Abstract
Background and purpose:
Although tramadol is known to exhibit a local anaesthetic effect, how tramadol exerts this effect is not understood fully.
Experimental approach:
The effects of tramadol and its metabolite mono-O-demethyl-tramadol (M1) on compound action potentials (CAPs) were examined by applying the air-gap method to frog sciatic nerves, and the results were compared with those of other local anaesthetics, lidocaine and ropivacaine.
Key results:
Tramadol reduced the peak amplitude of the CAP in a dose-dependent manner (IC50=2.3 mM). On the other hand, M1 (1–2 mM), which exhibits a higher affinity for μ-opioid receptors than tramadol, did not affect CAPs. These effects of tramadol were resistant to the non-selective opioid receptor antagonist naloxone and the μ-opioid receptor agonist, DAMGO, did not affect CAPs. This tramadol action was not affected by a combination of the noradrenaline uptake inhibitor, desipramine, and the 5-hydroxytryptamine uptake inhibitor, fluoxetine. Lidocaine and ropivacaine also concentration-dependently reduced CAP peak amplitudes with IC50 values of 0.74 and 0.34 mM, respectively.
Conclusions and implications:
These results indicate that tramadol reduces the peak amplitude of CAP in peripheral nerve fibres with a potency which is less than those of lidocaine and ropivacaine, whereas M1 has much less effect on CAPs. This action of tramadol was not produced by activation of μ-opioid receptors nor by inhibition of noradrenaline and 5-hydroxytryptamine uptake. It is suggested that the methyl group present in tramadol but not in M1 may play an important role in producing nerve conduction block.
Keywords: tramadol, lidocaine, ropivacaine, local anaesthetic, compound action potential, frog
Introduction
Tramadol, (1RS; 2RS)-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl)-cyclohexanol hydrochloride, is a clinically used, orally active, analgesic drug that is considered to act in the central nervous system (Klotz, 2003). Tramadol is metabolized to various compounds via N- and O-demethylation in humans and animals (Lintz et al., 1981), and its major metabolite, mono-O-demethyl-tramadol (M1), is therapeutically active as an analgesic (Klotz, 2003). One of the cellular mechanisms for the antinociceptive effect of tramadol is the activation of μ-opioid receptors (Hennies et al., 1988; Raffa et al., 1992). In support of this idea, M1 has the highest affinity for μ-opioid receptors among the metabolites of tramadol. We have demonstrated that M1 produces a membrane hyperpolarization by activating μ-opioid receptors in substantia gelatinosa (SG; lamina II of Rexed) neurones, which play a pivotal role in regulating nociceptive transmission to the spinal dorsal horn from the periphery, resulting in a decrease in the excitability of the SG neurones (Koga et al., 2005). In addition to centrally acting analgesic effects, tramadol is known to exhibit a local anaesthetic effect following intradermal injection in patients (Pang et al., 1998; Le Roux and Coetzee, 2000; Altunkaya et al., 2003, 2004). Consistent with this finding, in vivo studies have demonstrated that a direct application of tramadol on rat sciatic nerves reduced spinal somatosensory-evoked potentials (Tsai et al., 2001). Further, tramadol reduced the amplitude of compound action potentials (CAPs) recorded extracellularly from sciatic nerve fibres of frogs (Mert et al., 2002) and rats (Mert et al., 2003; Güven et al., 2005); but the involvement of μ-opioid receptors in this action of tramadol was not examined. On the other hand, we did not note a block of conduction of action potentials (APs) in primary-afferent fibres when the effect of M1 on dorsal root-evoked excitatory postsynaptic currents was examined by applying the patch-clamp technique to SG neurones in rat spinal cord slices (Koga et al., 2006). These results suggest that, unlike tramadol, M1 may not block the conduction of APs in nerve fibres, although both of them exhibit an affinity for μ-opioid receptors (Gillen et al., 2000). It is possible that the reduction of CAP amplitude produced by tramadol is not mediated by μ-opioid receptors.
As a first step in addressing this issue, we examined the effects of tramadol and M1 on frog sciatic nerve CAPs; these potentials are easily measured and have been well characterized. The results were quantitatively compared with those of the local anaesthetics, lidocaine, which is well known to block AP conduction (Hille, 1984; Mert et al., 2002, 2003; Güven et al., 2005), and ropivacaine, which reportedly exhibits a longer duration of action in terms of nerve conduction block than lidocaine (Chan et al., 1999; McClellan and Faulds, 2000). The present study revealed that tramadol reduced the peak amplitude of CAP in peripheral nerve fibres with a potency less than those of lidocaine and ropivacaine and that this action of tramadol was not produced by activation of μ-opioid receptors.
