Abstract
Background and purpose:
Female sex hormones may protect pre-menopausal women from sudden cardiac death. We therefore investigated the effects of the main female sex hormone, 17β-estradiol, on ischaemia-induced cardiac arrhythmias and on the L-type Ca2+ current (ICaL).
Experimental approach:
In vivo experiments were performed in pentobarbital-anaesthetized rats subjected to acute coronary artery occlusion. ICaL was measured by the whole-cell patch-clamp technique, in rat isolated ventricular myocytes.
Key results:
Acute intravenous administration of 17β-estradiol as a bolus dose followed by a continuous infusion, commencing 10 min before coronary artery occlusion, had dose-dependent antiarrhythmic activity. In female rats 300 ng kg-1 + 30 ng kg−1 min−1 17β-estradiol significantly reduced the number of ventricular premature beats (VPBs) and the incidence of ventricular fibrillation (VF). A ten fold higher dose of 17β-estradiol was required to cause similar effects in male rats. In vitro 17β-estradiol reduced peak ICaL in a concentration-dependent manner. The EC50 was ten-fold higher in male myocytes (0.66 μM) than in females (0.06 μM).
Conclusions and implications:
These results indicate that 17β-estradiol has marked dose-dependent antiarrhythmic activity that is greater in female rats than in males. A similar differential potency in blocking ICaL in myocytes from female and male rats can account for this effect. This provides an explanation for the antiarrhythmic activity of 17β-estradiol and gender-selective protection against sudden cardiac death.
Keywords: arrhythmias, cardiac myocytes, coronary artery occlusion, estradiol, L-type calcium current, myocardial ischaemia
Introduction
When patients with heart disease die suddenly it is usually because of lethal arrhythmias, such as ventricular fibrillation (VF). Premenopausal women are much less susceptible to coronary heart disease and sudden cardiac death than men of a similar age (Welty, 2001; Wenger, 2002). After the menopause, however, the incidence of coronary heart disease in women increases such that by age 75 years it is similar to that in men (Rosano et al., 2001). In patients with coronary heart disease the risk of sudden death was twice as high in men than it was in women (Kannel et al., 1998). Such observations have led to the suggestion that female sex hormones, in particular estrogens, protect premenopausal women from sudden cardiac death (Welty, 2001).
17β-Estradiol has a number of actions which may be antiarrhythmic including both direct and indirect vasodilator activity (Kitazawa et al., 1997; Teoh et al., 1999). Although there is a lot of evidence to suggest that estrogens may reduce arrhythmias indirectly, for example, by reducing the severity of ischaemia as a consequence of vasodilation, the possibility of direct antiarrhythmic actions on cardiac myocytes cannot be excluded. Acute administration of 17β-estradiol reduced the slow inward Ca2+ current in guinea-pig isolated cardiac myocytes (Jiang et al., 1992) although this study only examined concentrations of 17β-estradiol far in excess of those that occur endogenously.
Ca2+ channel blockers have antiarrhythmic activity during acute myocardial ischaemia (Coker and Parratt, 1983; Curtis and Walker, 1988). Ischaemic damage can partially depolarize ventricular cells, thus inactivating the fast Na+ current, which results in the cells becoming dependent on L-type calcium current (ICaL) for the upstroke of their action potential. These cells may then act as ectopic pacemakers. Reduction of ICaL can prevent this abnormal automaticity (Waldo and Wit, 1993).
The first aim was therefore to examine whether acute administration of 17β-estradiol had antiarrhythmic activity in an established rat model of coronary artery occlusion and to measure the serum concentrations of 17β-estradiol. The second aim was to determine if a range of concentrations of 17β-estradiol had any actions on ICaL in single ventricular myocytes.
Methods
In vivo experiments
Animal preparation
Experiments were conducted in either male or female Wistar rats (Charles River, Margate, UK) within the weight range of 250–350 g. Animals were housed in rooms maintained at 20°C on a 12 h light/dark cycle with food and water available ad libitum. Rats were anaesthetized with sodium pentobarbital 60 mg kg−1, i.p., and prepared for coronary artery occlusion using the methods described previously (Clark et al., 1980; Barnes and Coker, 1995). All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986; under the authority of Project Licence number PPL 40/1702.
