Abstract
Background and purpose:
The thiazolidine carboxylic acid, BML-241, has been proposed as a lead compound in development of selective antagonists at the sphingosine-1-phosphate receptor (S1P3), based on its inhibition of the rise in intracellular calcium concentrations ([Ca2+]i) in HeLa cells overexpressing S1P receptors. We have studied the antagonistic properties of BML-241 for the S1P3 receptor in more detail.
Experimental approach:
Chinese hamster ovary (CHO) cells stably transfected with the S1P3, S1P2 or α1A-adrenoceptors were used to investigate the effect of BML-241 on increases in [Ca2+]i mediated via different receptors. CHO-K1 cells were used to study ATP-induced [Ca2+]i elevations. Effects on S1P3-mediated inhibition of forskolin-induced cAMP accumulation and on binding to α1A-adrenoceptors were also investigated. In addition, the effect of BML-241 on contractions of rat mesenteric artery induced by phenylephrine was studied in an organ bath.
Key results:
High concentrations of BML-241 (10 μM) inhibited the rise in [Ca2+]i induced by S1P3 and S1P2 receptor stimulation; lower concentrations were ineffective. This high concentration of BML-241 also inhibited [Ca2+]i increases via P2 (nucleotide) receptor or α1A-adrenoceptor stimulation. Moreover, BML-241 (10 μM) inhibited α1-adrenoceptor-mediated contraction of rat mesenteric artery but did not displace [3H]-prazosin from α1A-adrenoceptors in concentrations up to 100 μM. BML-241 (10 μM) did not affect the S1P3-mediated decrease of forskolin-induced cAMP accumulation.
Conclusions and Implications:
We conclude that BML-241 is a low potency, non-selective inhibitor of increases in [Ca2+]i, rather than a specific antagonist at the S1P3 receptor.
Keywords: α1-adrenoceptor, BML-241, sphingosine-1-phosphate, S1P receptor, S1P3 antagonist
Introduction
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid found in high concentrations in blood (Yatomi et al., 1997) and it is produced and stored in platelets, from which it is released upon stimulation. S1P can also be produced by many other cell types from membrane phospholipids to act in an auto- and/or paracrine manner. S1P regulates many different biological functions like cell growth, proliferation, apoptosis, lymphocyte recirculation and angiogenesis via specific receptors. The first S1P receptor was discovered as an abundantly expressed gene in differentiating endothelial cells (Hla and Maciag, 1990). Originally described as an orphan receptor, the product of the endothelial differentiation gene 1 turned out to have high affinity for S1P and was, therefore, renamed S1P1 (Lee et al., 1998). The family of S1P receptors currently consists of five members with close homology. The S1P1, S1P2 and S1P3 receptors are ubiquitously expressed, whereas the S1P4 and S1P5 receptors show a more restricted expression pattern (Alewijnse et al., 2004). The S1P1 receptor predominantly activates Gi, the S1P2 and S1P3 receptor activate Gi, Gq/11 and G12/13 proteins and the S1P4 and S1P5 receptors activate Gi and G12/13 proteins (Spiegel and Milstien, 2003).
The specific role of S1P receptor subtypes has mostly been studied using genetic approaches because of the limited availability of selective agonists and antagonists. Although research with knockout mice has shown a crucial role for, for example, the S1P1 receptor in the formation of blood vessels (Liu et al., 2000), there is still a clear need for pharmacological tools in this field.
In 2002, a new compound, BML-241 (2(R,S)-undecylthiazolidine-4(R)-carboxylic acid), was identified in a search for inhibitors of S1P-induced rises in intracellular calcium concentrations ([Ca2+]i) in HeLa cells overexpressing S1P receptors. This compound was proposed as a lead compound in developing selective antagonists of the S1P3 receptor (Koide et al., 2002). However, those conclusions were based on the measurements with one BML-241 concentration and on the comparison of only the S1P1 and S1P3 receptor in one assay. Therefore, we have investigated the proposed selectivity of BML-241 as an antagonist at the S1P3 receptor, in greater detail.