Methods
Preparation of frog sciatic nerves
This study was approved by the Animal Care and Use Committee of Saga University, and was conducted in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science of the Physiological Society of Japan. All efforts were made to minimize animal suffering and the number of animals used. Frogs (Rana nigromaculata; weight: 30–55 g) of either sex were decapitated and then pithed; thereafter, the sciatic nerve (length: 4–5 cm; diameter: 0.6–1 mm) was dissected from the lumbar plexus to the knee in Ringer solution. The isolated sciatic nerve was carefully desheathed under a binocular microscope and then loosely placed on five platinum wires (diameter: 0.5 mm), separated by about 0.8 cm from each other, that were glued to a Lucite plate, where the two ends of the nerve were tied to the wires by using threads. The plate was put in a beaker containing Ringer solution (100 ml) to cover the sciatic nerve. Throughout the experiment, the Ringer solution was continuously stirred at a rate of about 200 r.p.m. with a Teflon-covered magnetic stirrer bar in order to maintain a uniform composition of Ringer solution around the sciatic nerve. The composition of Ringer solution used was (mM): NaCl, 112.0; KCl, 2.0; CaCl2, 1.8; and NaHCO3, 2.4 (pH=7.0). Before the start of the experiment, the sciatic nerve was preincubated for at least 15 min with Ringer solution.
Recordings of CAPs from frog sciatic nerve fibres
The Lucite plate with platinum wires attached to the sciatic nerve was moved from the beaker containing Ringer solution to an empty beaker and then CAPs were recorded in air, with a preamplifier (Model LI-75A, NF Electronics Instruments, Yokohama, Japan). Two of the platinum wires were used to record CAPs, and other two were for stimulating the sciatic nerve. The stimulation was performed at a frequency of 1 Hz with a stimulator (SEN-3201; Nihon Kohden, Tokyo, Japan), where rectangular pulses having 0.1 ms duration and various strengths less than 2.3 V were used. In order not to dry out the sciatic nerve in air, this procedure was quickly (about 30 s) performed and repeated at intervals of 2 min with the plate and nerve being returned to Ringer solution between recordings. The data were monitored on a storage oscilloscope (VC-6724, Hitachi Electronics Instruments, Tokyo, Japan) while being recorded on a thermal array recorder (Omnilight 8M36, NEC san-ei Instruments, Tokyo, Japan) having a wave form storage module and stored on magnetic tape with a PCM tape recorder (RD-125T, TEAC Corporation, Tokyo, Japan) for later analyses. In several cases, the data were analysed with pCLAMP 8.0 software (Axon Instruments, Foster City, CA, USA).
Stimulating the sciatic nerve produced a CAP following a stimulus artefact, as shown in Figure 1a. The peak amplitude of the CAP, which was measured as a difference between baseline and CAP peak levels, remained constant over at least 1 h (about 30 recordings; Figure 1b); this result was confirmed in other four sciatic nerve preparations. Each of the nerve preparations was used only once to examine the effect of a drug on CAPs, unless otherwise mentioned. A conduction velocity (CV) value was determined by using the fifth electrode as an additional stimulation site and then by measuring a change in time between stimulus artefact and the peak of CAP. All experiments were carried out at room temperature (22–27°C).
Figure 1.
Recordings of CAPs from frog sciatic nerve fibres by using the air-gap method. (a) Representative recording of CAP. This illustrates how the peak amplitude and HPD of the CAP were measured. (b) Recordings of CAPs for a period of 60 min. In this and subsequent figures, dashed line in recordings denotes the peak level of CAP in the control.
Data analysis
The reduction of the peak amplitude of CAP was analysed using the following Hill equation:
where [Drug] is drug concentration, IC50 is the concentration of drug for half-maximal inhibition and nH is the Hill coefficient.
Data in the text are given as mean±s.e.m. and statistical significance of a difference between means was set at P<0.05 using a paired Student's t-test. In all cases, n refers to the number of sciatic nerves studied.
Materials
Drugs used were tramadol HCl, (±)-M1 HCl (given kindly by Grünenthal GmbH, Aachen, Germany), ropivacaine HCl (provided kindly by AstraZeneca R&D, Södertälje, Sweden), tetrodotoxin (TTX; Wako, Osaka, Japan), [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), and naloxone, lidocaine HCl, desipramine HCl and fluoxetine HCl (Sigma, St Louis, MO, USA). These drugs except for lidocaine were first dissolved in distilled water at more than 100 times the final concentrations to be used, and then diluted to the desired concentrations in Ringer solution immediately before use. Lidocaine (2 mM) was dissolved in Ringer solution. Tramadol and M1 at 5 mM, lidocaine at 2 mM and also ropivacaine at 1 mM did not affect the pH of Ringer solution.
Results
The peak amplitude of CAP depended on the strength of stimulus given to the sciatic nerve. As shown in Figure 2a, the CAP peak amplitude increased with stimulus strength and attained a maximal value at about 1 V stimulus. Although a further increase in stimulus strength resulted in slow-conducting CAPs, which had smaller values of peak amplitude and CV than those of fast-conducting CAPs elicited at the lower stimulus strengths (data not shown), only the maximal amplitude of the fast-conducting CAP was analysed in the present study. Effects of drugs on the fast CAPs were examined in a total of 121 sciatic nerves; these CAPs had CV values of 25.8±0.7 m s−1 (n=93; range: 10.7–42.5 m s−1). The fast CAP disappeared within 4 min after putting the sciatic nerve in Ringer solution containing TTX (1 μM); washing out the TTX-treated nerve in drug-free Ringer solution for 24 min resulted in a complete recovery of CAP peak amplitude (data not shown; n=4).