Briefly, the trachea, the carotid artery and the left femoral vein were cannulated to permit artificial ventilation, recording of arterial blood pressure and infusion of drug or vehicle, respectively. Needle electrodes were inserted so that a lead I ECG could be recorded. The arterial cannula was attached to a Bell and Howell Type 4-422 pressure transducer. The ECG and arterial blood pressure were recorded via Lectromed 5340 and 5240 preamplifiers connected to a Biopac MP30 data acquisition and analysis system or via Gould 6615–65 and 6615–30 amplifiers connected to a Po-Ne-Mah P3 data acquisition and analysis system. Body temperature was maintained at 38±1°C by means of a heated table and a rectal thermometer. A left thoracotomy was performed, at which point the rats were artificially ventilated with room air at a rate of 54 strokes min−1 and a stroke volume of 10–15 ml kg−1 to maintain arterial PO2 above 70 mm Hg. An incision was made in the pericardium to expose the heart and a fine silk ligature (6/0) attached to a 10 mm reverse cutting needle (Mersilk W812, Ethicon Ltd., Livingston, UK) was placed around the left anterior descending coronary artery close to its origin. A 10 min stabilization period followed, during which time arterial blood gases were measured and the ventilation volume adjusted if required.
Infusion protocol and coronary artery occlusion
Four separate studies were carried out. In each study, rats were assigned randomly to one of four groups (n=12 per group) which received either vehicle (2% ethanol in saline) or one of three different doses of 17β-estradiol administered as an intravenous bolus dose (0.33 ml kg−1) followed by a continuous infusion at a rate of 0.01 ml min−1. In the first study, male rats received either 30 ng kg−1+3 ng kg−1 min−1, 100 ng kg−1+10 ng kg−1 min−1 or 300 ng kg−1+30 ng kg−1min−1 17β-estradiol and in the second study the same doses were given to female rats. These doses were chosen with the aim of achieving serum concentrations of 17β-estradiol that would be approximately one, three and 10 times those found in female rats. The third and fourth studies looked at the effects of 300 ng kg−1+30 ng kg−1 min−1, 1000 ng kg−1+100 ng kg−1 min−1 or 3000 ng kg−1+300 ng kg−1 min−1 17β-estradiol in female and male rats, respectively. After 10 min of drug or vehicle infusion, the ligature was tied to occlude the coronary artery, resulting in regional myocardial ischaemia. Data were recorded for a further 25 min. The data from studies 1 and 4 (males) and 2 and 3 (females) have been combined resulting in n=24 for the groups that received vehicle or the middle dose of 17β-estradiol with n=12 for each of the other four doses of 17β-estradiol.
A 5 ml blood sample was collected at the end of each experiment, allowed to clot, then centrifuged for 10 min (MSE Centaur 2, London, UK, 3800 r.p.m.). The serum was collected and stored at −20°C until the time of analysis. 17β-Estradiol concentrations were determined using a double antibody estradiol radioimmunoassay kit (KE2D, Diagnostic Products Corporation, Llanberis, North Wales, UK). Inter-assay precision was <5% throughout the range of the assay.
Arrhythmia analysis and definition
The arrhythmias that occurred during the first 25 min of myocardial ischaemia were classified in accordance with the Lambeth Conventions (Walker et al., 1988). In all animals surviving 25 min of myocardial ischaemia the total number of ventricular premature beats (VPBs) occurring as singles, bigeminy and salvos, and those occurring as ventricular tachycardia (VT – defined as four or more VPBs) were counted. The incidences of VT, self-terminating VF and mortality, due to VF sustained for at least 3 min, were recorded.
Experiments were terminated and excluded from data analysis if any of the following occurred: arrhythmias before coronary artery occlusion; no evidence of ECG changes indicative of ischaemia after occlusion, that is no changes in ST segment or R wave amplitude, or arrhythmias; mean arterial blood pressure <60 mm Hg before drug/vehicle administration; heart failure during the first 5 min of ischaemia; blood gases outside of normal limits, that is PCO2 25–35 mm Hg and PO2 no less than 70 mm Hg. All animals that were excluded were replaced immediately.
In vitro experiments
Isolation of ventricular cardiac myocytes
Ventricular myocytes from male or female Wistar rats (250–340 g, Charles River, Margate, UK) were isolated by enzymatic digestion. The methods used for cell isolation have been described previously by Frampton et al. (1991). In brief, Wistar rats were killed by stunning followed by cervical dislocation. The heart was removed and Langendorff-perfused for 2–3 min with HEPES-buffered physiological Tyrode solution containing 750 μM Ca2+ (see below for composition), followed by perfusion with Ca2+-free HEPES-buffered physiological Tyrode solution supplemented with 0.1 mM EGTA for 5 min. Finally, the heart was perfused for 7–8 min with HEPES-buffered physiological Tyrode solution containing collagenase (0.8–0.9 mg ml−1; Worthington Type I, Lorne Laboratories, Reading, UK) and protease (0.05 mg ml−1; Type XIV, Sigma, Poole, UK). The left ventricle was removed, opened and gently agitated while being gassed with 100% O2 at 37°C in the enzyme solution supplemented with 1% bovine serum albumin (Sigma). Myocytes were harvested by filtering the digest through a piece of fine muslin gauze at 5 min intervals over a period of 25 min, so that a total of five batches of myocytes were obtained from each isolation. Myocytes were collected by centrifugation of the filtrate for 40 s at 400 r.p.m., then resuspended in 750 μM Ca2+ solution, transferred to Petri dishes and stored at 4±1°C until use, usually within 6–8 h of isolation. Only myocytes that had a well-defined rectangular shape and showed clear cross-striations were used for recording ionic currents. All experiments were carried out at 35±1°C.
