Abstract
Background and purpose:
PAF antagonists inhibit ischaemia-induced ventricular fibrillation (VF) in animals. However, unfavourable ancillary actions (on QT interval and coronary flow) have been reported with the PAF antagonist, BN-50739. If these are class actions, they would preclude development of PAF antagonists as novel anti-VF drugs. Our purpose was to examine this proposition using the hitherto untested PAF antagonist, nupafant.
Experimental approach:
Two rat heart preparations (Langendorff and ‘dual coronary' perfusion) were used to assay nupafant's effects on ischaemia-induced VF, coronary flow and QT interval, and to test for the site-selectivity necessary if any effects on VF are caused by PAF antagonism.
Key results:
Global (whole-heart) delivery of 10 μM nupafant, reduced the incidence of ischaemia-induced VF and widened QT interval without affecting coronary flow. Importantly, lower concentrations (0.1 and 1 μM) had no effect on VF, yet widened QT almost identically to 10 μM nupafant. When nupafant was delivered selectively to (and entrapped within) the involved region it partially protected against VF (P<0.05). This occurred without change in QT interval. Selective nupafant delivery to the uninvolved region was without effect.
Conclusions and implications:
Nupafant protects against ischaemia-induced VF primarily by site-selective actions in the ischaemic region but, unlike BN-50739, the effect is unrelated to its QT widening action, and is not compromised by any effect on coronary flow. This establishes proof of concept that VF suppression by PAF antagonism need not invariably be associated with QT prolongation or vasodilatation, justifying further development of this drug class.
Keywords: antiarrhythmic, nupafant, heart, myocardial ischaemia, platelet activating factor, ventricular fibrillation
Introduction
Most antifibrillatory drugs tested against sudden cardiac death act directly on cardiac ion channels (Rosen, 1995). The Survival With Oral d-Sotalol (SWORD) clinical trial demonstrated an increased death rate and susceptibility to life-threatening arrhythmias following treatment with a potassium channel blocking drug that delays ventricular repolarization (Waldo et al., 1996). This, together with the widely recognized risk of proarrhythmia with non-cardiac drugs that prolong quantitative transmission (QT) interval, means that for prototype antiarrhythmic drugs, delay in ventricular repolarization is regarded as a hindrance rather than a help (Pugsley, 2002).
Despite an overall fall in mortality in patients with coronary artery disease over the last decade, mortality from ventricular fibrillation (VF) is not declining (Zheng et al., 2001). Inhibition of the actions of endogenous chemical mediators of the initiation of ventricular arrhythmia (Curtis et al., 1993) is an underexploited alternative to targeting cardiac ion channels as an approach to antiarrhythmic drug development (Clements-Jewery and Curtis, 2003).
Platelet activating factor (PAF) has a range of indirect actions that are liable to exacerbate acute myocardial ischaemia, including thrombogenic (Braquet et al., 1987; Mueller et al., 1995; Koller et al., 1996), coronary vasoconstrictor (Piper and Stewart, 1986) and negative inotropic actions (Robertson et al., 1987). Importantly it is also one of numerous putative endogenous chemical mediators of ischaemia-induced VF (Flores and Sheridan, 1990; Curtis et al., 1993). It complies with several necessary criteria for it to have a role as a mediator of VF, as elevated PAF levels are found in the ischaemic myocardium of baboons (Annable et al., 1985) and coronary vasculature of patients with atherosclerosis (Mueller et al., 1995), and exogenous PAF has proarrhythmic effects in Langendorff perfused, ischaemic guinea pig hearts (Flores and Sheridan, 1990). Moreover, when delivered selectively to the left coronary bed, PAF had proarrhythmic actions even in the absence of ischaemia (Baker and Curtis, 2004).
In view of this, preclinical investigations have been undertaken to evaluate the effects of PAF antagonists in ischaemia-induced VF models. One such antagonist, BN-50739, has antiarrhythmic effects (Koltai et al., 1991; Baker and Curtis, 1999), and antagonizes the proarrhythmic effects of PAF delivered selectively to the left coronary bed of perfused rat hearts in the absence of ischaemia (Baker and Curtis, 2004), but it widens QT interval at antiarrhythmic concentrations (Baker and Curtis, 1999). Regardless of mechanism (which could be linked to PAF antagonism, or could be PAF-independent and owing to potassium channel blockade), if QT widening is a class effect of PAF antagonists, and the concentration dependence of the QT effect is intimately linked with the concentration dependence of VF suppression (implying a cause–effect relationship), then there would be little scope for developing PAF antagonists as antiarrhythmics.
BN-50739 was also found to increase coronary flow at concentrations that suppressed VF in previous studies (Baker and Curtis, 1999). Drugs that relax coronary vascular smooth muscle at the concentration required for VF suppression will inevitably also cause peripheral vasodilatation, and the associated hypotensive effect will preclude their use in suppression of ventricular arrhythmias; verapamil is a good example of this profile of activity (Farkas et al., 1999). Therefore, and importantly, if the BN-50739 profile (with QT widening and vasodilatation inevitably occurring at antiarrhythmic concentrations) is the profile of a class effect of PAF antagonists, then PAF antagonists as a class have no future as VF preventive agents.