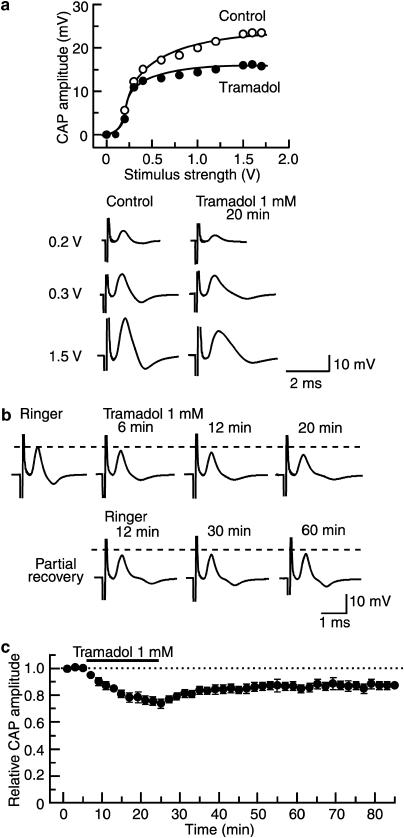
Figure 2.
Tramadol (1 mM) reduces the peak amplitude of CAP recorded from frog sciatic nerve fibres with a slow time course. (a) The peak amplitudes of CAP before (open circles) and under the action of tramadol for a period of 20 min (closed circles), which are plotted against stimulus strength used to elicit the CAP. Recordings of the CAPs elicited at 0.2, 0.3 and 1.5 V under the two conditions are shown in the lower. (b) Recordings of CAPs in the control, at 6, 12 and 20 min after exposure to tramadol and thereafter 12, 30 and 60 min in the absence of tramadol. (c) Average time course of changes in CAP peak amplitudes following exposure to tramadol for 20 min, relative to that before the soaking, obtained from four sciatic nerves. In this and subsequent figures, each point with vertical bars represents the mean and s.e.m. and dotted line denotes the control value. The s.e.m. of the values without a vertical bar was within the size of symbol. All data points after washout of tramadol differed from that before drug treatment (control).
Effect of tramadol on frog sciatic nerve CAPs
As shown in Figure 2b, exposing the sciatic nerve to Ringer solution containing tramadol (1 mM) reduced the peak amplitude of the CAP and the reduction was proportional to the time of exposure. This reduction was accompanied by an increase in the half-peak duration (HPD) of the CAP, which was measured as shown in Figure 1a. This effect of tramadol was seen for CAPs evoked at a maximal stimulus strength, while the threshold to elicit CAPs was not changed by tramadol (see Figure 2a; n=4). Figure 2c demonstrates an average time course of changes in CAP peak amplitude following exposure to tramadol, relative to control, obtained from four sciatic nerves. The tramadol-induced reduction in CAP peak amplitude attained a steady value by 20 min of exposure. At 20 min after treatment with tramadol, the peak amplitude of CAP was 74±4% of control (17.6±2.0 mV; n=4) and its HPD was 136±7% of control (0.434±0.076 ms; n=4). In nerves treated with tramadol for 20 min and then returned to drug-free Ringer solution (washout) for up to 1 h, the CAP amplitude did not recover to control levels, as shown in Figure 2b and c.
In the subsequent experiments, the sciatic nerves were treated for a fixed time of 20 min to examine further the effect of tramadol on CAPs. As seen in Figure 3a, the reduction of CAP peak amplitude produced by tramadol was concentration-dependent and these effects are summarized in Figure 3b, over a range of concentrations from 0.2 to 5 mM. The minimum (18±8%; n=4) of the relative CAP amplitude, seen at 5 mM, was not significantly different from zero. Analysis based on the Hill equation showed that the IC50 value for tramadol was 2.3 mM with an nH-value of 1.7.
Figure 3.
Tramadol reduces CAP peak amplitude in a dose-dependent manner in the frog sciatic nerve. (a) Recordings of CAPs in the control (left) and 20 min after exposure to tramadol at concentrations of 0.2, 2 and 5 mM (right); these were obtained from different sciatic nerves. (b) The peak amplitude of CAP recorded from fibres of sciatic nerves exposed to tramadol at various concentrations for 20 min, relative to that in the control, plotted against tramadol concentration. Each of the data points was obtained from four sciatic nerves. The s.e.m. of the values without a vertical bar was within the size of symbol. The dose–response curve was drawn according to the Hill equation (IC50: 2.3 mM; nH: 1.7).
As tramadol exhibits a high affinity for opioid receptors (Hennies et al., 1988), we next investigated whether the effect of tramadol was mediated by opioid receptors. For this we used the non-selective opioid receptor antagonist naloxone and the results of these experiments are shown in Figure 4a and b. Treatment with naloxone (10 μM) for 20 min did not affect the CAP amplitude and, further, did not affect the reduction in CAP induced by tramadol (1 mM). Thus, the peak amplitude of CAP in nerves exposed to tramadol together with naloxone was 62±6% of control (21.9±1.5 mV; n=4) and this value was not significantly different from that obtained from nerves exposed to tramadol only (74±4%; n=4; see above).
Figure 4.