Electrophysiological recordings
Patch-clamp experiments were performed in the conventional whole-cell configuration. Patch pipettes were pulled from filamented borosilicate glass capillaries (type GC150TF-15, Harvard Apparatus) using a microelectrode puller (PP-830, Narishige International Ltd, London, UK). Pipettes were fire polished using a Microforge (MF830, Narishige International Ltd.) to give a resistance of 3–5 MΩ when filled with the internal pipette solution (see solutions and drugs section for composition). Myocytes were layered and allowed to settle on to a coverslip, which formed the base of the heated perfusion chamber situated on the stage of an inverted microscope (Diaphot, Nikon, Kingston, UK). Cells were superfused with external physiological salt solution at a flow rate of 1–2 ml min−1 under gravity. Membrane currents were recorded using the Axopatch 200 patch clamp amplifier (Axon Instruments, Foster, CA, USA). Analogue signals (current and voltage) were digitised by an A/D converter (TL-1 direct memory access (DMA) interface, Axon Instruments) and stored on-line to a computer hard disk for subsequent off-line analysis using pCLAMP 8.1, clampfit software (Axon Instruments). Voltage clamp protocols were applied using the pCLAMP 6.0 software (Axon Instruments).
Experimental protocols
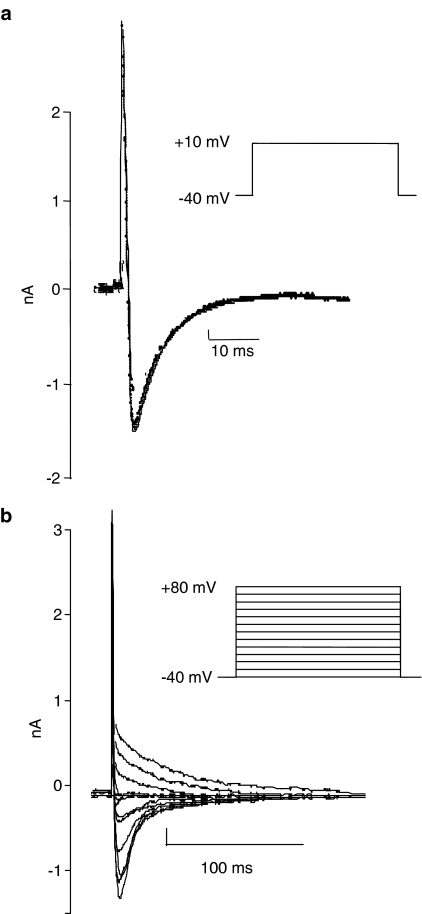
ICaL measurements were elicited in voltage clamp mode. Cells were held at −40 mV to inactivate the rapid inward Na+ current. Membrane currents were acquired at 5 kHz and cells were stimulated at 0.5 Hz. To record ICaL, a depolarization step to +10 mV (peak Ca2+ currents are elicited between 0 and +10 mV) was applied for 300 ms before returning to the holding potential of −40 mV (Figure 1a). To obtain data for the current–voltage (IV) relationship, cells were depolarised from −40 to +80 mV in steps of 10 mV increments for 700 ms (Figure 1b).
Figure 1.
The square wave pulse protocols used to elicit (a) ICaL at +10 mV and (b) current–voltage curves for ICaL. The lower traces in (a) and (b) show the corresponding current recordings.
The following concentrations of 17β-estradiol were tested, in male rat myocytes 0.1, 1, 10, 30 or 100 μM 17β-estradiol, and in female rat myocytes 0.001, 0.01, 0.1, 1, 10, 30 or 100 μM 17β-estradiol. Vehicle (0.2% ethanol in external physiological salt solution) was applied to cells and left for 3 min after which time the peak ICaL and the IV relationship for ICaL were recorded. A concentration of 17β-estradiol was then applied to the cell and left for 3 min after which time the peak ICaL and the IV relationship for ICaL in the presence of 17β-estradiol were recorded. The solution was finally reverted back to vehicle alone and after a further 3 min washout values were recorded.