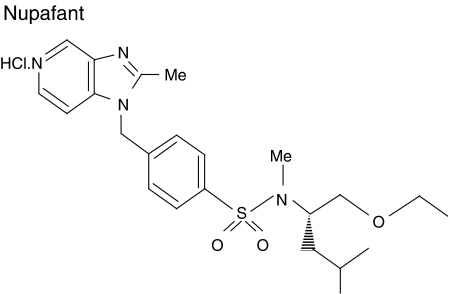
In view of this, we have examined the antiarrhythmic activity of a second specific PAF antagonist nupafant (Figure 1; Whittaker et al., 1992) in a concentration–response study designed to assess whether possible arrhythmia suppression is linked (interdependent), or unrelated, to possible QT interval widening and vasodilatation by the drug. The rat Langendorff preparation, a standardized model for detection of drug action on ischaemia-induced arrhythmias (Curtis and Hearse, 1989a; Curtis, 1998) was again used. In a separate study, to test for a relationship between any antiarrhythmic effect and blockade of the action of endogenous PAF, the site of action of nupafant (involved versus uninvolved zone) was examined by selective perfusion of nupafant to separate coronary beds using the rat dual coronary perfusion model (Avkiran and Curtis, 1991; Baker and Curtis, 2004).
Figure 1.
The chemical structure of nupafant (N-4-(1-H-2-methylimidazo[4,5-c]pyridylmethyl)phenylsulphonyl-L-leucinyl ethyl ether hydrochloride).
Methods
All experiments were performed in accordance with the United Kingdom Home Office ‘Guide on the operation of the Animals (Scientific Procedures) Act 1986'. A total of 96 rats were used in these experiments.
Arrhythmias in Langendorff perfused hearts
A standardized and validated method for evoking ischaemia-induced VF in perfused rat hearts (Curtis, 1998) was used. Hearts were excised from male Wistar rats (Bantin and Kingman, UK; 250–300 g) following induction of surgical-depth anaesthesia with pentobarbitone (60 mg kg−1 intraperitoneal) and sodium heparin (250 international units) to preclude coronary and intracardiac thrombosis. Excised hearts were immediately arrested in cold (4°C) perfusion solution containing (in mM): NaCl 118.5, NaHCO3 25.0, MgSO4 1.2, NaH2PO4 1.2, CaCl2 1.4, KCl 3.0 and glucose 11.1, then attached to an aortic Langendorff cannula. Perfusion at constant pressure (70 mm Hg) with similar solution (at 37°C and pH 7.4), was begun within 60 s of opening the chest cavity in all experiments. Rapid onset of perfusion after rapid cardiac excision and cold arrest precludes the development of ischaemic preconditioning against development of arrhythmia susceptibility, as attested by the close to 100% incidence of ischaemia-induced and reperfusion-induced VF routinely generated in the preparation (Curtis, 1998). All perfusion solutions were filtered (5 μM pore size) before use to remove particulate matter.
A uni-polar electrocardiogram (ECG) was recorded by implanting one stainless steel electrode into the centre of the anterior aspect of the left ventricle (the centre of the region destined to become ischaemic) with a second electrode connected to the aortic cannula (Curtis et al., 1997). Signals recorded in this manner are dominated by local activity, with recordings from adjacent perfusion beds detecting local events, for example, ST depression and elevation (Curtis, 1991) and regional QT changes (Rees and Curtis, 1995). A reversible traction-type coronary occluder consisting of a silk suture (Mersilk, 4/0) threaded through a polythene guide was used for coronary occlusion, was positioned as in previous studies (Baker and Curtis, 1999). Subsequently, after recording of baseline variables and switch to test solution (see below), regional ischaemia was induced by tightening the occluder. After a designated period, reperfusion was achieved by releasing the occluder. Coronary flow was measured by timed collection of coronary effluent (1 ml weighs 1 g). A balance (Ohaus Corporation, Cambridge, UK) accurate to ±1 mg (approximately 0.5% of the minimum volume collected) was used. Occlusion was verified by comparing flow at 1 min before ischaemia with flow after 1 min of ischaemia.
Experimental protocol for Langendorff experiments
In the first series of experiments, hearts were perfused for an initial 10 min with drug-free solution, then the solution was switched to one of 4 solutions containing vehicle (0.01% dimethyl sulphoxide (DMSO) in perfusion solution) or 0.1, 1 or 10 μM nupafant. Hearts were randomized to groups of 12 using a coded randomization table with the identity of groups unknown to the experimental operator. Test solution was delivered for a further 10 min perfusion, during which time a minimum of 80 ml of solution passed through the coronary circulation of each heart, equivalent to a minimum exposure of 100 ml solution per gram heart weight, that is, 0, 0.01, 0.1 or 1 μmol g−1 in the 0, 0.1, 1 or 10 μM nupafant groups. Then the left coronary artery was occluded to induce regional ischaemia. Perfusion of the uninvolved region continued with the same test solution. After 30 min ischaemia the occluder was released to achieve reperfusion. Hearts were superfused (10 ml min−1; 37°C) throughout the experiment with drug-free oxygenated perfusion solution, to minimise cardiac cooling (Pabla and Curtis, 1996).