CAP peak amplitude reduction produced by tramadol (1 mM) in the frog sciatic nerve is not due to the activation of μ-opioid receptors. (a) Recordings of CAPs in control conditions or with naloxone (10 μM) with or without tramadol. (b) Average time course of changes in CAP peak amplitudes following treatment with naloxone and with both naloxone and tramadol, relative to that before drug treatment, obtained from four sciatic nerves. The s.e.m. of the values without a vertical bar was within the size of symbol. (c) Recordings of CAPs under control conditions and 20 min after treatment with the μ-opioid receptor agonist DAMGO (1 μM).
We also investigated whether the CAPs were changed by a μ-opioid receptor agonist DAMGO at 1 μM, a concentration maximally activating μ-opioid receptors in rat SG neurones (Fujita and Kumamoto, 2006). The peak amplitude of CAP in the sciatic nerves was not affected by 20 min exposure to DAMGO (101±1% of control (30.2±1.2 mV); n=4; Figure 4c).
Tramadol is also known to inhibit noradrenaline (NA) and 5-hydroxytryptamine (5-HT) uptake at concentrations similar to those that activate μ-opioid receptors (Driessen and Reimann, 1992; Driessen et al., 1993). We therefore examined the effects of a combination of inhibitors of the uptake of NA and 5-HT (desipramine and fluoxetine, respectively; each 10 μM) on the reduction of CAP peak amplitude produced by tramadol (1 mM). Treatment of the sciatic nerve with the combination of desipramine and fluoxetine for 20 min did not affect the peak amplitude of CAP (99±6% of control (23.8±3.3 mV); n=4) nor did it affect the reduction of CAP peak amplitude induced by subsequent exposure to tramadol (1 mM) (56±7% of control, n=4). This reduction was not significantly different from that obtained from nerves treated with tramadol only (see above).
Effect of M1 on frog sciatic nerve CAPs
The effects of tramadol on CAP peak amplitude might also be shown by its major metabolite, M1, which is similar in chemical structure to tramadol (see Figure 5a) while having a higher affinity for the μ-opioid receptors than the parent compound (Gillen et al., 2000). We therefore tested M1 in our preparations and found that the CAPs were not affected by exposure of nerves to M1 at a concentration of 1 or 2 mM (Figure 5b, d and e). Thus, at 20 min after exposure to M1 (1 mM), the peak amplitude of CAP was 100±2% of control (24.1±1.6 mV; n=4) and its HPD was 97±2% of control (0.530±0.055 ms; n=4); neither of these values was significantly different from 100%. Raising the concentration of M1 to 5 mM for 20 min did reduce CAP peak amplitude slightly to 91±2% of control (29.1±1.9 mV; n=4) with a tendency for HPD to increase (121±7% of control (0.784±0.044 ms; n=4); Figure 5b and c).
Figure 5.
The tramadol metabolite, M1, reduces the peak amplitudes of CAPs recorded from frog sciatic nerve fibres less effectively than tramadol. (a) Chemical structures of tramadol and M1. (b) The peak amplitude of CAP recorded from fibres in sciatic nerves treated with M1 at various concentrations for 20 min, relative to that in the control, plotted against M1 concentration. (c) Recordings of CAPs in control conditions and 20 min after treatment with M1 (5 mM). (d) Recordings of CAPs in the control conditions, at 10 and 20 min after exposure to M1 and then at 10 and 20 min exposure to tramadol. (e) Average time course of changes in CAP peak amplitude following treatment with M1 and then tramadol, relative to that before drug treatment, obtained from four sciatic nerves. The s.e.m. of each data point was within the size of symbol.
We then looked for interactions between M1 and tramadol by treating nerves first with M1 (1 mM) for 20 min and then adding tramadol (1 mM). The results (Figure 5d and e) showed a small inhibition of the tramadol-induced reduction in CAP. Peak amplitude and HPD of CAP at 20 min after M1 and tramadol treatment were, respectively, 88±1% (n=4) and 113±1% (n=4) of control. These effects of tramadol, after M1 pretreatment, were significantly less than those obtained from sciatic nerves without M1 pretreatment, that is, which had been exposed to tramadol only (see above).
Effects of lidocaine and ropivacaine on frog sciatic nerve CAPs
In order to compare the effect of tramadol with those of other established local anaesthetics, we investigated the effects of lidocaine and ropivacaine on CAPs recorded from sciatic nerve fibres. Exposure of the sciatic nerve to lidocaine (1 mM) reduced the peak amplitude of the CAP and increased its HPD over time (Figure 6a). At 20 min after lidocaine treatment, the peak amplitude and HPD of CAP were, respectively, 18±3 and 165±19% of control values (20.3±2.0 mV and 0.470±0.054 ms; n=4). Figure 6a also shows an average time course of changes in CAP peak amplitude, relative to that before lidocaine, obtained from four sciatic nerves. This effect of lidocaine was completely reversed by returning the nerve to a lidocaine-free Ringer solution for 30 min. The effect of lidocaine was also concentration-dependent; at 2 mM, the relative CAP amplitude was not significantly different from zero (Figure 6b and c). Analysis based on the Hill equation showed that the IC50 value for lidocaine was 0.74 mM with an nH value of 1.7.
Figure 6.
