Abstract
Background and purpose:
Red wine polyphenols (RWPs) inhibit the expression of vascular endothelial growth factor (VEGF), a major pro-angiogenic and pro-atherosclerotic factor, in vascular smooth muscle cells (VSMCs). The aim of this study was to identify which red wine polyphenols were inhibitory and to determine the mechanism underlying the inhibitory effects.
Experimental approach:
Release of VEGF stimulated by platelet derived growth factorAB (PDGFAB), from human aortic VSMCs was measured by immunoassay and phosphorylation of kinases by Western blot analysis. The direct antioxidant properties of polyphenols were determined by electron paramagnetic resonance and the cellular formation of reactive oxygen species (ROS) by dichlorofluorescein.
Key results:
The inhibitory effect of RWPs on PDGFAB-induced release of VEGF was mimicked by delphinidin but not by quercetin, catechins, resveratrol, gallic acid or caffeic acid. In the anthocyanin class, not only delphinidin but also cyanidin prevented VEGF release whereas malvidin and peonidin were without effect. RWPs, delphinidin and cyanidin directly scavenged ROS and prevented the PDGFAB-induced formation of ROS in VSMCs. Malvidin and peonidin did not scavenge ROS but prevented the cellular formation of ROS. Although the p38 MAPK, ERK1/2 and JNK pathways have been involved in the PDGFAB-induced expression of VEGF, in our experiments, only phosphorylation of p38 MAPK and JNK was inhibited by RWPs, delphinidin and cyanidin.
Conclusions and implications:
Anthocyanins presenting a hydroxyl residue at position 3′ are able to inhibit PDGFAB-induced VEGF expression by preventing activation of p38 MAPK and JNK in VSMCs.
Keywords: Red wine polyphenols, vascular endothelial growth factor, vascular smooth muscle cells, reactive oxygen species, mitogen-activated protein kinases, atherosclerosis
Introduction
Epidemiological studies have suggested an inverse relation between moderate wine consumption, particularly red wine, and the risk of coronary heart disease (Renaud et al., 1998; Gronbaek et al., 2000). The beneficial effect of red wine on coronary disease might be attributable, in part, to its ability to retard the progression of early atherosclerotic lesions, as observed in human coronary arteries at childhood, to advanced plaques, which are prone to rupture with superimposed thrombosis as suggested by studies with experimental models of atherosclerosis (Hayek et al., 1997; da Luz et al., 1999; Vinson et al., 2001). As non-alcoholic wine products and the red wine polyphenols (RWPs), quercetin and catechin, were also able to prevent the progression of atherosclerotic lesions, polyphenols present in red wine account, at least in part, for the protective effect of moderate wine consumption (Hayek et al., 1997; da Luz et al., 1999; Vinson et al., 2001). However, the mechanism underlying the beneficial effect of RWPs on coronary diseases remains unclear.
Experimental evidence has suggested that the protective effect might be attributable to the ability of RWPs to prevent oxidation of low-density lipoprotein (Frankel et al., 1993), activation of platelets and matrix metalloproteinase (Wang et al., 2002; Oak et al., 2004), and expression of pro-thrombotic and pro-atherosclerotic molecules such as monocyte chemoattractant protein-1 and tissue factor (Feng et al., 1999; Pendurthi et al., 1999). Our recent findings suggest that it may also be owing to the ability of RWPs to prevent the expression of vascular endothelial growth factor (VEGF), a major angiogenic factor, in vascular smooth muscle cells (VSMCs) (Oak et al., 2003). Indeed, VEGF is strongly expressed in human atherosclerotic plaques (Couffinhal et al., 1997; Chen et al., 1999), and the number of VEGF-positive cells increases gradually with the progression of lesions (Chen et al., 1999). The cellular sources of VEGF in human atherosclerotic plaques are predominantly VSMCs and to some extent foamy macrophages (Couffinhal et al., 1997; Chen et al., 1999). VEGF has been suggested to contribute to the growth of atherosclerotic lesions by stimulating the formation of neointimal blood vessels and also by inducing the expression of pro-coagulant and pro-atherosclerotic factors (Clauss et al., 1990; Melder et al., 1996; Marumo et al., 1999). Although RWPs are potent inhibitors of VEGF expression, the active compounds still remain to be determined. Analysis of red wine has indicated the presence of more than 500 polyphenolic compounds, which belong either to the flavonoid group or the non-flavonoid group (Soleas et al., 1997). Therefore, the aim of the present study was to determine the ability of major RWPs to inhibit VEGF expression and, if so, to elucidate the underlying mechanism in VSMCs. The findings indicate that among nine major RWPs, only the anthocyanins of the flavonoid group, delphinidin and cyanidin, are able to inhibit the platelet-derived growth factorAB (PDGFAB)-induced expression of VEGF by preventing activation of the redox-sensitive p38 mitogen-activated protein kinase (p38 MAPK) and c-Jun N-terminal kinase (JNK) pathways.