Experiments were conducted until n⩾9 cells, per concentration of drug, for both male and female rat myocytes had been achieved. Only one concentration of drug or vehicle was tested per cell and myocytes from at least three rats were used to acquire data for each drug concentration. Concentrations of 10 and 30 μM were examined first, as these had been reported to be effective previously in guinea-pig myocytes (Jiang et al., 1992) then further concentrations were examined to explore the full concentration–response relationship.
Experimental analysis
Measurements of the ICaL were made by placement of on screen cursors at the peak of the elicited current and at the end of the current during the pulse. Peak minus the end current was obtained by setting the current at the end of the pulse to zero. Data were normalized for cell capacitance and expressed as pA/pF.
Drugs
17β-Estradiol (Sigma, Poole, UK) was dissolved in 100% ethanol (BDH, Poole, UK) and diluted down with 0.9% w/v NaCl solution so that the final percentage of ethanol injected into the animal did not exceed 2% in the in vivo studies. For the in vitro experiments a 10 mM stock solution of 17β-estradiol was made up in 20% ethanol and external physiological salt solution. This was then diluted down so that the final percentage of ethanol in the external solution did not exceed 0.2%.
Electrophysiology solutions
All the following chemical concentrations are in millimolar unless stated otherwise. The HEPES-buffered Tyrode solution was prepared fresh each day and contained NaCl 130; KCl 5.4; MgCl2 1.4; NaH2PO4 0.4; creatine 10; taurine 20; glucose 10; HEPES 10; CaCl2 0.75 and titrated to pH 7.3 with 1 M NaOH. The Ca2+-free solution contained no added calcium and 0.1 mM EGTA. The enzyme solution contained 50 μM CaCl2 plus collagenase (0.8–0.9 mg ml−1; Worthington, Reading, UK) and protease (0.05 mg ml−1; Sigma). The external physiological salt solution was composed of NaCl 140; KCl 5.4; MgCl2 1, CaCl2 1; glucose 10; HEPES 10 and titrated to pH 7.35 with 1 M TEA OH. The internal pipette solution contained KCl 20; Cs glutamate 120 (prepared from equimolar L-glutamic acid and CsOH); MgCl2 5; Na2ATP 5; EGTA 1, HEPES 10 and titrated to pH 7.25 with CsOH.
Statistical analysis
Where appropriate, values are expressed as mean±standard error (s.e.) mean of n observations. All data were tested for normal distribution using Shapiro-Wilk tests. For data that were not distributed normally, nonparametric tests were used. Between group differences in haemodynamics, blood gas values, 17β-estradiol concentrations and the number of VPBs were compared by Kruskal Wallis tests. Fisher's exact test was used to compare incidences of VT, VF and mortality between groups. The Mann–Whitney U test was used to compare 17β-estradiol concentrations between male and female rats. For data that were distributed normally parametric tests were used. Pre- and postdrug comparisons measured in the same cells for the peak ICaL and IV relationship data were compared using Student's 2-tailed paired t-test. The percentage changes in the peak ICaL between different concentrations of drug were compared with one-way ANOVA using Dunnett's method for multiple comparisons with a control group. Comparisons between male and female data were made using Student's 2-tailed unpaired t-test. A probability value of P<0.05 was considered to be statistically significant.
Results
Effects of 17β-estradiol on ischaemia-induced arrhythmias
The induction of acute myocardial ischaemia by coronary artery occlusion, in vehicle group rats, resulted in the occurrence of arrhythmias, which varied in severity from single VPBs to lethal VF. The onset of arrhythmias occurred between 4 and 8 min postocclusion and arrhythmias persisted for up to 15 min. After 20 min normal sinus rhythm returned and remained in all animals surviving the 25 min period of myocardial ischaemia.
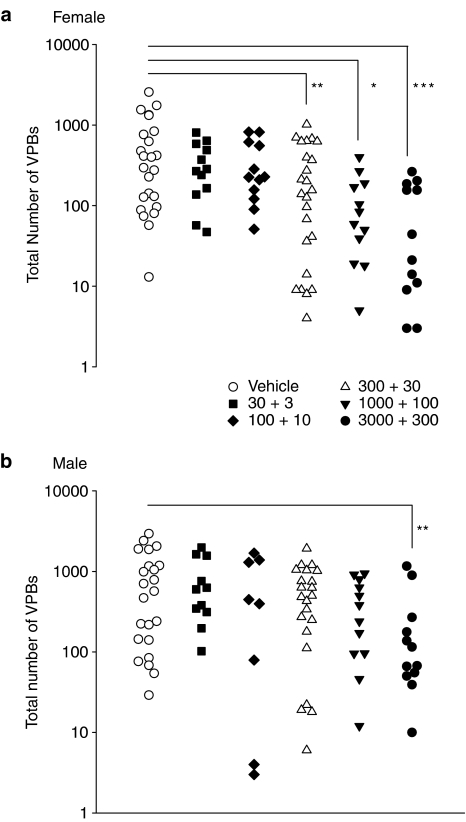
Administration of 17β-estradiol reduced the total number of VPBs in a dose-dependent manner (Figure 2). This effect was greater in female rats where doses of 300 ng kg−1+30 ng kg−1 min−1 and above significantly reduced the total number of VPBs. A significant effect on the total number of VPBs was not observed in male rats until a dose of 3000 ng kg−1+300 ng kg−1 min−1 was used. 17β-Estradiol suppressed the VPBs that occurred as singles, bigeminy or salvos to a similar extent to those that occurred as VT. For both of these subcategories, significant effects were seen in female rats with doses of 300 ng kg−1+30 ng kg−1 min−1 and above whereas in male rats only the highest dose of 17β-estradiol had significant effects.