Effects of lidocaine and ropivacaine on CAPs recorded from frog sciatic nerve fibres. (a, d) Average time course of a change in CAP peak amplitude following treatment with (a) lidocaine (1 mM) or (d) ropivacaine (0.2 mM), relative to that in the control, obtained from four sciatic nerves. Insets in (a) and (d) show CAPs in the control (dotted line) and 20 min under the action of lidocaine (1 mM, a) or ropivacaine (0.2 mM, d; straight line). An asterisk (*) shown below the data point indicates that there is no difference from the dotted line (value before drug treatment). (b, e) Recordings of CAPs in the control (left) and 20 min after the beginning of soaking the sciatic nerve into lidocaine (0.1, 0.5 and 2 mM; b)- or ropivacaine (0.01, 0.5 and 1 mM; e)-containing solution (right); these were obtained from different sciatic nerves. (c, f) The peak amplitude of CAP recorded from fibres in sciatic nerves treated for 20 min with lidocaine (c) or ropivacaine (f) at various concentrations, relative to that in the control, plotted against the concentration of the local anaesthetic. Each of the data points in (c) and (f) was obtained from 3–4 sciatic nerves. The dose–response curves in (c) and (f) were drawn according to the Hill equation (c: IC50=0.74 mM, nH=1.7; f: IC50=0.34 mM, nH=1.7). In (a), (c), (d) and (f), the s.e.m. of the values without a vertical bar was within the size of symbol.
Like lidocaine, ropivacaine (0.2 mM) reversibly reduced the peak amplitude of CAP and increased its HPD (see inset of Figure 6d). At 20 min after ropivacaine treatment, the peak amplitude and HPD of CAP were, respectively, 67±5 and 149±13% of control (24.2±2.6 mV and 0.570±0.055 ms; n=4). Figure 6d shows the mean time course of effect and washout of ropivacaine, obtained from four sciatic nerves. As shown in Figure 6e and f, the effect on CAP amplitude was concentration-dependent with an IC50 value of 0.34 mM (nH=1.7) and ropivacaine at 1 mM completely blocked CAPs (n=4).
Discussion and conclusions
The present study has demonstrated that tramadol reduced the peak amplitude of CAPs recorded from frog sciatic nerve fibres by the air-gap method. This reduction was accompanied by an increase in the HPD of CAP, indicating a slowing of CV of a significant proportion of fibres in the sciatic nerve. A similar reduction of CAP amplitude produced by tramadol has been obtained by applying the sucrose-gap method to frog (Mert et al., 2002) and rat sciatic nerves (Mert et al., 2003; Güven et al., 2005). The IC50 values for tramadol in reducing CAP peak amplitude, estimated by the Hill equation, in our study was 2.3 mM, about threefold lower than that (6.6 mM) obtained previously for the frog sciatic nerve (Mert et al., 2002). Although tramadol is known to inhibit the activation of various types of receptors including acetylcholine (ACh) receptors, our IC50 values for the reduction of CAP amplitudes were much higher than those (3.4 and 1.2 μM, respectively) for tramadol in reducing the amplitudes of ACh (1 μM)-induced Cl− currents in Xenopus oocytes expressing cloned M1 muscarinic ACh receptors (Shiraishi et al., 2001) and of currents produced by nicotine (10 μM) in bovine adrenal chromaffin cells (Shiraishi et al., 2002).
No study has previously reported a time course for the tramadol-induced inhibition of CAPs and we have demonstrated that the tramadol effect persisted for at least 1 h after returning the sciatic nerve to tramadol-free solution. Tramadol is known to be metabolized, in vivo, to various compounds including its major metabolite, M1. Although M1 has analgesic activity and a higher affinity for the μ-opioid receptor than tramadol, we found that M1 was less potent in inhibiting CAPs in frog sciatic nerve fibres than tramadol. The effect of tramadol on CAPs was slightly inhibited in sciatic nerves pretreated with M1 at a concentration that had no effect on CAPs by itself. Although the hyperpolarizing effects of M1 in SG neurones persisted for at least 30 min after washout of M1 (1 mM; Koga et al., 2005), suggesting a strong binding of M1 to its site of action, the reasons why sub-threshold amounts of preapplied M1 affected tramadol-induced reduction in CAP peak amplitude in our preparations remain to be established.
As it is well known that opioids such as fentanyl and sufentanil reduce the peak amplitudes of CAPs recorded from peripheral nerve fibres (Gissen et al., 1987; Jaffe and Rowe, 1996) and binding studies have demonstrated the presence of opioid receptors in peripheral nerve fibres (Fields et al., 1980), the effect of tramadol on CAP amplitude could have been mediated by opioid receptors. Jurna and Grossmann (1977) have reported that the inhibitory effect of morphine on CAPs in mammalian peripheral nerve fibres was antagonized by the non-selective opioid receptor antagonist, naloxone, indicating the involvement of opioid receptors. However, in the present study, such involvement of opioid receptors is unlikely because M1, which has a higher affinity for μ-opioid receptors than tramadol, was less effective in reducing CAP peak amplitude than tramadol. Furthermore, the effect of tramadol was not sensitive to inhibition by naloxone nor was it mimicked by the μ-opioid receptor agonist, DAMGO. Consistent with our observations, Tsai et al. (2001) have reported that a reduction in spinal somatosensory-evoked potentials following the application of tramadol to rat sciatic nerves in vivo was resistant to naloxone. The concentration of naloxone we used was based on the results of Gillen et al. (2000). Tramadol has a Ki of 2.4 μM at the μ-opioid receptor, determined by competition with [3H]naloxone in membranes obtained from CHO-K1 cells transfected with the μ-opioid receptor. Thus, naloxone should inhibit responses produced by tramadol (1 mM) at the μ-opioid receptor with an IC50 of 3.4 μM, calculated according to Cheng and Prusoff (1973). This concentration is well below the concentration of naloxone (10 μM) used in the present study.