Arrhythmias in hearts perfused in the dual coronary perfusion model
In order to identify the site of any antiarrhythmic action of nupafant (involved versus uninvolved tissue), independent manipulation of the left and right coronary beds was achieved by dual coronary perfusion. This technique uses a specially made cannula for separate perfusion of left and right coronary beds and induction of regional ischaemia without the need for coronary artery ligation (Avkiran and Curtis, 1991). Perfusion was achieved with solution identical to that used for the Langendorff experiment. Independent left and right coronary perfusion was verified and monitored with a pair of in-line, ultra-sonic flow meters (Transonic T206 Flowmeter, Transonic Systems Inc., USA). Left regional ischaemia was induced by complete occlusion of the flow line into the left coronary bed at source. All other techniques and analyses were identical to those for the Langendorff arrhythmia experiments.
Experimental protocol for dual perfusion experiments
Hearts were perfused for an initial 10 min with drug-free solution, before solutions were randomly switched to allow (i) perfusion of left and right coronary beds with vehicle, (ii) perfusion of the left coronary bed with a concentration of nupafant chosen from the Langendorff studies (10 μM), (iii) perfusion of the right coronary bed with 10 μM nupafant or (iv) perfusion of left and right coronary beds with 10 μM nupafant. After 10 min perfusion with test solution, flow into the left coronary bed was stopped to induce regional ischaemia. This was imposed for 30 min, following which the left coronary perfusion line was unclamped to permit reperfusion and assessment of reperfusion-induced arrhythmias.
Arrhythmia diagnosis and ECG analysis
For Langendorff and dual perfusion experiments, MacLab software for the Apple MacIntosh computer was used in the identification and analysis of the ECG and diagnosis of arrhythmias. The diagnostic criteria for discriminating between different ventricular arrhythmias were those described by the Lambeth Conventions (Walker et al., 1988) with the exception of VF which was defined as described by Tsuchihashi and Curtis (1991). From the ECG, the incidence of arrhythmias, the heart rate, PR interval and QT interval, measurement at point of 90% repolarization (Ridley et al., 1992) were obtained.
Measurement of perfusion bed size and regional coronary flow
For Langendorff and dual perfusion experiments, the size of the left (involved) and right (uninvolved) coronary beds was quantified at the end of 10 min reperfusion using the disulphine blue dye exclusion method (Curtis and Hearse, 1989a). Involved zone size was expressed as % total ventricular weight. Values of coronary flow (ml min−1 g−1) in the involved and uninvolved zones, were calculated from the total coronary flow (ml min−1) and the weight of the two zones (Curtis and Hearse, 1989a).
Exclusion criteria
For Langendorff and dual perfusion experiments, hearts were excluded 5 min before the onset of ischaemia if found to have a sinus rate of less than 250 beats min−1 or more than 420 beats min−1, or a global coronary flow of more than 18 ml min−1 or less than 8 ml min−1. Likewise, hearts were excluded at the end of the experiment if found to have an involved zone size of less than 30% or more than 60% of the total ventricular weight. Each excluded heart was replaced immediately to preserve the randomization. Exclusion criteria and the basis for their use have been described previously (Curtis and Hearse, 1989a, 1989b).
Statistics
Gaussian distributed variables were expressed as mean ±s.e.m. and were subjected to one way analysis of variance. If treatment constituted a significant source of variance, each group was compared with the control using Dunnett's test. Mainland's contingency Tables were used for non-parametric analysis of arrhythmia incidence. P<0.05 was taken as indicative of a statistically significant difference between means.
Drugs and materials
Nupafant (structure in Figure 1 supplied by British Biotech, Oxford, UK) was stored as a 10 mM stock solution dissolved in DMSO, at 0°C. All salts were obtained from Sigma Chemicals (Poole, UK). Water for preparing perfusion solutions was supplied using a reverse osmosis system (Milli-RO 10 and Milli-Q 50, Millipore Ltd, UK). Disulphine blue dye was supplied by BDH Chemicals (Poole, UK).
Results
Actions of nupafant in Langendorff perfused hearts
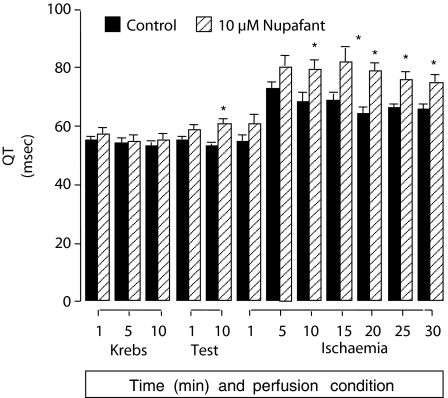
In Langendorff perfused hearts, nupafant 10 μM greatly reduced the incidence of ischaemia-induced VF, whereas concentrations of less than 10 μM had no effect (Table 1). Other types of ventricular arrhythmia were not suppressed even by 10 μM nupafant (Table 1). Nupafant widened the QT interval, an effect that was significant and similar for all three concentrations, with values at 10 min of ischaemia in control and 0.1, 1 and 10 μM nupafant groups of 60±2, 73±3, 75±3 and 76±2 msec, respectively (P<0.05 for all three concentrations versus controls). The effect was sustained during ischaemia, and the time course of QT interval change is shown in Figure 2. Nupafant had no effects on any other variables (values shown here are controls, 0.1, 1 and 10 μM nupafant, respectively at 10 min of ischaemia) including coronary flow, (−1±0.3, −1±0.3, −1±0.2 and −1±0.3 ml min−1 g−1 change from predrug values), heart rate (280±13, 265±13, 271±13 and 269±10 beats min−1), or PR interval (41±2, 45±3, 37±1 and 40±2 msec; n=12 throughout).