Figure 2.
The effects of vehicle and five doses of 17β-estradiol (expressed as bolus dose in ng kg−1+infusion rate in ng kg−1 min−1) on the total number of ventricular premature beats (VPBs) in each individual anaesthetized (a) female and (b) male rat which survived the 25 min period of myocardial ischaemia. *P<0.05, **P<0.01, ***P<0.001, compared to vehicle, Kruskal Wallis test.
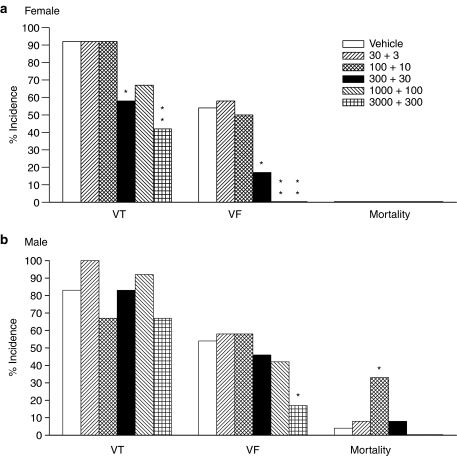
Figure 3 illustrates the effects of 17β-estradiol on the incidences of VT, VF and mortality due to sustained VF in male and female rats. Again, the data from female rats clearly showed significant dose dependent reductions in the incidences of VT and VF at doses above 300 ng kg−1+30 ng kg−1 min−1 17β-estradiol. Doses of 1000 ng kg−1+100 ng kg−1 min−1 and above eradicated VF completely. No mortality was observed in any of the female rats used in this study. The data from male rats showed that 17β-estradiol exerted no effect on the incidence of VT, however, the incidence of VF was significantly reduced with the highest dose of 17β-estradiol. Mortality was absent only in male rats that received the two highest doses of 17β-estradiol, however, this was not significantly different from the vehicle group. There was a statistically significant increase in mortality in the group that received the second dose of 17β-estradiol, compared to the vehicle group, but the biological significance of this finding is not clear. Overall, the male data show a 10-fold difference in the dose of 17β-estradiol needed to exert a significant reduction in the number of VPBs and the incidence of VF, compared to female data.
Figure 3.
The effects of vehicle and five doses of 17β-estradiol (expressed as bolus dose in ng kg−1+infusion rate in ng kg−1 min−1) on the incidences of ventricular tachycardia (VT), ventricular fibrillation (VF) and mortality (due to sustained VF) in anaesthetized (a) female and (b) male rats. *P<0.05, **P<0.01 compared to vehicle, Fisher's exact test.
No significant effects of 17β-estradiol were observed on heart rate, systolic, diastolic, or mean arterial blood pressures, blood gas data or pH values in rats receiving hormone compared to vehicle-treated rats of either sex. Mean arterial blood pressure data are detailed in Table 1. Baseline heart rates were 400±8 and 408±10 beats min−1 in female and male vehicle group rats, respectively. Similar heart rates were recorded in the other groups and no significant changes occurred during the course of the experiments. PCO2, PO2 and pH values were 28.9±0.6 mm Hg, 86.4±2.0 mm Hg and 7.48±0.01 U for the female vehicle group. Male data were very similar to the female data and values did not change significantly during the experiments.
Table 1.
