingen, Switzerland; 1 mg min−1), and 3b) l-NMMA with acetylcholine 7.5 μg min−1, and finally, 3c) l-NMMA with acetylcholine 15 μg min−1. Each dose of the drug was infused for 6 min, and was separated from the next by 18 min infusion of saline ( = basal value for next drug infusions).
Statistical analysis
Quantitative data (mean±s.e.mean) were compared by a t-test or Mann-Whitney’s test and proportions by the chi-square or Fisher′s exact test. Confidence intervals 95% (CI) on differences were calculated for selected variables. Because, absolute measurements of forearm blood flow are subject to error due to minor external factors, it is appropriate in follow up studies to describe the drug effects as the percentage change from the preceding basal value of blood flow in the infused arm as a ratio of the same percentage change in the control arm [17–20]. That is:
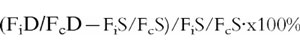 |
where Fi and Fc represents measured blood flows in the infused and control arms, during periods of drug (D) and saline (S) administration [20]. Two-way ANOVA with repeated measurements was used to compare baseline, placebo and UDCA treatments. Period effects and carry-over effects were calculated for each value [21]. A value of P < 0.05 was considered significant.
Results
Clinical characteristics
All screened laboratory values were normal before and after UDCA treatment. Plasma thromboxane B2-like immunoreactivity showed a lowering trend during UDCA: baseline 1.59±0.47 ng ml−1, placebo 0.72±0.45 ng ml−1 and UDCA 0.49±0.22 ng ml−1 (mean difference from placebo −0.22 [95% CI −0.84, 0.39], P = 0.1). Abdominal ultrasound examinations were normal. The anti-anginal medication did not change during the trial.
Compliance and adverse effects
One patient was withdrawn during the placebo period due to non-compliance before the first visit. Serum bile acids concentrations rose during UDCA therapy, confirming that patients did take their medication: baseline 2.5±0 μmol l−1, placebo 3.8±0.9 μmol l−1 and UDCA 18.5±5.0 μmol l−1 (mean difference from placebo 14.8 μmol l−1 [95% CI 4.6, 24.9], P = 0.0016). No clinically significant adverse events were reported.
Vascular responses
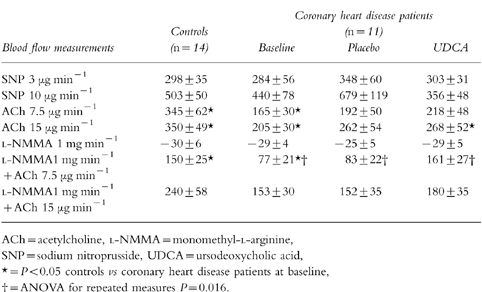
Forearm blood flow in the non-infused arm did not change significantly during the infusions of nitroprusside, acetylcholine or l-NMMA, indicating lack of systemic effects. Flow values in the control subjects during acetylcholine and l-NMMA+acetylcholine were higher (P = 0.04) than those of coronary heart disease patients at baseline (Table 1). The percentage changes from the preceding basal value of blood flow in the infused arm as a ratio of the same percentage change in the control arm are given in Table 2. The vascular responses to nitroprusside, acetylcholine or l-NMMA were not influenced by UDCA treatment. However, when low dose-acetylcholine was infused with l-NMMA (vasodilatation due to factors other than NO-production), vasodilatation was more pronounced during UDCA therapy; 161±27%vs 83±22% during placebo (mean difference 91% [95% CI 35%, 147%], P = 0.016). UDCA therapy failed to change significantly the vasodilatation response of l-NMMA+high dose-acetylcholine. Although the flow values of controls during l-NMMA+low dose-acetylcholine were significantly higher than those of coronary heart disease patients at baseline (Table 2), after UDCA therapy they were similar (P > 0.05).
Table 1.
Absolute flow values at baseline and during the trial.
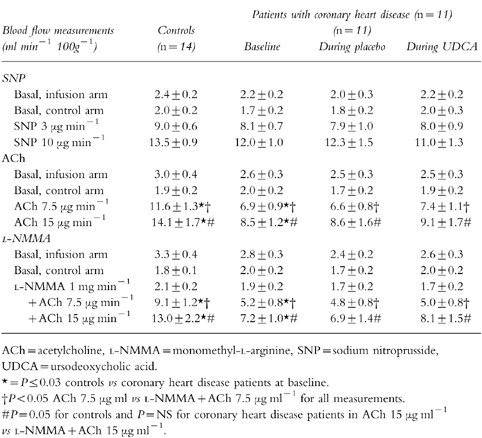
Table 2.
The percentage change from the preceding basal value of blood flow in the infused arm as a ratio of the same percentage change in the control arm at baseline and during the trial.
Discussion
Depressed endothelial function in coronary heart disease patients can be improved by UDCA. Acetylcholine increased the forearm blood flow of the controls significantly more than blood flow of coronary heart disease patients, as has been shown previously [22]. UDCA treatment of coronary heart disease patients failed to change vascular responses to either nitroprusside or acetylcholine. However, when the formation of NO was blocked by l-NMMA, acetylcholine-induced vasodilatation was augmented during the UDCA period. Several explanations are possible.
Acetylcholine-induced vasodilatation during NO-blockade may be due to increased production of arachidonic acid-derived vasodilators, e.g. endothelium-derived hyperpolarizing factor and prostacyclin [2], or due to a decreased production of vasoconstrictors, e.g. thromboxane A2and superoxide anion. Decreased release of contracting metabolites of arachidonic acid [23] could result from the anti-inflammatory effect of UDCA. This possibility was tested by measuring plasma thromboxane B2-like immuno-reactivity (ir-TXB2). In comparison with the basal levels, ir-TXB2 was clearly lowered in nine out of eleven subjects during the UDCA period. In two patients the values were slightly decreased, and in none increased. However, in comparison to the placebo ir-TXB2, levels at the end of UDCA periods did not differ significantly.