Table 1.
Group incidence (%) of VPB, bigeminy, salvo, VT and VF during 30 min of ischaemia
|
Arrhythmia incidence |
|||||
|---|---|---|---|---|---|
| VPB | Bigeminy | Salvo | VT | VF | |
| Control | 92 | 75 | 92 | 100 | 100 |
| 0.1 μM Nupafant | 100 | 92 | 100 | 92 | 92 |
| 1 μM Nupafant | 100 | 100 | 100 | 100 | 83 |
| 10 μM Nupafant | 100 | 100 | 100 | 100 | 25* |
Abbreviations: VF, ventricular fibrillation; VPB, ventricular premature beats; VT, ventricular tachycardia.
Nupafant was delivered to hearts perfused in the Langendorff mode. n=12 per group.
P<0.05 versus control.
Figure 2.
Effects of nupafant on the QT interval in Langendorff perfused rat hearts after coronary artery occlusion. The QT interval was measured at 90% repolarization. Nupafant (10 μM) or vehicle delivery (‘test') began 10 min before onset of ischaemia. *P<0.05 versus control (n=12). Involved zone sizes were 45±1% of total ventricular weight in the nupafant group and 44±1% in controls. Data for 0.1 and 1 μM PAF were similar to data for the 10 μM group and have been omitted for clarity.
Actions of nupafant delivered selectively to left or right coronary beds
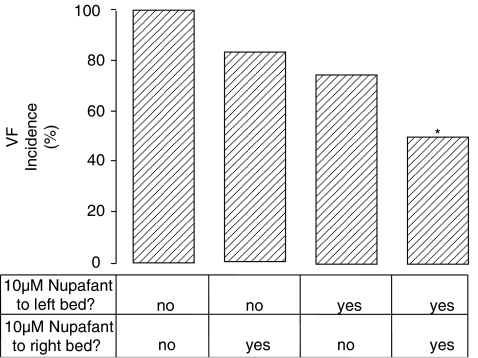
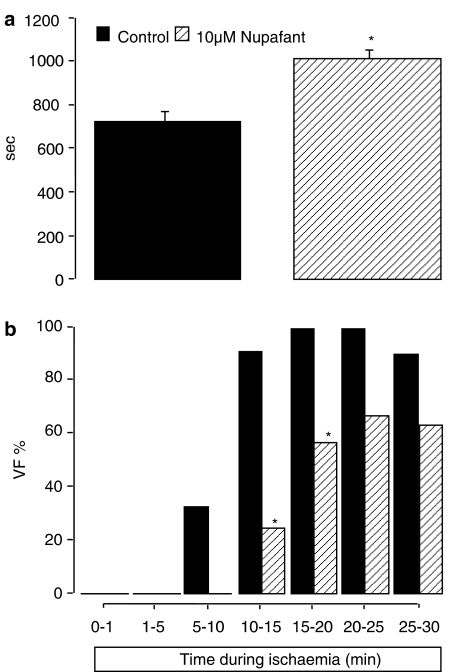
Based on the outcome of Langendorff perfusion studies, 10 μM nupafant was chosen for use in dual perfusion studies. Nupafant significantly reduced VF incidence when delivered simultaneously to left and right coronary beds (Figure 3) in a manner similar to its effects in the equivalent Langendorff perfusion study (Table 1). However, selective delivery of nupafant to either the left or right coronary bed alone had no effect on the incidence of VF (Figure 3). Nupafant did however significantly delay VF onset when administered selectively to the left coronary bed and trapped there during ischaemia whereas drug-free solution was delivered throughout to the uninvolved region (Figure 4a), such that fewer hearts developed VF during the first 15 min of ischaemia (Figure 4b). In contrast, selective delivery of nupafant to the right coronary bed had no effect on VF incidence or onset (data not shown), whereas delivery of nupafant to both beds reduced VF incidence (as noted above) precluding assessment of group mean VF onset. Perfusion of both the left and right coronary beds jointly with nupafant prolonged the QT interval measured from an electrode placed in the left ventricle (Table 2) as it did in the Langendorff experiments. However, nupafant did not prolong the QT interval if delivered selectively to only the left or right coronary bed (Table 2).
Figure 3.
Group incidence (%) of VF during 30 min of ischaemia (dual perfusion experiment) in rat perfused hearts Krebs containing vehicle or nupafant was delivered to both coronary beds, selectively to the coronary bed or selectively to the right coronary bed using the dual perfusion cannula; n=12 per group. Delivery to the two sites is indicated (‘yes' or ‘no'). *P<0.05 versus control (no nupafant in left or right perfusion bed).
Figure 4.