Mean±s.e. mean values of mean arterial blood pressure (mm Hg) for male and female rats
|
Time in min pre/postocclusion |
|||||||
|---|---|---|---|---|---|---|---|
| n | −11 | −9 | −1 | 1 | 5 | 25 | |
| Females | |||||||
| Vehicle | 24 | 82±4 | 76±4 | 89±4 | 65±2 | 70±4 | 72±5 |
| 30+3 | 12 | 80±6 | 73±5 | 93±6 | 62±4 | 57±5 | 66±5 |
| 100+10 | 12 | 75±5 | 69±5 | 85±6 | 60±5 | 62±5 | 86±7 |
| 300+30 | 24 | 81±4 | 76±4 | 88±4 | 68±3 | 74±5 | 75±5 |
| 1000+100 | 12 | 78±5 | 75±5 | 84±6 | 64±5 | 70±7 | 72±8 |
| 3000+300 | 12 | 78±7 | 75±6 | 84±6 | 64±4 | 70±6 | 67±6 |
| Males | |||||||
| Vehicle | 24 | 83±4 | 76±3 | 88±4 | 71±3 | 75±4 | 83±5 |
| 30+3 | 12 | 86±6 | 75±5 | 87±5 | 69±3 | 68±6 | 76±6 |
| 100+10 | 12 | 84±6 | 76±6 | 88±5 | 69±4 | 67±5 | 83±9 |
| 300+30 | 24 | 86±4 | 78±4 | 94±4 | 73±3 | 76±6 | 90±4 |
| 1000+100 | 12 | 81±5 | 77±5 | 88±5 | 77±4 | 81±5 | 88±6 |
| 3000+300 | 12 | 85±4 | 80±4 | 96±4 | 78±3 | 86±4 | 87±6 |
Values after 25 min of ischemia are in surviving animals only. Doses are expressed as bolus+infusion in ng kg−1+ng kg−1 min–1.
17β-Estradiol measurements
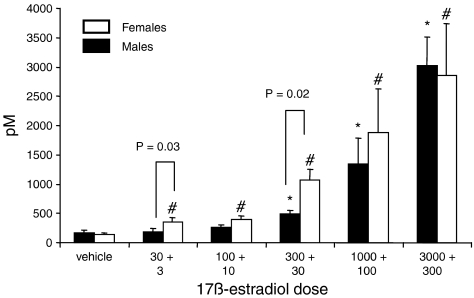
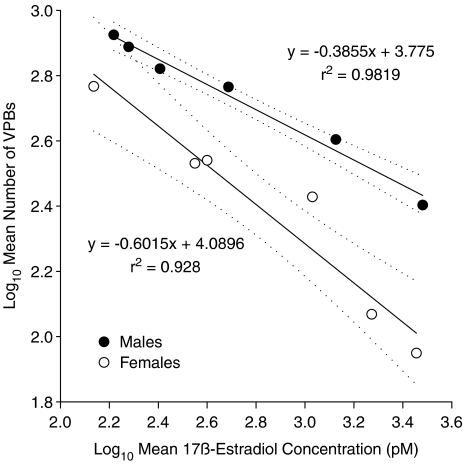
In both sexes, increasing the dose of 17β-estradiol dose-dependently increased the serum concentrations of 17β-estradiol (Figure 4). In female rats, all concentrations were significantly higher than the vehicle group, whereas in males only doses above 300 ng kg−1+30 ng kg−1 min−1 increased the serum concentrations significantly compared to vehicle. There were strong correlations between the mean number of VPBs in each group and the measured mean serum 17β-estradiol concentrations, plotted on logarithmic scales (Figure 5). This graph also revealed a significant difference in the slopes of the regression lines.
Figure 4.
Mean±s.e. mean values for serum 17β-estradiol concentrations for vehicle and five 17β-estradiol dosed groups (doses expressed as bolus+infusion in ng kg−1+ng kg−1 min−1, respectively) in male and female rats. Sample numbers in each group range from 6 to 23 as it was not always possible to obtain a blood sample from each rat. *P<0.05 compared to the male vehicle group, #P<0.05 compared to the female vehicle group, Kruskal Wallis test. P-values indicated on the histogram for male/female comparisons were obtained using the Mann–Whitney U test.
Figure 5.
Correlations between the log of the mean number of ventricular premature beats (VPBs) in each group vs the log of the measured mean serum concentrations of 17β-estradiol in male and female rats. Dotted lines indicate the 95% confidence intervals for the regression lines. An unpaired t-test (assuming unequal variances) indicated that the slopes of the regression lines were significantly different (P=0.036).
In vitro experiments
In total, 75 cells from 22 female rats and 67 cells from 17 male rats were used in this study. The mean weight of the female rats was 284±4 g and that of the male rats 294±4 g. The mean series resistance for females and males was 9.3±0.5 and 10.5±0.5 MΩ, respectively, and the mean cell capacitance was 171±4.6 pF in females and 180±4.5 pF in males.