Although tramadol is also known to inhibit NA and 5-HT uptake (Driessen and Reimann, 1992; Driessen et al., 1993), a combination of NA and 5-HT uptake blockers (desipramine and fluoxetine, respectively) did not affect the reduction of CAP amplitude produced by tramadol, indicating no involvement of inhibition of the uptake of NA and 5-HT. With respect to concentrations of the blockers used, desipramine at 10 μM was enough to completely block Na+-dependent [3H]NA uptake in cultured rat astrocytes (Inazu et al., 2003); fluoxetine at 10 μM maximally reversed 5-HT-induced currents in Xenopus oocytes expressing human 5-HT transporters (Wang et al., 2006).
The opioid-induced reduction in CAP amplitude has been generally reported to be insensitive to naloxone (Gissen et al., 1987; Jaffe and Rowe, 1996). Not only local anaesthetics but also alcohols, anticonvulsants, barbiturates and narcotics block AP conduction in peripheral nerve fibres (Staiman and Seeman, 1974). Thus, the effects of tramadol on CAPs in the present study may have been due to nonspecific interactions with membrane bilayers or with ion channels, such as voltage-gated Na+ and K+ channels (see Scholz, 2002, for a review). In support of the latter idea, Wagner et al. (1999) have reported that the opioid, meperidine, which is used for AP conduction blockade, and thus analgesia, reduced voltage-gated Na+ channel currents in a manner similar to that of lidocaine. Very recently, Tsai et al. (2006) have demonstrated that tramadol suppresses the current amplitude of delayed rectifier K+ channels (Kv3.1a types) expressed in NG 108-15 cells with an IC50 value of 25 μM (nH=1.1). This IC50 value was much lower than that we obtained (2.3 mM) for tramadol in reducing CAP amplitudes with nH-values different from each other (1.7 vs 1.1). Values of nH greater than unity, as were obtained in the present study, may indicate more than one site, including voltage-gated K+ channels, at which tramadol acts. In support of this idea, many of local anaesthetics are known to reduce both voltage-gated Na+ and K+ channel current amplitudes (Scholz, 2002). It remains to be examined how tramadol interacts with voltage-gated Na+ channels.
When compared with reductions of CAP amplitude produced by other local anaesthetics, the IC50 value (2.3 mM) for tramadol was higher by 3.1- and 6.8-fold than those (0.74 and 0.34 mM, respectively) for lidocaine and ropivacaine, and reversal by soaking the nerve preparation in drug-free solution (washout) was much slower with tramadol than with lidocaine or ropivacaine. Recovery from a comparable effect (about 30% reduction in CAP amplitude) was complete after 30 min washout of ropivacaine (0.2 mM), but was still incomplete after 60 min washout of tramadol (1 mM; see Figures 2c and 6d). Recovery from lidocaine (1 mM)-induced reduction in CAP amplitude occurred within 30 min in a lidocaine-free solution (Figure 6a). Mert et al. (2002) have reported that lidocaine reduces CAP amplitudes in the frog sciatic nerve, with an IC50 value of 6.6 mM, a value threefold higher than that of tramadol. This ratio is comparable to that found in our study, although the actual IC50 values for lidocaine were different. Although IC50 values for ropivacaine in reducing CAP amplitude are not available in other preparations, the effects of 0.2 mM ropivacaine in our preparations (about 30% reduction in CAP amplitude) were comparable to its inhibition of A fibres in the rabbit vagus nerve, at the same concentration (Bader et al., 1989).
We found the inhibitory actions of tramadol and lidocaine on CAPs to be quite distinct in terms of recovery after washout and Mert et al. (2002, 2003) have reported that the effects of tramadol and lidocaine on CAPs also differ in their sensitivity to 4-aminopyridine and the extracellular Ca2+ concentration. The differences in CAP inhibition between tramadol and lidocaine remain to be examined at single-cell levels.