Effect of nupafant on VF onset and incidence in discrete time bins. In (a) VF onset time (mean sec) and in (b), Group incidence (%) of VF during 30 min of ischaemia in discrete time bins. Krebs containing vehicle or nupafant was delivered selectively to the left coronary bed in hearts perfused with the dual perfusion cannula; n=12 per group. * P<0.05 versus control.
Table 2.
The data represent the QT intervals at 90% repolarization (msec), 1 min before the onset of ischaemia (−1 min) and 1, 10 and 29 min after the start of ischaemia
|
Time relative to ischaemia onset |
||||
|---|---|---|---|---|
| +1 min | +10 min | +10 min | +29 min | |
| Nupafant | ||||
| Control | 51±1 | 57±3 | 77±5 | |
| Right bed | 61±3 | 65±5 | 64±7 | |
| Nupafant | ||||
| Control | 53±1 | 55±2 | 67±4 | |
| Left bed | 50±1 | 57±1 | 65±1 | |
| Nupafant | ||||
| Control | 55±1 | 60±2 | 65±2 | |
| Both beds | 67±3 | 79±3 | 79±3 | 74±3 |
Abbreviation: QT, quantitative transmission.
Nupafant (10 μM), was delivered to both coronary beds, selectively to the left coronary bed or selectively to the right coronary bed using the dual perfusion cannula; n=12 per group. The QT interval was recorded from the left coronary bed. Values are means±s.e.m. *P<0.05 vs control (no nupafant in either bed). Missing values at 29 min reflect the occurrence of persistent arrhythmias in all but one group.
Discussion
Overview
The main purpose of the present study was to generate a data array to explore whether it may be possible to synthesize a PAF antagonist that can suppress ischaemia-induced VF by antagonizing the effects of endogenous PAF and without dilating blood vessels or prolonging the QT interval. By showing that the PAF antagonist nupafant could reduce ischaemia-induced VF without increasing coronary flow, by an action mediated primarily in the involved zone, and in a manner unrelated to its effects on QT interval, we have established a proof of concept that may allow nupafant to serve as a start compound for new antiarrhythmic drug discovery.
Concentration dependence of VF prevention by nupafant
Nupafant 10 μM protected against ischaemia-induced VF in Langendorff perfused isolated rat hearts. It is extremely difficult to link this VF suppression to antagonism of endogenous PAF. This is partly owing to the uncertainty over how much drug is necessary for achieving antagonism of endogenous PAF, as opposed to antagonism of exogenously applied PAF. In superfused preparations, antagonism of exogenous PAF occurs with considerably lower concentrations of nupafant than we used (Whittaker et al., 1992). We found similar protection against arrhythmias, similarly requiring a higher drug concentration than would have been expected on the basis of superfusion analysis, in previous work with another PAF antagonist, BN-50739 (Baker and Curtis, 1999). The likelihood that endogenous PAF mediates some of its pathophysiological actions intracellularly (Hwang, 1990) may explain this. The effects of exogenous PAF are likely to be much more sensitive to blockade by antagonists than effects of endogenous PAF, owing to issues of drug access to intracellular targets.
Comparison with previous findings and key implications
In previous studies we found that BN-50739 could partially antagonize the proarrhythmic action of exogenously applied PAF in the rat dual perfusion model (Baker and Curtis, 2004). We did not attempt to mimic this experiment with nupafant as this approach had only limited value in previous studies with BN-50739 (Baker and Curtis, 2004) with high drug doses required for effects.
The advantage of the present studies is that we can rule out two important factors which complicated interpretation of findings with BN-50739. Nupafant reduced the incidence of VF without affecting coronary flow (unlike BN-50739 which, in previous studies, causes coronary vasodilatation; Baker and Curtis, 1999). Like BN-50739 (Baker and Curtis, 1999) nupafant did widen the QT interval, but the effects on QT and VF of nupafant were unrelated as lower concentrations of nupafant had similar effects on QT interval without having any effect on VF, and nupafant delivered to the left coronary bed delayed VF onset without affecting the QT interval. This means that it is unlikely that nonspecific (flow/QT) effects contributed to the actions of nupafant on VF. This is a significant advantage over the situation found previously with BN-50739 in which QT and flow effects were more closely linked with effects on VF, making it difficult to attribute benefit to PAF antagonism (Baker and Curtis, 1999), and raising the possibility that effects of PAF antagonists on the QT interval are class effects. The key implication from the present study, therefore is that it may be possible to separate PAF antagonism-related antiarrhythmic activity from rhythm effects related to nonspecific drug actions on QT interval and coronary flow, allowing development of new PAF antagonists that are better (more selective) antifibrillatory agents with a lower liability for adverse effects related to changes in QT interval (torsades de pointes).