Methods
Preparation of RWPs
RWPs dry powder from red French wine (Corbières AOC) was provided by Dr M Moutounet (Institut National de la Recherche Agronomique, Montpellier, Montpellier, France) and analyzed by Dr P-L Teissedre (Département d'œnologie, Université de Montpellier, France). The procedures used to prepare and analyze RWPs have been described previously (Oak et al., 2003). One liter of red wine produced 2.7 g of RWPs, which contained 471 mg g−1 of total phenolic compounds expressed as gallic acid equivalents.
Cell culture
Human aortic VSMCs were obtained from Clonetics (Tebu, Le Perray, France) and cultured as recommended. All experiments were performed with VSMCs from passages 5 to 15, which were serum-deprived for 24 h. Cell viability was assayed with the Cell Titer MDSU kit.
Determination of VEGF
A commercially available human VEGF immunoassay was used for the determination of VEGF content in human VSMCs-conditioned medium (24 h).
Electron paramagnetic resonance measurements
The direct reactive oxygen species (ROS)-scavenging property of polyphenols was assessed by the electron paramagnetic resonance (EPR)-spin trapping method. RWPs were introduced into the reaction mixture containing 1 mM 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine (TEMPONE-H), and 0.1 M diethyltetraaminopentaacetic acid in aqueous buffered solution (0.1 M phosphate, pH=7.4). The reaction was started by adding 0.8 μM potassium superoxide, a source of superoxide ions. After 5 min, the level of TEMPONE was detected by EPR in glass capillary. EPR spectra were recorded on a MS100 spectrometer (Magnettech, Berlin) under the following conditions: room temperature, microwave frequency 9.34 GHz, microwave power 20 mW, modulation frequency 100 kHz, modulation amplitude 0.1 mT and time constant 100 ms.
Determination of ROS formation in VSMCs
The medium of treated and untreated VSMCs grown in 96-well plates was replaced by Hank's balanced salt solution (HBSS), and the cells were loaded with dichlorofluorescein (DCF) diacetate (10 μM) dissolved in HBSS for 30 min at 37°C. The extracellular dye was removed, and HBSS containing PDGFAB (30 ng ml−1) or solvent was added. DCF fluorescence was measured in a Wallac Victor 1420 fluorescence plate reader (EG&G Wallac, PerkinElmer, Courtaboeuf, France) at 37°C at an excitation wavelength of 488 nm and an emission wavelength of 535 nm.
Western blot analysis
Total proteins (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide electrophoresis (12%) and blotted on polyvinylidine difluoride membrane. Immunodetection was carried out using antibodies directed against phosphorylated p38 MAPK, extracellular signal-regulated kinase (ERK)1/2 and JNK, and the respective non-phosphorylated proteins. The immunoreactive band was detected by enhanced chemiluminescence.
Statistical analysis
Results are shown as mean±s.e.m. Statistical analyses were performed using ANOVA followed by Fisher's protected least significant difference test to compare two treatments. P<0.05 was considered statistically significant.
Materials
Authentic polyphenols were purchased from Extrasynthese (Lyon, France). The purity of resveratrol was >90% and that of all other polyphenols was >98%. The human VEGF immunoassay kit (VEGFs 165 and 120) was supplied by R&D Systems (Lille, France), TEMPONE-H by Alexis Biochemicals (Lausen, Switzerland) and DCF diacetate by Invitrogen (Cergy Pontoise, France). The antibodies against phosphorylated p38 MAPK, ERK1/2 and JNK, and the respective non-phosphorylated proteins were from Cell Signalling (Danvers, MS, USA) and the enhanced chemiluminescence reagent was from Amersham (Orsay, Paris). The cell viability kit, CellTiter 96 MDSU aqueous one solution cell proliferation assay, was purchased from Promega (Charbonnières, France).