Materials and methods
Cells and cell culture
Chinese hamster ovary (CHO)-K1 cells were purchased from ECACC (Zwijndrecht, The Netherlands). CHO cells stably expressing the human α1A-adrenoceptor at a density of approximately 2 pmol mg protein−1 were as described previously (Keffel et al., 2000). Flp-In-CHO cells for transfection (see below) were obtained from Invitrogen (Breda, The Netherlands).
CHO-K1 cells were passaged 1:10 every 2 or 3 days in F-12 Nutrient Mixture (Ham) with L-glutamine, supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 10% foetal calf serum (FCS). CHO-K1 cells stably expressing α1A-adrenoceptor were cultured as described previously (Keffel et al., 2000). Flp-In-CHO cells stably expressing the S1P2 or S1P3 receptor (see below) were passaged 1:5 every 2 or 3 days in F-12 Nutrient Mixture (Ham) with L-glutamine, supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 313 μg ml−1 hygromycin B and 10% charcoal-stripped FCS. All cell lines were cultured at 37°C in humidified air containing 5% CO2.
Molecular cloning and transfection
An N-terminal HisG-tag was added to the S1P2 or S1P3 receptor via cloning into pcDNA3.1/HisC or pcDNA3.1/HisA, using BamHI and ApaI or BamHI and XhoI digestion, respectively. A second cloning step was performed using HindIII and ApaI or HindIII and XhoI to clone the HisG-tagged S1P2 or S1P3 receptor into the expression vector pcDNA5/FRT/TO, using standard cloning techniques.
Transfection of plasmids into Flp-In-CHO cells was performed according to the manufacturer's protocol with Lipofectamine 2000. Hygromycin B (600 μg ml−1) was used to select for positive clones, which were subsequently separately grown to confluency. Expression of the S1P2 or S1P3 receptor was confirmed by immunofluorescence against the His-tag.
Measurement of intracellular calcium
Intracellular calcium measurements were performed according to Bakker et al. (2004) with minor changes. CHO cells were plated in a black, clear bottom 96-well plate at 40 000 cells well−1. After growth overnight, cells were loaded for 1 h with 4 μM Fluo-4 AM ester in Hank's balanced salt solution (HBSS) containing 20 mM N-2-hydroxyl piperazine-N′-2-ehane sulphonic acid (HEPES), 0.42% v v−1 pluronic acid and 2.5 mM probenecid and incubated at 37°C. After loading, cells were washed twice with HBSS containing 20 mM HEPES and 2.5 mM probenecid and incubated at 37°C with HBSS containing 20 mM HEPES and 2.5 mM probenecid in the presence or absence of BML-241 for 1 h. The increase in [Ca2+]i upon ligand stimulation was calculated as the difference between the [Ca2+]i for the basal level and after adding a ligand.
Cyclic adenosine monophosphate assay
The LANCE cAMP 384 kit was used to determine cyclic adenosine monophosphate (cAMP) concentrations according to the manufacturer's protocol. Flp-In-CHO cells stably expressing the S1P3 receptor were incubated for 1 h with or without 10 μM BML-241 in F-12 Nutrient Mixture (Ham) with L-glutamine and detached from the surface using enzyme-free cell dissociation buffer. Cells were washed once with HBSS and subsequently resuspended in stimulation buffer, containing HBSS with 0.5 mM 3-isobutyl-1-methylxanthine, 0.05% bovine serum albumin (BSA) and 5 mM HEPES.
Stimulation mixtures consisted of stimulation buffer with 3 μM forskolin, 10 μM BML-241 and the concentration–response curve of S1P ranging from 10 pM to 1 μM. Cells were added to the stimulation mixtures 1:1 in a 384-well optiplate at 2500 cells well−1 and stimulated for 60 min at room temperature. Detection of the cAMP formed during stimulation was performed according to the manufacturer's protocol. Measurements were carried out on the Victor (Wallac, Perkin-Elmer, Zaventem, Belgium) 3 h after adding detection buffer and antibody mixture.