Reiterative findings
By themselves the results from the Langendorff preparations do not prove that nupafant prevents VF by antagonizing the actions of endogenous PAF. However the case for a cause–effect relationship may be strengthened by consideration of reiterative findings from other types of experiment. Thus, the site of action of nupafant (ischaemic versus non-ischaemic zones) was examined by selective delivery of drug to the left versus right coronary bed using the dual perfusion preparation. The results showed that delivery of nupafant to the left bed and entrapment of nupafant within this region during left regional ischaemia delayed but did not prevent the occurrence of VF. Nevertheless, selective and continuous delivery to the right (uninvolved) bed had no effect at all on VF susceptibility. Moreover, the delay in VF caused by left local delivery of nupafant was not accompanied by QT prolongation. This means that, although at the concentration studied the selective delivery of nupafant to the involved region had only partial protective actions, it was possible to detect the effects unaccompanied by widening of the QT interval. In previous studies with BN-70539 the effect on VF incidence was more pronounced, but it was accompanied by significant concomitant QT widening (Baker and Curtis, 1999). These data further support the argument that PAF antagonists can suppress ischaemia-induced arrhythmias by an action mediated largely in the involved region, and moreover they also show, for the first time, that the action is not dependent on a QT-widening effect, which is neither sufficient (with low concentrations of nupafant) nor necessary (with selective left coronary delivery of nupafant) to suppress VF.
Rat QT interval – irrelevant?
Many arrhythmia investigators rarely, if ever, cite studies of rat heart arrhythmias, owing to scepticism about the clinical relevance of results obtained in rat heart. Arguments to counter this scepticism have been presented in detail (Curtis, 1998). In the present study, one issue of possible concern is the meaning of the QT interval data. Very few investigators attempt to measure rat QT intervals because it is difficult to determine the point of 100% repolarization. We have found that measurement of rat QT interval at 90% repolarization has allowed generation of a series of robust data sets with good precision, and that the QT interval at 90% repolarization is a variable that allows robust and credible detection of drug-induced repolarization delay (e.g., Baker and Curtis, 2004; Clements-Jewery et al., 2002, 2005, 2006; Farkas et al., 1999; Rees and Curtis, 1993; Rees and Curtis, 1996). The only outcome that is at odds with outcomes from studies using other species is the lack of QT widening with selective blockers of the rapid delayed rectifying potassium current (IKr) in the rat heart, although this is entirely expected given that rat ventricular IKr is not functional (Rees and Curtis, 1996). This caveat can be assimilated in global project design. On the other hand, QT interval at 90% repolarization in the rat Langendorff preparation is an excellent variable for evaluating IKr-independent drug-induced repolarization delay (Farkas and Curtis, 2003), which is the presumed mechanism of nupafant's actions on the QT interval in the present study. Of course were a drug to have ancillary actions on IKr, these actions would not be detected by QT interval measurement in a rat heart bioassay. This defines the limit of the clinical relevance of the QT interval in rat hearts as a biomarker.
Relevance to the role of PAF as a VF mediator and the mechanism of this effect
The present study provides further support for the hypothesis that antagonism of endogenously produced PAF accounts for the antiarrhythmic actions of PAF antagonists. It also reinforces the notion that endogenous PAF functions as a mediator of ischaemia-induced VF, direct evidence for which was provided recently (Baker and Curtis, 2004). Lately, the role of PAF in ischaemic heart disease has received renewed attention, although study outcomes have been contradictory. Transfection of PAF-acetylhydrolase (to facilitate local degradation of endogenous PAF) limits experimental atherogenic alteration in rabbits (Arakawa et al., 2005), an observation that would benefit from confirmation by use of newly synthesized PAF-acetylhydrolase inhibitors (Quistad et al., 2005). However, in a large cohort of coronary artery disease patients, PAF-acetylhydrolase was found to be, itself, an independent risk factor (Winkler et al., 2005). Thus, a better picture may be obtained from further studies with selective PAF antagonists. This may explain the renewed interest in novel PAF antagonists (Jantan et al., 2004). Full elucidation of the role of PAF in cardiovascular pathology (including VF) requires antagonists that are fully free from PAF-independent effects on QT interval as well as coronary flow. It will be important to factor establishment of this into future research protocols.
Transduction of PAF-mediated pathology
It was not our purpose to address the transduction pathway linking PAF receptor antagonism to VF suppression. This is a separate question. The mechanism of action of PAF is complex and of the known tissue effects (Piper and Stewart, 1986, Yamanaka et al., 1992; Chao and Olson, 1993; Izumi and Shimizu, 1995), perhaps the one that has received the most attention in the present context is its ability to shorten the duration of the action potential in isolated hearts (Flores and Sheridan, 1990). This effect could be related to activation of K+ channels. Ventricular repolarization in rat heart is dependent on the transient outward current, Ito (Rees and Curtis, 1996), and Ito is modulated by changes in intracellular calcium concentrations (Rosen, 1995). It is therefore possible that a PAF-induced increase in cellular calcium (Izumi and Shimizu, 1995), is sufficient to increase the opening of the channels responsible for Ito, and thereby shorten action potential duration regionally in the ischaemic tissue. This action may be sufficient to shorten refractory period.
Conclusion
Nupafant has a substantial effect on ischaemia-induced VF that is accompanied by – but is unrelated to – prolongation of QT interval. The separation between actions on QT interval and antifibrillatory actions implies separate mechanisms. Differences between nupafant and BN-50739 (which affected coronary flow as well as having more strongly linked effects on QT interval and on arrhythmias) suggests that it may be possible to synthesize a PAF antagonist capable of suppressing VF without having any effect on QT interval. A drug with this profile of action would be potentially more promising as a tool for probing PAF mechanisms in arrhythmogenesis, and a safer and more effective antiarrhythmic option than the currently available PAF antagonists. As is typical in pathophysiologically based pharmacology, elucidation of the role of PAF as an arrhythmogenic mediator and the realization of a selective antiarrhythmic PAF antagonist, are two goals that are likely to be achieved in tandem, or not al all.