Results
The anthocyanins delphinidin and cyanidin inhibit VEGF expression
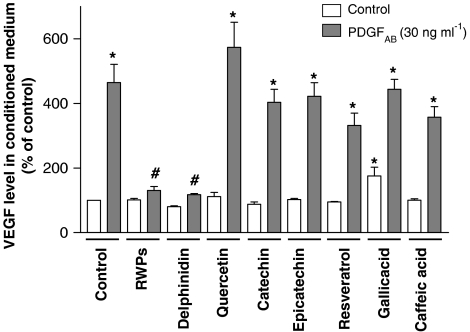
Exposure of VSMCs to PDGFAB for 24 h markedly increased the modest basal release of VEGF (Figure 1). The stimulatory effect of PDGFAB was abolished by pretreatment of VSMCs with RWPs for 30 min before the addition of the growth factor (Figure 1). Among polyphenolic compounds belonging to the flavonoid group, delphinidin (a member of the anthocyanin class) mimicked the inhibitory effect, whereas catechin and epicatechin (members of the flavanol class) and quercetin (a member of the flavonol class) did not have such an effect (Figure 1). The stimulatory effect of PDGFAB was also not affected by three members of the non-flavonoid group: gallic acid (a member of the benzoic acid class), caffeic acid (a member of the cinnamic acid class) and resveratrol (a member of the distilbene class; Figure 1). Exposure of VSMCs to a polyphenol alone did not affect the basal release of VEGF, with the exception of gallic acid, which caused a 175±27% increase (Figure 1). In addition, a higher concentration of quercetin alone (100 μM) also increased VEGF release by 302±16%.
Figure 1.
Effect of RWPs and several authentic RWPs on PDGFAB-stimulated release of VEGF into conditioned medium of VSMCs. VSMCs were exposed to either solvent (0.1% dimethylsulphoxide (DMSO)), RWPs (30 μg ml−1) or a polyphenol (30 μM) for 30 min before the addition of PDGFAB for 24 h. Thereafter, the amount of VEGF protein into conditioned medium was determined by immunoassay. Results are shown as mean±s.e.m. of three different experiments performed in triplicate. *versus control, #versus PDGFAB treatment alone.
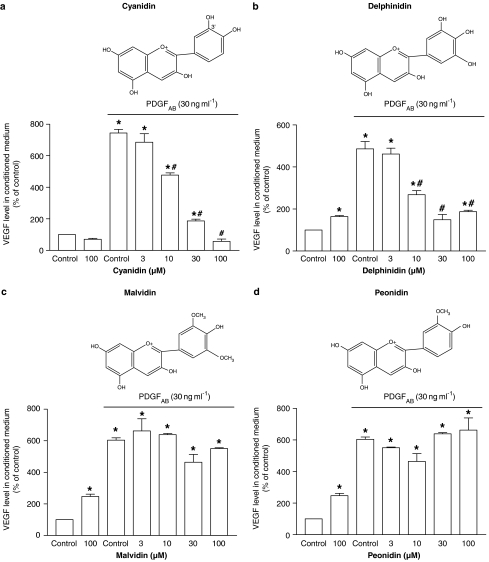
Next, experiments were planned to determine whether, besides delphinidin, other members of the anthocyanin class are able to prevent PDGFAB-induced VEGF release. Both delphinidin and cyanidin inhibited the stimulatory effect of PDGFAB in a concentration-dependent manner, whereas malvidin and peonidin were without effect at concentrations as high as 100 μM (Figure 2). The inhibitory effect of the active anthocyanins was observed at concentrations of 10 μM or higher (Figure 2).
Figure 2.
Effect of (a) cyanidin, (b) delphinidin, (c) malvidin and (d) peonidin (all belonging to the anthocyanin class) on PDGFAB-induced release of VEGF into conditioned medium of VSMCs. VSMCs were exposed to either solvent (0.1% DMSO) or an anthocyanin compound for 30 min before the addition of PDGFAB for 24 h. Thereafter, the amount of VEGF protein in conditioned medium was determined by immunoassay. The chemical structure of each anthocyanin tested is also shown. Results are shown as mean±s.e.m. of three different experiments performed in triplicate. *versus PDGFAB treatment alone, #versus PDGFAB treatment alone.