Radioligand binding studies
Competition binding studies with α1A-adrenoceptors were performed as described previously (Keffel et al., 2000).
Vascular contraction studies
Rat mesenteric artery was mounted into the organ baths of a 4-channel wire myograph according to Koop et al. (2005). Additionally, 10 μM BML-241 was added to the bathing solution 30 min before a cumulative concentration–response curve (30 nM–30 μM) for phenylephrine. Isometric force of contraction was measured continuously and all data presented are normalized to the contractile response obtained by the third 100 mM KCl-induced contraction.
Data analysis
Concentration–response curves were analysed by fitting sigmoidal functions to the experimental data using Prism 4 (Graphpad Software Inc., San Diego, CA, USA). Data are expressed as means±s.e.m. Differences between two groups were compared using a paired Student's t-test. One-way analysis of variance with a Dunnett's correction was used where appropriate. Values of P<0.05 were considered to show significant differences between means.
Materials
pcDNA3.1 containing the entire coding region of the human S1P2 or S1P3 receptor was purchased from UMR cDNA Resource Center (Rolla, MO, USA). Cell culture media, hygromycin B, Lipofectamine 2000, Optimem, HBSS, pOG44, pcDNA3.1/HisA/C and pcDNA5/FRT/TO were obtained from Invitrogen (Breda, The Netherlands). Pluronic acid and Fluo-4 AM were obtained from Molecular Probes (via Invitrogen). FCS, enzyme-free cell dissociation buffer and penicillin/streptomycin were obtained from Gibco (via Invitrogen). LANCE cAMP 384 kit, white 384-well optiplates and Microscint-O were obtained from Perkin-Elmer (Zaventem, Belgium). Restriction enzymes (BamHI, ApaI, XhoI and HindIII) were obtained from Fermentas Life Sciences (St Leon-Rot, Germany). S1P was obtained from Avanti-Polar Lipids Inc. (Delfzijl, The Netherlands). Probenecid, HEPES, ethyleneglycoltetraacetate (EGTA), Triton X-100, 3-isobutyl-1-methylxanthine, BSA (fatty acid free) and activated charcoal were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands). BML-241 was obtained from Biomol (via Tebu-Bio, Heerhugowaard, The Netherlands). [3H]-prazosin (specific activity 2.96TBq mmol−1) was obtained from Amersham (Diegem, Belgium). Clear bottom black 96-well plates were obtained from Greiner Bio One (Alphen aan den Rijn, The Netherlands).
Results
Effect of BML-241 on S1P-induced elevation of [Ca2+]i in CHO cells stably expressing the S1P3 receptor
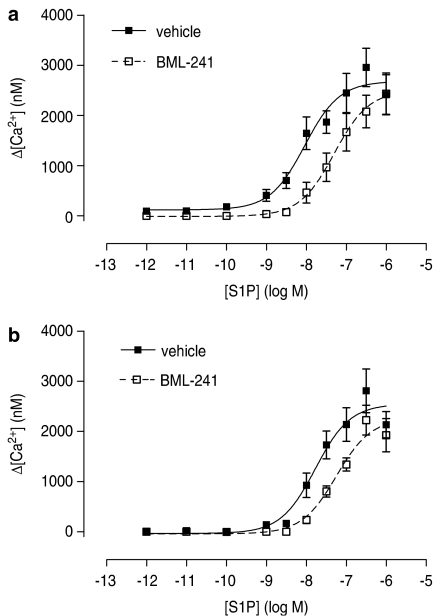
Exogenous S1P concentration-dependently increased the [Ca2+]i in Flp-In-CHO cells stably expressing the S1P3 receptor. The experimental record (Figure 1a) shows a rapid ligand-induced increase followed by a decrease. The rapid increase was used to characterize the response over a range of S1P concentrations and these results are summarized in Figure 2a, with pEC50 and Emax values in Table 1. A 60 min preincubation with 10 μM BML-241 caused a rightward shift of the concentration–response curve without affecting the maximum responses (Figure 2a; Table 1). Lower concentrations of BML-241 (1 or 3 μM) did not significantly influence the [Ca2+]i response (data not shown).