Acknowledgments
Some of the studies described were funded by the British Heart Foundation (FS/95040). KEB was a British Heart Foundation PhD student.
Abbreviations
- DMSO
dimethyl sulphoxide
- ECG
electrocardiogram
- i.p.
intraperitoneal
- i.u.
international units
- PAF
platelet activating factor
- QT
quantitative transmission
- SWORD
Survival With Oral d-Sotalol
- VF
ventricular fibrillation
Conflict of interest
The authors state no conflict of interest.
References
- Annable CR, Mcmanus LR, Carey KR, Pinckard RN. Isolation of platelet activating factor from ischaemic baboon myocardium. Fed Proc. 1985;44:1271. [Google Scholar]
- Arakawa H, Qian J-Y, Baatar D, Karasawa K, Asada Y, Sasaguri Y, et al. Local expression of platelet-activating factor-acetylhydrolase reduces accumulation of oxidized lipoproteins and inhibits inflammation, shear stress-induced thrombosis, and neointima formation in balloon-injured carotid arteries in nonhyperlipedemic rabbits. Circulation. 2005;111:3302–3309. doi: 10.1161/CIRCULATIONAHA.104.476242. [DOI] [PubMed] [Google Scholar]
- Avkiran M, Curtis MJ. Independent dual perfusion of left and right coronary arteries in isolated rat hearts. Am J Physiol. 1991;261:H2082–H2090. doi: 10.1152/ajpheart.1991.261.6.H2082. [DOI] [PubMed] [Google Scholar]
- Baker KE, Curtis MJ. Protection against ventricular fibrillation by the PAF antagonist, BN-50739, involves an ischaemia-selective mechanism. J Cardiovasc Pharmacol. 1999;34:394–401. doi: 10.1097/00005344-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Baker KE, Curtis MJ. Left regional cardiac perfusion in vitro with platelet-activating factor, norepinephrine and K+ reveals that ischaemic arrhythmias are caused by independent effects of endogenous ‘mediators' facilitated by interactions, and moderated by paradoxical antagonism. Br J Pharmacol. 2004;142:352–366. doi: 10.1038/sj.bjp.0705767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet activating factor research. Pharm Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- Chao W, Olson MS. Platelet activating factor: receptors and signal transduction. Biochem J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Jewery H, Curtis MJ.Biochemical mediators of ventricular arrhythmias in ischaemic heart disease Cardiac Drug Development Guide 2003The Humana Press Inc: Totowa, NJ, USA; 203–226.In: MK Pugsley (ed) [Google Scholar]
- Clements-Jewery H, Hearse DJ, Curtis MJ. The isolated blood-perfused rat heart: an inappropriate model for the study of ischaemia-induced ventricular fibrillation. Br J Pharmacol. 2002;137:1089–1099. doi: 10.1038/sj.bjp.0704977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Jewery H, Hearse DJ, Curtis MJ. Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic drug development and safety pharmacology evaluation. Br J Pharmacol. 2005;145:551–564. doi: 10.1038/sj.bjp.0706231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Jewery H, Kanaganayagam GS, Kabra R, Curtis MJ. Actions of flecainide on susceptibility to phase-2 ventricular arrhythmias during infarct evolution in rat isolated perfused hearts. Br J Pharmacol. 2006;147:468–475. doi: 10.1038/sj.bjp.0706633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ. The rabbit dual coronary perfusion model: a new method for assessing the pathological relevance of individual products of ischaemia and reperfusion: role of potassium in arrhythmogenesis. Cardiovasc Res. 1991;25:1010–1022. doi: 10.1093/cvr/25.12.1010. [DOI] [PubMed] [Google Scholar]
- Curtis MJ. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Baczko I, Baker KE.The ECG in man and laboratory animals: recording methods, analysis and interpretation Methods in Cardiac Electrophysiology 1997CRC Press: Basel; 133–158.In Walker MJA, Pugsley MK, (eds) [Google Scholar]
- Curtis MJ, Hearse DJ. Ischaemia induced and reperfusion induced arrhythmias differ in their sensitivity to potassium: implications for mechanism of initiation and maintenance of ventricular fibrillation. J Mol Cell Cardiol. 1989a;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hearse DJ. Reperfusion induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J Mol Cell Cardiol. 1989b;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Pugsley MK, Walker MJA. Endogenous chemical mediators of ventricular arrhythmias in ischaemic heart disease. Cardiovasc Res. 1993;27:703–719. doi: 10.1093/cvr/27.5.703. [DOI] [PubMed] [Google Scholar]
- Farkas A, Curtis MJ. Does QT widening in the Langendorff-perfused rat heart represent the effect of repolarization delay or conduction slowing. J Cardiovasc Pharmacol. 2003;42:612–621. doi: 10.1097/00005344-200311000-00006. [DOI] [PubMed] [Google Scholar]