Neither delphinidin nor cyanidin affected cell viability; viabilities were 102±4 and 106±3% in delphinidin- and cyanidin-treated cells (both were tested at 30 μM) after a 24-h incubation period, respectively (n=6). RWPs (30 μg ml−1) did also not affect cell viability (Oak et al., 2003).
Antioxidant properties of polyphenols
Previous studies have indicated that growth factor-induced expression of VEGF is critically dependent on the formation of ROS by a p22phox-containing nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase in VSMCs (Bassus et al., 2001; Gorlach et al., 2001; Oak et al., 2003). Therefore, experiments were planned to determine the ability of the different polyphenols to directly scavenge ROS and to prevent the PDGFAB-induced formation of ROS in VSMCs.
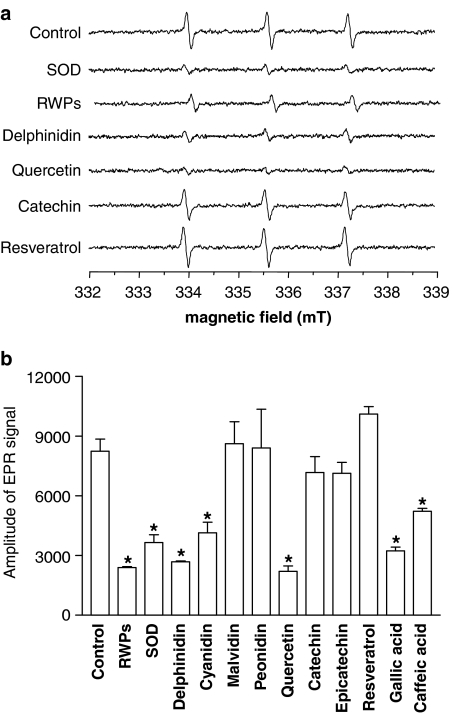
The direct scavenging properties of polyphenols were assessed using the EPR-spin trapping method with TEMPONE-H and potassium superoxide. The EPR signal was markedly reduced by the addition of either RWPs or superoxide dismutase (Figure 3). A reduced signal was also obtained with delphinidin and cyanidin, whereas malvidin and peonidin were without effect (Figure 3). In addition, the EPR signal was markedly reduced by quercetin and gallic acid and, a lesser extent, by caffeic acid, whereas catechin, epicatechin and resveratrol did not have such an effect (Figure 3).
Figure 3.
Direct ROS-scavenging activity of RWPs and several authentic RWPs. (a) Representative EPR signals induced by the reaction of TEMPONE-H with potassium superoxide in the absence and presence of superoxide dismutase (SOD), RWPs (30 μg ml−1) or an authentic RWP (30 μM). (b) Bar graph showing cumulative data. Results are shown as mean±s.e.m. of four different experiments. *versus control.
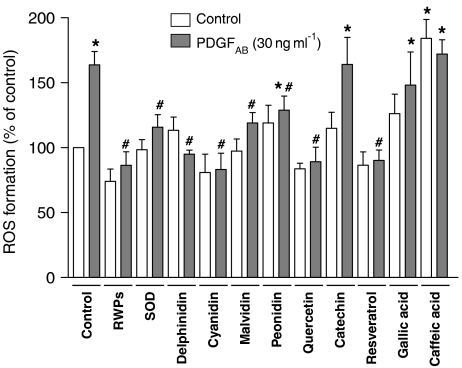
Exposure of VSMCs to PDGFAB caused the formation of ROS as assessed by DCF (Figure 4). The stimulatory effect of PDGFAB was abolished by RWPs and superoxide dismutase (Figure 4). Among compounds from the flavonoid group, delphinidin, cyanidin, malvidin, peonidin and quercetin markedly reduced the response to PDGFAB, whereas catechin had no effect (Figure 4). Among compounds from the non-flavonoid group, resveratrol abolished the pro-oxidant response of PDGFAB, whereas gallic acid and caffeic acid did not have such an effect (Figure 4). All polyphenols alone affected only slightly the basal formation of ROS, with the exception of caffeic acid, which caused a 1.8-fold increase (Figure 4).
Figure 4.