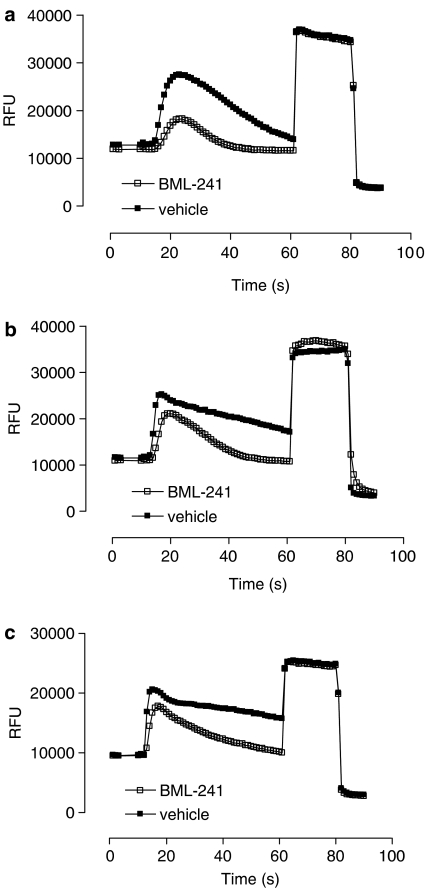
Figure 1.
Kinetics of increases of [Ca2+]i induced by S1P (10 nM) (a), ATP (100 nM) (b) or phenylephrine (10 nM) (c) in the presence (open squares) or absence (closed squares) of BML-241 (10 μM) in CHO cells. Ten per cent v v−1 Triton was added after 60 s, 0.1 M EGTA after 80 s. Graphs are representative for one ligand concentration. Data are shown as relative fluorescent units (r.f.u.).
Figure 2.
Concentration–response curve of S1P-induced increase of [Ca2+]i in CHO-Flp-In cells stably expressing the S1P3 receptor (n=9) (a) or the S1P2 receptor (b) in the presence (open squares) or absence (closed squares) of BML-241 (10 μM) (n=6–7).
Table 1.
Pharmacodynamic parameters for the effects of BML-241 (10 μM) on ligand-induced increases in [Ca2+]i in CHO cells
| (A) | ||||||
|---|---|---|---|---|---|---|
| Receptor | Ligand | pEC50 | Emax | n | ||
| S1P3 | ||||||
| S1P | 8.1±0.2 | 2837±419 | 9 | |||
| S1P+BML-241 | 7.2±0.2* | 2697±403 | 8 | |||
| S1P2 | ||||||
| S1P | 7.7±0.1 | 2698±257 | 6 | |||
| |
S1P+BML-241 |
7.3±0.1* |
|
2309±359 |
|
7 |
| (B) | ||||||
| Receptor | Ligand |
pEC50 |
Emax |
n | ||
| |
|
Early |
Late |
Early |
Late |
|
| P2 | ||||||
| ATP | 6.9±0.1 | 6.3±0.1 | 1463±72 | 972±43 | 5 | |
| ATP+BML-241 | 6.4±0.1* | 6.1±0.1 | 1536±152 | 697±62* | 5 | |
| α1A-Adrenoceptor | ||||||
| PE | 8.4±0.1 | 7.9±0.1 | 1719±98 | 1241±58 | 5 | |
| PE+BML-241 | 8.1±0.1* | 7.7±0.1* | 1693±148 | 889±43* | 5 | |
Abbreviations: ATP, adenosine triphosphate; BML-241, 2(R,S)-undecylthiazolidine-4(R)-carboxylic acid; CHO, Chinese hamster ovary; S1P, sphingosine-1-phosphate; PE, phenylephrine.