- Farkas A, Qureshi A, Curtis MJ. Inadequate ischaemia-selectivity limits the antiarrhythmic efficacy of mibefradil during regional ischaemia and reperfusion in the rat isolated perfused heart. Br J Pharmacol. 1999;128:41–50. doi: 10.1038/sj.bjp.0702778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores NA, Sheridan DJ. Electrophysiological and arrhythmogenic effects of PAF during normal perfusion, myocardial ischaemia and reperfusion in the guinea pig. Br J Pharmacol. 1990;101:734–738. doi: 10.1111/j.1476-5381.1990.tb14149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SB. Specific receptors of platelet activating factor, receptor heterogenicity and signal transduction mechanisms. J Lipid Med. 1990;2:123–158. [PubMed] [Google Scholar]
- Izumi T, Shimizu T. Platelet activating receptor: gene expression and signal transduction. Biochim et Biophys Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Jantan I, Pisar M, Mohd H, Sirat M, Basar N, Jamil S, et al. Inhibitory effects of compounds from zingiberaceae species on platelet activating factor receptor binding. Phytotherapy Res. 2004;18:1005–1007. doi: 10.1002/ptr.1608. [DOI] [PubMed] [Google Scholar]
- Koller M, Hilger RA, Konig W. Effect of PAF receptor antagonist SM-12502 on human platelets. Inflammation. 1996;20:71–85. doi: 10.1007/BF01487746. [DOI] [PubMed] [Google Scholar]
- Koltai M, TosakiI A, Hosford D, Esanu A, Braquet P. Effect of BN-50739, a new platelet-activating-factor antagonist, on ischemia induced ventricular arrhythmias in isolated working rat hearts. Cardiovasc Res. 1991;25:391–397. doi: 10.1093/cvr/25.5.391. [DOI] [PubMed] [Google Scholar]
- Mueller HW, Haught CA, McNatt JM. Measurement of platelet activating factor in a canine model of coronary thrombosis and in endarterectomy samples from patients with advanced coronary artery disease. Circ Res. 1995;77:54–63. doi: 10.1161/01.res.77.1.54. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Effect of endogenous nitric oxide on cardiac systolic and diastolic function during ischaemia and reperfusion in the rat isolated perfused heart. J Mol Cell Cardiol. 1996;28:2111–2121. doi: 10.1006/jmcc.1996.0203. [DOI] [PubMed] [Google Scholar]
- Piper PJ, Stewart AG. Coronary vasoconstriction in the rat, isolated perfused heart induced by PAF is mediated by LTC4. Br J Pharmacol. 1986;88:595–605. doi: 10.1111/j.1476-5381.1986.tb10240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley MK. Antiarrhythmic drug development: historical review and future perspectives. Drug Dev Res. 2002;55:3–16. [Google Scholar]
- Quistad GB, Fisher KJ, Oweb SC, Klintenberg R, Casida JE. Platelet-activating factor acetylhydrolase: selective inhibition by potent n-alkyl methylphosphonofluoridates. Toxicol Appl Pharmacol. 2005;205:149–156. doi: 10.1016/j.taap.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Specific IK1 blockade, a new antiarrhythmic mechanism? Effect of RP58866 on ventricular arrhythmias in rat, rabbit and primate. Circulation. 1993;87:1979–1989. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Further investigation into the mechanism of antifibrillatory action of the specific IK1 blocker, RP58866, assessed using the rat dual coronary perfusion model. J Mol Cell Cardiol. 1995;27:2579–2606. doi: 10.1006/jmcc.1995.0046. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Which cardiac potassium channel subtype is the preferable target for suppression of ventricular arrhythmias. Pharmacol Ther. 1996;69:199–217. doi: 10.1016/0163-7258(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Ridley PH, Yacoub MH, Curtis MJ. A Modified model of global ischaemia: application to the study of syncytial mechanisms of arrhythmogenesis. Cardiovasc Res. 1992;26:313–323. doi: 10.1093/cvr/26.4.309. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Genovese A, Levi R. negative inotropic effect of platelet activating factor on human myocardium: a pharmacological study. J Pharmacol Exp Therapeut. 1987;243:834–839. [PubMed] [Google Scholar]
- Rosen MR. Cardiac arrhythmias and anti-arrhythmic drugs: recent advances in our understanding of mechanisms. J Cardiovasc Electrophysiol. 1995;6:868–879. doi: 10.1111/j.1540-8167.1995.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi K, Curtis MJ. Influence of tedisamil on the initiation and maintenance of ventricular fibrillation: chemical defibrillation by Ito blockade. J Cardiovasc Pharmacol. 1991;18:455–456. doi: 10.1097/00005344-199109000-00018. [DOI] [PubMed] [Google Scholar]
- Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Whittaker M, Beauchamp CL, Bowles KS, Cackett MS, Christodoulou W, Galloway A, et al. BB-823, a PAF receptor antagonist with picomolar activity. Pharmacol Commun. 1992;1:251–257. [Google Scholar]
- Winkler K, Winkelmann BR, Scharnagl H, Hoffmann MM, Grawitz AB, Nauck M, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: The Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;111:980–987. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- Yamanaka S, Miura K, Yukimura T, Okumura M, Yamamoto K. Putative mechanism of hypotensive action of platelet activating factor in dogs. Circ Res. 1992;70:893–900. doi: 10.1161/01.res.70.5.893. [DOI] [PubMed] [Google Scholar]
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]