Effect of RWPs and several authentic RWPs on the PDGFAB-induced formation of ROS in VSMCs. VSMCs were exposed to either solvent (0.1% DMSO), RWPs (30 μg ml−1) or an authentic RWP (30 μM) for 30 min before the addition of PDGFAB. After a 1-h incubation period, the generation of intracellular ROS was assessed using DCF and expressed in arbitrary units as a fold increase of the signal obtained with control cells. Results are shown as mean±s.e.m. of three different experiments performed in triplicate. *versus control, #versus PDGFAB treatment alone. SOD: superoxide dismutase.
RWPs and the redox-sensitive p38 MAPK, ERK1/2 and JNK pathways
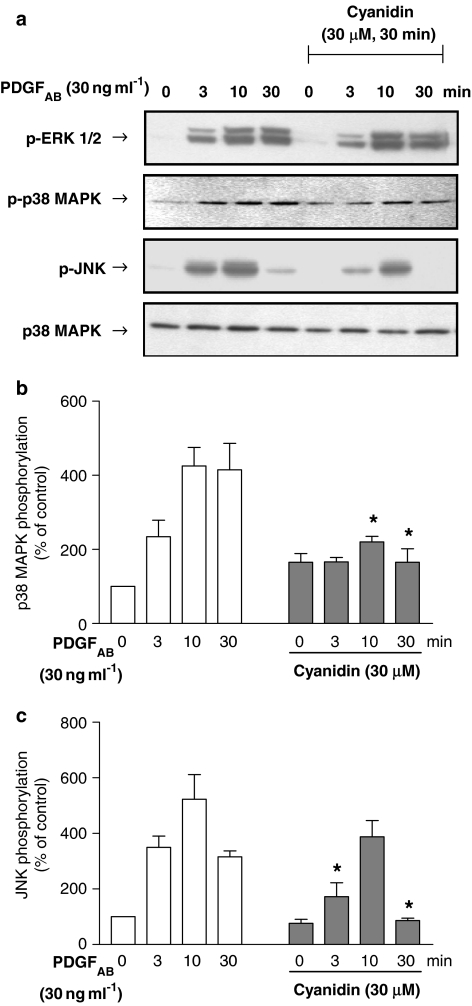
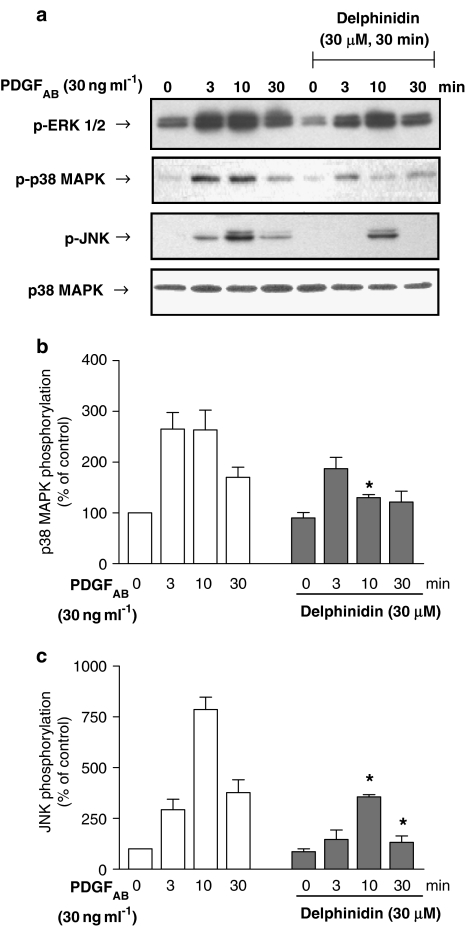
The stimulatory effect of PDGFAB on VEGF expression is critically dependent on the redox-sensitive activation of the p38 MAPK pathway and also, to some extent, the ERK1/2 pathway in VSMCs (Oak et al., 2003). In addition, the JNK inhibitor SP 600125 (30 μM) also significantly reduced the stimulatory effect of PDGFAB (30 ng ml−1: from 427 to 156%, n=4), indicating also the involvement of the JNK pathway. As RWPs have been shown to be potent inhibitors of the PDGFAB-induced p38 MAPK activation (Iijima et al., 2002; Oak et al., 2003), the effect of cyanidin and delphinidin on the activation of p38 MAPK, JNK and ERK1/2 was determined. Immunoblot analysis indicated that PDGFAB caused a time-dependent phosphorylation of p38 MAPK, JNK and ERK1/2 (Figures 5 and 6). Both cyanidin and delphinidin significantly reduced the stimulatory effect of PDGFAB on p38 MAPK and JNK but not on ERK1/2 (Figures 5 and 6). The stimulatory effect of PDGFAB on p38 MAPK phosphorylation was also inhibited by quercetin, whereas malvidin, catechin and resveratrol were without effect (data not shown).