Clinical significance of the effects of tramadol, lidocaine and ropivacaine on nerve CAPs
The reduction of CAP peak amplitude produced by tramadol in frog sciatic nerves may provide a mechanistic basis for the local anaesthetic effect of tramadol, following its intradermal injection in patients (Pang et al., 1998; Altunkaya et al., 2003, 2004). Consistent with our finding that the IC50 value for tramadol in reducing CAP peak amplitude was threefold higher than that of lidocaine, is that sensory block after intradermal injection of 5% tramadol was similar to that of 1% lidocaine (Pang et al., 1998). Further, in our study, the ratio of IC50 values for lidocain and ropivacaine was about 2.2 and, for equivalent levels of surgical anaesthesia, the intravenous dose ratio was 2.5 (0.2% ropivacaine and 0.5% lidocaine) (Atanassoff et al., 2001). Although sensory information is transmitted by not only fast- but also slow-conducting fibres in sciatic nerves, the present study did not examine the effects of the local anaesthetics on slow-conducting APs. In order to establish more firmly the clinical significance of CAP amplitude reduction produced by local anaesthetics, their effects on slow-conducting CAPs such as those resistant to TTX (for instance, see Kobayashi et al., 1993) should be examined. The CV values of the fast-conducting APs in the present study (25.8±0.7 m s−1; n=93) were less than those (42.5±1.8 m s−1, n=6) of TTX-sensitive fast CAPs in frog sciatic nerves (Kobayashi et al., 1993). A variation in CV values obtained in the present study and also discrepancies between our and Kobayashi et al.'s studies may be due to the fact that the distribution of nerve CVs is not uniform along an isolated nerve (Pehlivan et al., 2004) and thus CVs depend on where along the nerve they are measured.
It is of interest to note that the methyl group, present in tramadol but not M1 (see Figure 5a; tramadol has a hydrophobic group (–OCH3) in the benzene ring while its metabolite has a hydrophilic substituent (–OH)), may play an important role in causing the reduction in CAP peak amplitudes in sciatic nerve. This difference in chemical structure could prove to be important in the molecular mechanism(s) underlying the inhibition of AP conduction by tramadol.
Conflict of interest
The authors state no conflict of interest.
Acknowledgments
We thank Dr Takeru Nose and Professor Yasuyuki Shimohigashi at the Department of Chemistry, Faculty and Graduate School of Sciences, in Kyusyu University for their comments about a difference in chemical structure between tramadol and M1.
Abbreviations
- ACh
acetylcholine
- AP
action potential
- CAP
compound action potential
- CV
conduction velocity
- DAMGO
[D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin
- HPD
half-peak duration
- 5-HT
5-hydroxytryptamine
- M1
mono-O-demethyl-tramadol
- NA
noradrenaline
- SG
substantia gelatinosa
- tramadol
(1RS; 2RS)-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl)-cyclohexanol hydrochloride
- TTX
tetrodotoxin
References
- Altunkaya H, Ozer Y, Kargi E, Babuccu O. Comparison of local anaesthetic effects of tramadol with prilocaine for minor surgical procedures. Br J Anaesthet. 2003;90:320–322. doi: 10.1093/bja/aeg079. [DOI] [PubMed] [Google Scholar]
- Altunkaya H, Ozer Y, Kargi E, Ozkocak I, Hosnuter M, Demirel CB, et al. The postoperative analgesic effect of tramadol when used as subcutaneous local anesthetic. Anesth Analg. 2004;99:1461–1464. doi: 10.1213/01.ANE.0000135640.21229.A0. [DOI] [PubMed] [Google Scholar]
- Atanassoff PG, Ocampo CA, Bande MC, Hartmannsgruber MWB, Halaszynski TM. Ropivacaine 0.2% and lidocaine 0.5% for intravenous regional anesthesia in outpatient surgery. Anesthesiology. 2001;95:627–631. doi: 10.1097/00000542-200109000-00013. [DOI] [PubMed] [Google Scholar]
- Bader AM, Datta S, Flanagan H, Covino BG. Comparison of bupivacaine- and ropivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth Analg. 1989;68:724–727. [PubMed] [Google Scholar]
- Chan VWS, Weisbrod MJ, Kaszas Z, Dragomir C. Comparison of ropivacaine and lidocaine for intravenous regional anesthesia in volunteers: a preliminary study on anesthetic efficacy and blood level. Anesthesiology. 1999;90:1602–1608. doi: 10.1097/00000542-199906000-00016. [DOI] [PubMed] [Google Scholar]
- Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Driessen B, Reimann W. Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro. Br J Pharmacol. 1992;105:147–151. doi: 10.1111/j.1476-5381.1992.tb14226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen B, Reimann W, Giertz H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol. 1993;108:806–811. doi: 10.1111/j.1476-5381.1993.tb12882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RFT, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980;284:351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Fujita T, Kumamoto E. Inhibition by endomorphin-1 and endomorphine-2 of excitatory transmission in adult rat substantia gelatinosa neurons. Neuroscience. 2006;139:1095–1105. doi: 10.1016/j.neuroscience.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human μ-opioid receptor. Naunyn-Schmiedeberg's Arch Pharmacol. 2000;362:116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Gissen AJ, Gugino LD, Datta S, Miller J, Covino BG. Effects of fentanyl and sufentanil on peripheral mammalian nerves. Anesth Analg. 1987;66:1272–1276. [PubMed] [Google Scholar]