The data in (A) are derived from CHO cells selectively transfected with the S1P receptors shown in the table and stimulated with a range of concentrations of sphingosine-1-phosphate (S1P), with or without BML-241 (10 μM).
In (B), data are from cells expressing P2 receptors or α1A-adrenoceptors (as shown) and stimulated with ATP or phenylephrine (PE), which show a two-phase response in [Ca2+]i (see Figure 1b and c).
Significantly different from corresponding value without BML-241; n=as shown in the Table; P<0.05; Student's t-test.
Effect of BML-241 on S1P-induced elevation of [Ca2+]i in CHO cells stably expressing the S1P2 receptor
S1P also concentration-dependently increased the [Ca2+]i in Flp-In-CHO cells stably expressing the S1P2 receptor. The concentration–response curve was significantly shifted to the right after a preincubation with 10 μM BML-241, whereas the maximal response was not affected (Figure 2b; Table 1). However, lower concentrations of BML-241 (1 or 3 μM) did not show such inhibition (data not shown).
Effect of BML-241 on elevation of [Ca2+]i in CHO cells via P2 receptors and α1A-adrenoceptors
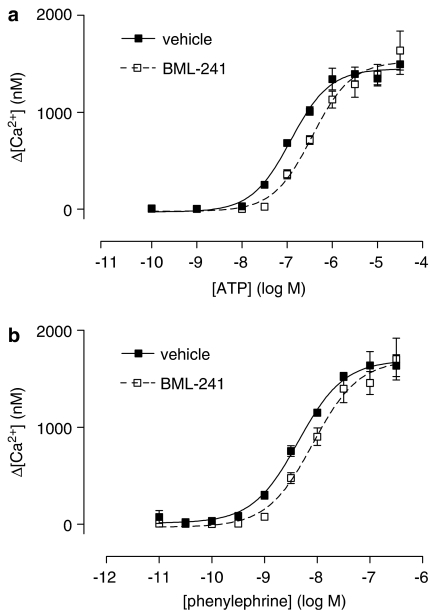
Ligand-stimulated increases in [Ca2+]i were also obtained with adenosine triphosphate (ATP) via the native P2 receptors in CHO-K1 cells or with phenylephrine in CHO cells, expressing α1A-adrenoceptors (Keffel et al., 2000). However, with either of these ligands, the fluorescent signal remained elevated longer (Figure 1b and c) compared to that after stimulation with S1P (Figure 1a). Therefore, we measured the effect of BML-241 on peak responses (‘early') as well as after 60 s (‘late'). Preincubation with BML-241 (10 μM) caused a significant rightward shift of the ATP-induced [Ca2+]i elevation in the early phase (Figure 3a; Table 1), but had no significant effect on the late response (Table 1). On the other hand, this concentration of BML-241 did not significantly alter the Emax of the early response (Figure 3a), but significantly decreased that of the late response (Table 1). Similarly for phenylephrine, BML-241 (10 μM) significantly shifted the induced [Ca2+]i elevation for the early phase (Figure 3b), as well as for the late phase (Table 1). BML-241 (10 μM) did not alter the Emax of the early response (Figure 3b) but significantly decreased the late response (Table 1).
Figure 3.
Concentration–response curve of the early phase of ATP-induced (a) and phenylephrine-induced (b) increase of [Ca2+]i in CHO-K1 cells in the presence (open squares) or absence (closed squares) of BML-241 (10 μM) (n=5).