Figure 5.
Effect of cyanidin on the PDGFAB-induced phosphorylation of ERK1/2, p38 MAPK and JNK inVSMCs. VSMCs were exposed to either solvent (0.1% DMSO) or cyanidin (30 μM) for 30 min before the addition of PDGFAB for the indicated time. Thereafter, the phosphorylation level of p38 MAPK, JNK and ERK1/2 was assessed by Western blot analysis. Total amount of p38 MAPK is also shown. A representative Western blot is shown in (a). The corresponding cumulative data for p38 MAPK phosphorylation (zero time levels=100) is shown in (b) and the corresponding cumulative data for JNK phosphorylation is shown in (c). *Significantly different from the corresponding values without cyanidin; n=3; P<0.05.
Figure 6.
Effect of delphinidin on the PDGFAB-induced phosphorylation of ERK1/2, p38 MAPK and JNK in VSMCs. VSMCs were exposed to either solvent (0.1% DMSO) or delphinidin (30 μM) for 30 min before the addition of PDGFAB for the indicated time. Thereafter, the phosphorylation level of p38 MAPK, JNK and ERK1/2 was assessed by Western blot analysis. Total amount of p38 MAPK is also shown. A representative Western blot is shown in (a). The corresponding cumulative data for p38 MAPK phosphorylation (zero time levels=100) is shown in (b) and the corresponding cumulative data for JNK phosphorylation is shown in (c). *Significantly different from corresponding values without delphinidin; n=3; P<0.05.
Discussion
We recently reported that RWPs strongly inhibit growth factor-induced VEGF mRNA expression and the subsequent release of the pro-angiogenic and pro-atherosclerotic factor at concentrations that are likely to be achieved in blood after moderate consumption of red wine, in VSMCs (Oak et al., 2003). The present findings further extend the earlier results by demonstrating that two RWPs of the anthocyanin group, cyanidin and delphinidin, were able to mimic the inhibitory effect of RWPs. In contrast, several other RWPs including quercetin, catechin, gallic acid, caffeic acid and resveratrol were inactive. Moreover, other members of the anthocyanin group did not share the inhibitory effect as malvidin and peonidin did not affect VEGF release. The comparison of the chemical structure of the four anthocyanins studied indicates that the active compounds have a hydroxyl residue at position 3′, whereas the inactive ones have a methoxy residue. Thus, the presence of a hydroxyl residue at position 3′ of the anthocyanin structure appears to be essential to inhibit VEGF expression. Delphinidin and cyanidin effectively inhibited VEGF expression at concentrations as low as 10 μM. Such concentrations are likely to be achieved in blood after red wine consumption as the concentration of cyanidin and delphinidin in red wine is about 150 μM (Nyman and Kumpulainen, 2001), and the bioavailability of cyanidin and delphinidin has been estimated to be about 60% in healthy volunteers after consumption of red wine (Frank et al., 2003).