In this study, it was observed that the vehicle (0.2% ethanol) had no effect in either sex on the ICaL elicited at +10 mV (in males −6.84±0.66 pA/pF in external physiological salt solution vs –6.81±0.69 pA/pF in vehicle; in females, −5.02±1.05 pA/pF in external physiological salt solution vs −4.71±0.75 pA/pF in vehicle). The IV relationship was not altered by the vehicle. The baseline ICaL at +10 mV was significantly lower in females compared to males (P=0.001), indicating the possibility that the ICaL density could be lower in female rats.
Effects of 17β-estradiol on ICaL and IV relationship in myocytes from female and male rats
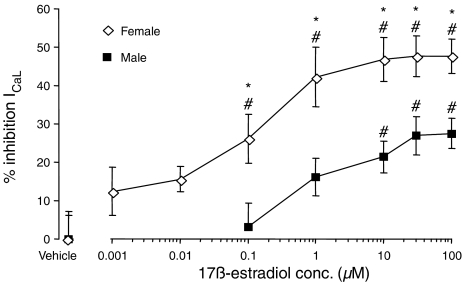
In the experiments carried out in female ventricular myocytes, concentrations of 0.1 μM. 17β-Estradiol and above had a pronounced, and significant, concentration-dependent inhibitory effect on the ICaL elicited at +10 mV (Figure 6). The inhibitory effect reached a plateau at 10 μM 17β-estradiol, so that the maximum inhibition recorded at 30 μM 17β-estradiol was 48±5%. In male ventricular myocytes, concentrations above 10 μM 17β-estradiol significantly inhibited the amplitude of ICaL at +10 mV (Figure 6). ICaL was decreased by 17β-estradiol, again, in a concentration-dependent manner. This effect reached a plateau between 30 and 100 μM, so that the maximum percentage inhibition observed was 28±4% at 100 μM 17β-estradiol in myocytes from male rats.
Figure 6.
The effects of acute administration of 17β-estradiol or vehicle (0.2% ethanol) on the L-type Ca2+ current (ICaL) elicited at +10 mV, in male and female ventricular myocytes. *P<0.05 compared to the respective 17β-estradiol concentration in male myocytes (Student's unpaired t-test). #P<0.05 compared to the respective gender vehicle group (one way ANOVA, using Dunnett's method for multiple comparisons with a control).
When the percentage inhibition of ICaL at +10 mV was compared between male and female myocytes it was evident that at all concentrations of 17β-estradiol the percentage inhibition was greater in female than male myocytes (Figure 6). The degree of inhibition of the peak ICaL did not reach 100% in either sex. Virtually no inhibition of the peak ICaL was observed with 0.1 μM 17β-estradiol in male myocytes whereas a significant inhibition of 26±6% was recorded in female myocytes. The concentrations of 17β-estradiol that produced a 50% reduction of the maximum effect of the hormone exerted in either sex on ICaL at +10 mV (EC50 values) were 0.66 μM in male myocytes and 0.06 μM in female myocytes. Thus, there was a 10-fold difference in the concentration of 17β-estradiol, needed to reduce the ICaL by 50%, between male and female myocytes.
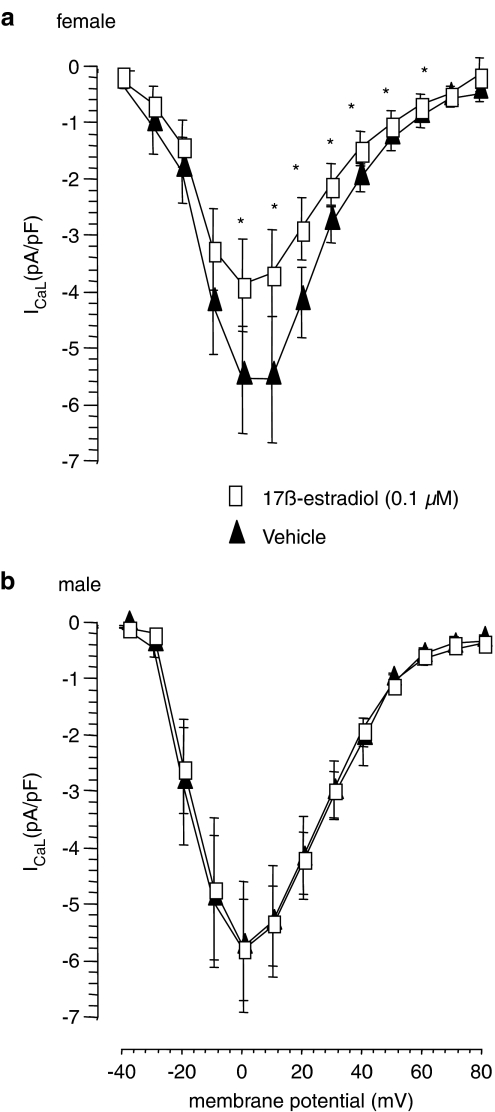
In myocytes from male rats, at concentrations above 1 μM, 17β-estradiol produced a concentration-dependent reduction of the IV relationship without altering the typical bell shape of the IV curve for ICaL obtained in the presence of vehicle alone. The lower concentration of 0.1 μM 17β-estradiol exerted no effect on the IV curve in male myocytes (Figure 7). The averaged reversal potential, obtained from the male vehicle data was 60.5±0.6 mV. 17β-Estradiol also produced a concentration-dependent reduction of the IV relationship in myocytes from female rats but the effect was greater. A significant reduction in current amplitude was achieved at concentrations above 0.01 μM 17β-estradiol and a significant reduction in the IV curve was observed over the voltage range of 0 to +60 mV with 0.1 μM 17β-estradiol (Figure 7). The averaged reversal potential, obtained from the female vehicle data was 60.4±0.9 mV. The maximum current density was recorded at 0 mV in all groups. The observed effects of 17β-estradiol on ICaL in both male and female myocytes are considered to be true effects of the hormone as the inhibitory effect of the sex hormone was reversed after a 3 min washout period with vehicle.