Effect of BML-241 on phenylephrine-induced contraction of the mesenteric artery
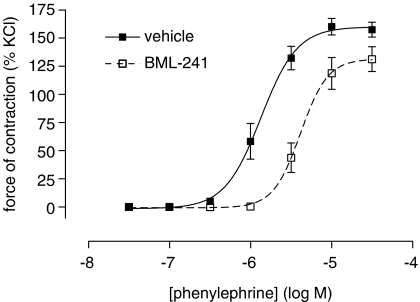
To investigate non-selective effects of BML-241 on a tissue level, an organ bath set-up was used. In rat mesenteric artery preparations, phenylephrine causes contraction via the α1A-adrenoceptor. Addition of BML-241 (10 μM) caused a significant rightward shift of the concentration–response curve for phenylephrine (Figure 4) and significantly decreased the Emax. BML-241 had no significant influence on KCl-induced constriction (data not shown).
Figure 4.
Phenylephrine-induced contraction of rat mesenteric artery in the presence (open squares) or absence (closed squares) of BML-241 (10 μM). Data are expressed as percentage of the third KCl (120 mM)-induced contraction. BML-241 shifted the pEC50 values (control pEC50=5.9±0.1; after BML-241, 5.4±0.1) and decreased Emax (control Emax=160±6%; after BML-241, 132±9%, n=6–7 for both parameters).
Effect of BML-241 on binding of [3H]-prazosin to the α1A-adrenoceptor
In competition binding experiments, BML-241 in concentrations up to 100 μM did not cause detectable inhibition of radioligand binding to α1A-adrenoceptors, whereas phenylephrine as the positive control exhibited a concentration-dependent displacement of the radioligand (data not shown).
Effect of BML-241 on S1P3-induced inhibition of cAMP accumulation
In CHO cells stably expressing the S1P3 receptor, S1P inhibited forskolin-induced cAMP accumulation in a concentration-dependent manner (pEC50=7.8±0.4, n=4). Preincubation with BML-241 (10 μM) did not have a significant influence on the S1P-induced inhibition of cAMP accumulation (pEC50=8.1±0.5, n=4). In mock-tranfected CHO cells, S1P did not inhibit forskolin-induced cAMP accumulation (data not shown).
Discussion
Research on S1P receptors has been hampered by a lack of specific ligands, particularly reagents that allow investigation of the role of endogenous S1P. The S1P3 receptor can stimulate phospholipase C, activate Rho and regulate extracellular signal-regulated kinase 1/2 activation (Watterson et al., 2005). Specific and particularly subtype-selective antagonists could help considerably to understand better the physiology of S1P responses as well as the mechanisms of drugs targeted at this system; moreover, such antagonists have the potential to become useful drugs in themselves.
In 2002, it was reported from a screening of HeLa cells that a single high concentration of BML-241 (10 μM) inhibited the S1P3-induced [Ca2+]i response by 37%, whereas S1P1-induced responses were not affected (Koide et al., 2002). Based upon these data, BML-241 was proposed as a lead compound to develop a selective antagonist of the S1P3 receptor. Our study confirms that a high concentration of BML-241 indeed did inhibit the S1P-induced increase of [Ca2+]i in cells overexpressing the S1P3 receptor. However, this high concentration (10 μM) was not selective for the S1P3 receptor but also affected the S1P2-mediated rise in [Ca2+]i. To determine the specificity of BML-241 for S1P receptors, effects on P2 receptors and α1-adrenoceptors were studied using ATP and phenylephrine, respectively. Interestingly, a difference in kinetics of the [Ca2+]i signal was observed with these two ligands, compared to S1P-stimulated cells stably expressing the S1P2 or S1P3 receptor. The P2 receptor agonist ATP and the α1-adrenoceptor agonist phenylephrine also rapidly increased [Ca2+]i, but the fluorescent signal remained elevated longer than after S1P receptor stimulation. Therefore, the effect of BML-241 was investigated in the ‘early' and ‘late' phases separately. BML-241 (10 μM) caused a significant rightward shift of the EC50 in the early phase, but not in the late phase for the ATP-induced [Ca2+]i response. However, for the phenylephrine-induced [Ca2+]i response, BML-241 caused a significant rightward shift of the EC50 of the early and the late phase. The maximal