The characterization of the signalling pathway leading to VEGF expression in response to growth factors has indicated a critical and early role of the NADPH oxidase-dependent formation of ROS (Bassus et al., 2001; Gorlach et al., 2001). Indeed, antioxidants including vitamin C and N-acetylcysteine, and selective inhibition of the NADPH oxidase using the p22phox antisense approach reduced the growth factor-induced pro-oxidant response and expression of VEGF (Bassus et al., 2001; Gorlach et al., 2001; Oak et al., 2003). The inhibitory effect of RWPs on VEGF expression has been explained, at least in part, by their ability to prevent the pro-oxidant response to PDGFAB (Oak et al., 2003). A similar mechanism may also account for the inhibitory effect of delphinidin and cyanidin as observed in the present study as both compounds directly scavenged superoxide anions and abolished the PDGFAB-induced intracellular formation of ROS. However, as malvidin, peonidin, quercetin and resveratrol also abolished or markedly reduced the pro-oxidant response to PDGFAB without affecting the release of VEGF, the inhibitory effect of polyphenols on VEGF expression requires, besides prevention of the pro-oxidant response, also inhibition of essential downstream mediators leading to VEGF expression. Growth factors are potent activators of protein kinases in VSMCs, including ERK1/2, p38 MAPK, JNK and the phosphatidylinositol-3-kinase/Akt pathway, which have all been involved in VEGF expression (Milanini et al., 1998; Jiang et al., 2000; Tanaka et al., 2000). Pharmacological inhibition of these signalling pathways has indicated that the PDGFAB-induced expression of VEGF is mediated predominantly by the p38 MAPK and JNK pathways and also, to some extent, ERK1/2 pathway (Oak et al., 2003, present findings). PDGFAB-induced activation of the p38 MAPK and JNK pathways is markedly reduced by antioxidants such as vitamin C, whereas that of ERK1/2 is not affected (Oak et al., 2003; Kyaw et al., 2004). Thus, the PDGFAB-induced expression of VEGF involves both a redox-sensitive pathway leading to p38 MAPK and JNK activation and an insensitive one leading to ERK1/2 activation. These previous findings in conjunction with the present ones suggest that both activator pathways may represent potential targets for the inhibitory effect of polyphenols on VEGF expression. Indeed, delphinidin and cyanidin markedly reduced the PDGFAB-induced phosphorylation of p38 MAPK and JNK without affecting that of ERK1/2. Similar observations were made with the RWP extract (Oak et al., 2003). The central role of the p38 MAPK is also supported by the fact that although malvidin and resveratrol inhibited the pro-oxidant response to PDGFAB, these compounds failed to affect activation of p38 MAPK and release of VEGF (present findings). Altogether, inhibition of p38 MAPK and JNK appears to be closely related to the inhibitory effect of polyphenols on the expression of VEGF. In addition, RWPs have been shown to inhibit PDGFBB binding to the PDGF β receptor and its phosphorylation (Rosenkranz et al., 2002). However, such an explanation is unlikely to account for the present observations as RWPs, cyanidin and delphinidin did not affect the PDGFAB-induced phosphorylation of ERK1/2.
Similarly to delphinidin and cyanidin, quercetin also abolished the PDGFAB-induced pro-oxidant response and phosphorylation of p38 MAPK, but this polyphenol did not affect the release of VEGF. Moreover, VEGF release was elicited by quercetin alone when the concentration was increased from 30 to 100 μM. An upregulation of VEGF expression by quercetin alone has also been observed in endometrial cancer cells and endothelial cells (Wilson and Poellinger, 2002; Kaneuchi et al., 2003). Altogether, these findings suggest that quercetin might either directly or indirectly affect key signalling molecules downstream of the formation of ROS and activation of p38 MAPK pathway leading to VEGF expression. The increased release of VEGF may be explained by the ability of quercetin to activate hypoxia-inducible factor, a key transcription factor controlling VEGF expression (Wilson and Poellinger, 2002).
In conclusion, the present findings indicate that the red wine anthocyanins, delphinidin and cyanidin, like RWPs, strongly inhibit PDGFAB-induced VEGF expression in VSMCs by preventing activation of the p38 MAPK and JNK pathways by both redox-sensitive and -insensitive mechanisms. Such an inhibitory effect is not shared by other anthocyanin compounds like malvidin and peonidin, indicating the crucial role of the hydroxyl residue at position 3′. The ability of the active anthocyanin compounds to maintain endothelial cells in a quiescent state by preventing the vascular expression of VEGF might contribute to a decreased rate of development of atherosclerotic lesions.
Acknowledgments
This study was supported in part by the French Ministry of Agriculture (ONIVINS).
Abbreviations
- ERK1/2
extracellular signal-regulated kinase 1/2
- JNK
c-Jun N-terminal kinase
- PDGFAB
platelet-derived growth factorAB
- p38 MAPK
p38 mitogen-activated protein kinase
- ROS
reactive oxygen species
- RWPs
red wine polyphenols
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cells
Conflict of interest
The authors state no conflict of interest.