Limitations of the study, such as size of series, short follow up, relatively short abstinence of coronary heart disease medication (24–72 h) and selection of patients with severe endothelial dysfunction to receive UDCA, may have affected the results. The study should be repeated in a normal population and in regard of endothelial function in non-selected coronary heart disease population before any clinical conclusions can be drawn. Basal blood flows were higher in the infusion arm than in the control arm. This is because forearm blood flow is usually higher in the dominant arm [17]. Basal blood flow reduction by l-NMMA did not differ between coronary heart disease patients and controls as shown earlier in patients at risk of coronary heart disease [24]. UDCA caused a significant increase of blood flow only in coronary heart disease patients with l-NMMA+low dose-acetylcholine, but l-NMMA did not diminish high dose acetylcholine-induced vasorelaxation. This was seen systematically in all measurements during the trial, and may be due to patient selection.
In conclusion, the present study showed for the first time that 6-week UDCA treatment enhanced NO-independent endothelial vasodilatation in coronary heart disease patients with depressed endothelial function. It has been suggested that, in severe endothelial dysfunction, there can be significant production of constrictor substances in response to acetylcholine and physiological vasodilatation stimulation. Therefore, drugs that inhibit constrictor release may prove beneficial in clinical use.
Acknowledgments
We greatly appreciate the help of Kimmo Malminiemi, MD, University of Tampere, for checking the statistics. This study was supported in part by Leiras Pharmaceuticals, Finland and the Aarno Koskelo Foundation.
References
- 1.Lüscher T, Tanner FC. Endothelial regulation of vascular tone and growth. Am J Hypertension. 1993;6:283S&–293S. doi: 10.1093/ajh/6.7.283s. [DOI] [PubMed] [Google Scholar]
- 2.Cohen R, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 3.Alexander R. Inflammation and coronary artery disease. N Engl J Med. 1994;331:468–469. doi: 10.1056/NEJM199408183310709. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Molecular basis of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 5.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide link. Lancet. 1997;349:1391–1392. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 6.Rubin R, Kowalski TE, Khandelwal M, Malet PF. Ursodiol for hepatobiliary disorders. Ann Intern Med. 1994;121:207–218. doi: 10.7326/0003-4819-121-3-199408010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Invernizzi P, Salzman AL, Szabo C, Ueta I, O,Connor M, Setchell KDR. Ursodeoxycholate inhibits induction of NOS in human intestinal epithelial cells and in vivo. Am J Physiol. 1997;273:G131–G138. doi: 10.1152/ajpgi.1997.273.1.G131. [DOI] [PubMed] [Google Scholar]
- 8.Güldütuna S, Deisinger B, Weiss A, et al. Ursodeoxycholate stabilizes phospholipid-rich membranes and mimics the effect of cholesterol:investigation on large unilamellar vesicles. Biochim Biophys Acta. 1997;1326:265–274. doi: 10.1016/s0005-2736(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 9.Poupon R, Balkau B, Eschwege E Poupon R and the UDCA-PBC study group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med. 1991;324:1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 10.Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators. Biochem Pharmacol. 1995;49:581–589. doi: 10.1016/0006-2952(94)00428-o. [DOI] [PubMed] [Google Scholar]
- 11.Alanko J, Riutta A, Mucha I, et al. Adrenaline stimulates thromboxane and inhibites leukotriene synthesis in man. Eicosanoids. 1992;5:169–175. [PubMed] [Google Scholar]
- 12.Sinisalo J, Mattila K, Nieminen MS, et al. The effect of prolonged doxycycline therapy on Chlamydia pneumoniae serological markers, coronary heart disease risk factors and forearm basal nitric oxide production. J Antimicrob Chemother. 1998;41:85–92. doi: 10.1093/jac/41.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Whitney R. The measurement of volume changes in human limbs. J Physiol (Lond) 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohme E, Graf H, Schultz G. Effects of sodium nitroprusside and other smooth muscle relaxant on cyclic-GMP formation in smooth muscle and platelets. Adv Cyclic Nucl Res. 1978;9:131–143. [PubMed] [Google Scholar]
- 15.Chowienczyk P, Cockcroft JR, Ritter JM. Blood flow responses to intra-arterial acetylcholine in man: effects of basal flow and conduit vessel length. Clin Sci. 1994;87:45–51. doi: 10.1042/cs0870045. [DOI] [PubMed] [Google Scholar]
- 16.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;ii:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 17.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme a reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 18.Green D, O’Driscoll G, Blanksby B, Taylor R. Lack of effect of vitamin E administration on basal nitric oxide function in male smokers and non-smokers. Clin Sci. 1995;89:343–348. doi: 10.1042/cs0890343. [DOI] [PubMed] [Google Scholar]
- 19.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb D. The pharmacology of human blood vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]
- 21.Pocock S. Crossover trials. In: Pocock S, editor. Clinical trials: A practical approach. Bath, Great Britan: A Wiley Medical Publication; 1983. pp. 110–121. [Google Scholar]
- 22.Zeiher A, Drexler H, Wollschläger H, Just H. Modulation of coronary vasomotor tone in human. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 23.Vane J, Änggård EE, Botting RM. Regulatory function of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 24.Casino P, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilatation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]