response for both the ATP-induced and the phenylephrine-induced rise in [Ca2+]i was significantly decreased by BML-241 (10 μM) in the late phase, but not in the early phase. The relevance of the lack of selectivity for S1P receptors was confirmed at the tissue level by the inhibitory effect of BML-241 on the phenylephrine-induced contraction in preparations of rat mesenteric artery. This combination of low potency and non-selectivity exhibited by the antagonism of [Ca2+]i responses could be due to BML-241 binding with low affinity to multiple receptors. However, BML-241 did not displace [3H]-prazosin from the α1A-adrenoceptor, even at very high concentrations (100 μM), indicating that BML-241 did not act at the receptor level. Based upon these results, we conclude that BML-241 is a low-potency, functional antagonist of increases in [Ca2+]i rather than a specific S1P3 receptor antagonist. In line with this conclusion, BML-241 showed a lack of antagonism towards the Gi-mediated inhibitory effect of the S1P3 receptor on forskolin-induced cAMP production. Recently, expression of S1P1,2,4 receptor mRNA was shown in CHO-K1 cells (Holdsworth et al., 2005). Hence, the S1P-induced inhibition of forskolin-induced cAMP accumulation could be caused via these endogenous receptors instead of the stably transfected S1P3 receptor. However, in our mock-transfected CHO-Flp-In cells, there was no effect of S1P on the forskolin-induced cAMP accumulation. Therefore, the effect of S1P could only be caused via the stably transfected S1P3 receptor and not any endogenous S1P receptors. Owing to the complexity of calcium signalling, identification of the low-affinity molecular target of BML-241 remains unclear and is beyond the scope of this paper. However, these results show that possible new S1P receptor antagonists, like BML-241, but also the new compound VPC23019 (Davis et al., 2005), a S1P1/S1P3 antagonist should be investigated thoroughly.
Acknowledgments
We thank Marie-Jeanne Mathy and Martina B. Michel-Reher for technical assistance.
Abbreviations
- ATP
adenosine triphosphate
- BML-241
2(R,S)-undecylthiazolidine-4(R)-carboxylic acid
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- CHO
Chinese hamster ovary
- EGTA
ethyleneglycoltetraacetate
- FCS
foetal calf serum
- HBSS
Hank's balanced salt solution
- HEPES
N-2-hydroxyl piperazine-N′-2-ehane sulphonic acid
- S1P
sphingosine-1-phosphate
Conflict of interest
The authors state no conflict of interest.
References
- Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RA, Weiner DM, ter Laak T, Beuming T, Zuiderveld OP, Edelbroek M, et al. 8R-lisuride is a potent stereospecific histamine H1-receptor partial agonist. Mol Pharmacol. 2004;65:538–549. doi: 10.1124/mol.65.3.538. [DOI] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Holdsworth G, Slocombe P, Hutchinson G, Milligan G. Analysis of endogenous S1P and LPA receptor expression in CHO-K1 cells. Gene. 2005;350:59–63. doi: 10.1016/j.gene.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Keffel S, Alexandrov A, Goepel M, Michel MC. α1-adrenoceptor subtypes differentially couple to growth promotion and inhibition in Chinese hamster ovary cells. Biochem Biophys Res Commun. 2000;272:906–911. doi: 10.1006/bbrc.2000.2850. [DOI] [PubMed] [Google Scholar]
- Koide Y, Hasegawa T, Takahashi A, Ende A, Mochizuki N, Nakagawa M, et al. Development of novel EDG3 antagonists using a 3D database search and their structure–activity relationships. J Med Chem. 2002;45:4629–4638. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- Koop EA, Gebbink MF, Sweeney TE, Mathy MJ, Heijnen HF, Spaan JA, et al. Impaired flow-induced dilation in mesenteric resistance arteries from receptor protein tyrosine phosphatase-mu-deficient mice. Am J Physio Heart Circ Physiol. 2005;288:H1218–H1223. doi: 10.1152/ajpheart.00512.2004. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem Tokyo. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]