References
- Bassus S, Herkert O, Kronemann N, Gorlach A, Bremerich D, Kirchmaier CM, et al. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: role of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2001;21:1550–1555. doi: 10.1161/hq0901.095148. [DOI] [PubMed] [Google Scholar]
- Chen YX, Nakashima Y, Tanaka K, Shiraishi S, Nakagawa K, Sueishi K. Immunohistochemical expression of vascular endothelial growth factor/vascular permeability factor in atherosclerotic intimas of human coronary arteries. Arterioscler Thromb Vasc Biol. 1999;19:131–139. doi: 10.1161/01.atv.19.1.131. [DOI] [PubMed] [Google Scholar]
- Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, et al. Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol. 1997;150:1673–1685. [PMC free article] [PubMed] [Google Scholar]
- da Luz PL, Serrano Junior CV, Chacra AP, Monteiro HP, Yoshida VM, Furtado M, et al. The effect of red wine on experimental atherosclerosis: lipid-independent protection. Exp Mol Pathol. 1999;65:150–159. doi: 10.1016/s0014-4800(99)80004-5. [DOI] [PubMed] [Google Scholar]
- Feng AN, Chen YL, Chen YT, Ding YZ, Lin SJ. Red wine inhibits monocyte chemotactic protein-1 expression and modestly reduces neointimal hyperplasia after balloon injury in cholesterol-Fed rabbits. Circulation. 1999;100:2254–2259. doi: 10.1161/01.cir.100.22.2254. [DOI] [PubMed] [Google Scholar]
- Frank T, Netzel M, Strass G, Bitsch R, Bitsch I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can J Physiol Pharmacol. 2003;81:423–435. doi: 10.1139/y03-038. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, et al. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- Iijima K, Yoshizumi M, Hashimoto M, Akishita M, Kozaki K, Ako J, et al. Red wine polyphenols inhibit vascular smooth muscle cell migration through two distinct signaling pathways. Circulation. 2002;105:2404–2410. doi: 10.1161/01.cir.0000016349.36385.fb. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneuchi M, Sasaki M, Tanaka Y, Sakuragi N, Fujimoto S, Dahiya R. Quercetin regulates growth of Ishikawa cells through the suppression of EGF and cyclin D1. Int J Oncol. 2003;22:159–164. [PubMed] [Google Scholar]
- Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin. 2004;25:977–985. [PubMed] [Google Scholar]
- Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2:992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- Milanini J, Vinals F, Pouyssegur J, Pages G. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem. 1998;273:18165–18172. doi: 10.1074/jbc.273.29.18165. [DOI] [PubMed] [Google Scholar]
- Nyman NA, Kumpulainen JT. Determination of anthocyanidins in berries and red wine by high-performance liquid chromatography. J Agric Food Chem. 2001;49:4183–4187. doi: 10.1021/jf010572i. [DOI] [PubMed] [Google Scholar]
- Oak MH, El Bedoui J, Anglard P, Schini-Kerth VB. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation. 2004;110:1861–1867. doi: 10.1161/01.CIR.0000142617.52881.F4. [DOI] [PubMed] [Google Scholar]
- Oak MH, Chataigneau M, Keravis T, Chataigneau T, Beretz A, Andriantsitohaina R, et al. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1001–1007. doi: 10.1161/01.ATV.0000070101.70534.38. [DOI] [PubMed] [Google Scholar]
- Pendurthi UR, Williams JT, Rao LV. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells: a possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler Thromb Vasc Biol. 1999;19:419–426. doi: 10.1161/01.atv.19.2.419. [DOI] [PubMed] [Google Scholar]
- Renaud SC, Gueguen R, Schenker J, d'Houtaud A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology. 1998;9:184–188. [PubMed] [Google Scholar]
- Rosenkranz S, Knirel D, Dietrich H, Flesch M, Erdmann E, Bohm M. Inhibition of the PDGF receptor by red wine flavonoids provides a molecular explanation for the ‘French paradox'. FASEB J. 2002;16:1958–1960. doi: 10.1096/fj.02-0207fje. [DOI] [PubMed] [Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11:287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kanai H, Sekiguchi K, Aihara Y, Yokoyama T, Arai M, et al. Induction of VEGF gene transcription by IL-1 beta is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1955–1967. doi: 10.1006/jmcc.2000.1228. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis. 2001;156:67–72. doi: 10.1016/s0021-9150(00)00625-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- Wilson WJ, Poellinger L. The dietary flavonoid quercetin modulates HIF-1 alpha activity in endothelial cells. Biochem Biophys Res Commun. 2002;293:446–450. doi: 10.1016/S0006-291X(02)00244-9. [DOI] [PubMed] [Google Scholar]