- Güven M, Mert T, Günay I. Effects of tramadol on nerve action potentials in rat: comparisons with benzocaine and lidocaine. Int J Neurosci. 2005;115:339–349. doi: 10.1080/00207450590520948. [DOI] [PubMed] [Google Scholar]
- Hennies H-H, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988;38:877–880. [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates Inc: Massachusetts; 1984. pp. 285–295. [Google Scholar]
- Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem. 2003;84:136–144. doi: 10.1046/j.1471-4159.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- Jaffe RA, Rowe MA. A comparison of the local anesthetic effects of meperidine, fentanyl, and sufentanil on dorsal root axons. Anesth Analg. 1996;83:776–781. doi: 10.1097/00000539-199610000-00021. [DOI] [PubMed] [Google Scholar]
- Jurna I, Grossmann W. The effect of morphine on mammalian nerve fibres. Eur J Pharmacol. 1977;44:339–348. doi: 10.1016/0014-2999(77)90308-9. [DOI] [PubMed] [Google Scholar]
- Klotz U. Tramadol – the impact of its pharmacokinetic and pharmacodynamic properties on the clinical management of pain. Arzneimittelforschung. 2003;53:681–687. doi: 10.1055/s-0031-1299812. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Ohta M, Terada Y. C fiber generates a slow Na+ spike in the frog sciatic nerve. Neurosci Lett. 1993;162:93–96. doi: 10.1016/0304-3940(93)90568-6. [DOI] [PubMed] [Google Scholar]
- Koga A, Fujita T, Liu T, Nakatsuka T, Kumamoto E. Effect of tramadol metabolite M1 on glutamatergic excitatory transmission in rat spinal dorsal horn neurons. Neurosci Res. 2006;55 Suppl 1:S190. [Google Scholar]
- Koga A, Fujita T, Totoki T, Kumamoto E. Tramadol produces outward currents by activating μ-opioid receptors in adult rat substantia gelatinosa neurones. Br J Pharmacol. 2005;145:602–607. doi: 10.1038/sj.bjp.0706225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux PJ, Coetzee JF. Tramadol today. Curr Opin Anaesth. 2000;13:457–461. doi: 10.1097/00001503-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Lintz W, Erlacin S, Frankus E, Uragg H. Metabolismus von Tramadol bei Mensch und Tier. Arzneimittelforschung. 1981;31:1932–1943. [PubMed] [Google Scholar]
- McClellan KJ, Faulds D. Ropivacaine: an update of its use in regional anaesthesia. Drugs. 2000;60:1065–1093. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- Mert T, Gunes Y, Guven M, Gunay I, Gocmen C. Differential effects of lidocaine and tramadol on modified nerve impulse by 4-aminopyridine in rats. Pharmacology. 2003;69:68–73. doi: 10.1159/000072358. [DOI] [PubMed] [Google Scholar]
- Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439:77–81. doi: 10.1016/s0014-2999(02)01368-7. [DOI] [PubMed] [Google Scholar]
- Pang W-W, Mok MS, Chang D-P, Huang M-H. Local anesthetic effect of tramadol, metoclopramide, and lidocaine following intradermal injection. Region Anesth Pain Med. 1998;23:580–583. doi: 10.1016/s1098-7339(98)90085-2. [DOI] [PubMed] [Google Scholar]
- Pehlivan F, Dalkilic N, Kiziltan E. Does the conduction velocity distribution change along the nerve. Med Eng Phys. 2004;26:395–401. doi: 10.1016/j.medengphy.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical' opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesthet. 2002;89:52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A. Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors. J Pharmacol Exp Ther. 2001;299:255–260. [PubMed] [Google Scholar]
- Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A, Shibuya I. Inhibitory effects of tramadol on nicotinic acetylcholine receptors in adrenal chromaffin cells and in Xenopus oocytes expressing α7 receptors. Br J Pharmacol. 2002;136:207–216. doi: 10.1038/sj.bjp.0704703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiman A, Seeman P. The impulse-blocking concentrations of anesthetics, alcohols, anticonvulsants, barbiturates, and narcotics on phrenic and sciatic nerves. Can J Physiol Pharmacol. 1974;52:535–550. doi: 10.1139/y74-071. [DOI] [PubMed] [Google Scholar]
- Tsai T-Y, Tsai Y-C, Wu S-N, Liu Y-C.Tramadol-induced blockade of delayed rectifier potassium current in NG108-15 neuronal cells Eur J Pain 2006(in press) [DOI] [PubMed]
- Tsai Y-C, Chang P-J, Jou I-M. Direct tramadol application on sciatic nerve inhibits spinal somatosensory evoked potentials in rats. Anesth Analg. 2001;92:1547–1551. doi: 10.1097/00000539-200106000-00040. [DOI] [PubMed] [Google Scholar]
- Wagner LE, II, Eaton M, Sabnis SS, Gingrich KJ. Meperidine and lidocaine block of recombinant voltage-dependent Na+ channels: evidence that meperidine is a local anesthetic. Anesthesiology. 1999;91:1481–1490. doi: 10.1097/00000542-199911000-00042. [DOI] [PubMed] [Google Scholar]
- Wang H-W, Li C-Z, Yang Z-F, Zheng Y-Q, Zhang Y, Liu Y-M. Electrophysiological effect of fluoxetine on Xenopus oocytes heterologously expressing human serotonin transporter. Acta Pharmacol Sin. 2006;27:289–293. doi: 10.1111/j.1745-7254.2006.00274.x. [DOI] [PubMed] [Google Scholar]