Figure 7.
Effects of vehicle or 0.1 μM 17β-estradiol on the IV relationship for ICaL in (a) female and (b) male rat ventricular myocytes. *P<0.05 compared to vehicle (Student's paired t-test).
Discussion
This is the first report of the effects of acute administration of a wide range of doses of 17β-estradiol in both male and female animals on ischaemia-induced arrhythmias, with parallel concentration–response studies on ICaL in vitro. The major findings from these studies are that 17β-estradiol had antiarrhythmic activity and reduced ICaL. Approximately 10-fold less hormone was required to produce these effects in female rats than in male rats. These actions of 17β-estradiol were dose-dependent and occurred rapidly, within minutes, both in vitro and in vivo.
Antiarrhythmic activity of 17β-estradiol
The antiarrhythmic activity was particularly prominent in female rats, with the three highest doses of 17β-estradiol all significantly reducing the numbers of VPBs and the incidence of the potentially life-threatening arrhythmias, VT and VF. In fact, VF was abolished completely by the two highest doses of 17β-estradiol and there was no mortality due to sustained VF in any of the female rats. This contrasts with the findings in male rats, where only the highest dose of 17β-estradiol significantly reduced VF and some rats in the control and lower dose groups died because of sustained VF.
Previously, it has been shown that a single intravenous dose of 100 μg of conjugated equine estrogens (McHugh et al., 1995) or intracoronary infusion of 17β-estradiol (Node et al., 1997) reduced ischaemia-induced arrhythmias in anaesthetized dogs. Another study in anaesthetized dogs suggested that a bolus dose of 10 μg kg−1 of 17β-estradiol resulted in a trend of more animals surviving ischaemia and reperfusion, but it is difficult to interpret the effects on arrhythmias in this study as animals with intractable VF were excluded from the analysis (Lee et al., 2000). Other work has focused on the effects of estrogens on reperfusion-induced arrhythmias rather than those observed during ischaemia (Node et al., 1997; McHugh et al., 1998; Tsai et al., 2002). Our studies in anaesthetized rats clearly show a dose-dependent antiarrhythmic effect of 17β-estradiol during acute myocardial ischaemia in anaesthetized rats.
Initially two separate studies were carried out with the three lower doses of 17β-estradiol in female and male rats. Statistical analysis of the data from these experiments, where there were 12 rats in each group, showed no significant effect of 17β-estradiol on either the number or severity of ischaemia-induced arrhythmias. However, the apparent effects of the highest dose of 17β-estradiol in the female rats only just failed to reach statistical significance. Power calculations suggested that the observed effects would be significant if n was ⩾19. It was therefore decided that two further randomized studies should be performed in which the control group and the highest dose of 17β-estradiol examined previously (300 ng kg−1+30 ng kg−1 min−1) were repeated and two additional higher doses of 17β-estradiol were included. This second pair of studies showed clearly that all three of the higher doses of 17β-estradiol had significant antiarrhythmic activity in female rats. When the data were combined (see Figures 2 and 3) a significant effect of the highest dose of 17β-estradiol in male rats was also revealed.
Concentrations of 17β-estradiol
Although the concentrations that caused significant Ca2+ channel blockade in vitro (100 nM in female cells) were not identical to those measured in serum at the end of the in vivo experiments (∼1 nM in female rats) there are very good parallels between the in vivo and in vitro experiments. The EC50 for 17β-estradiol to reduce ICaL was 10-fold lower in cells from female rats (0.06 μM) than in cells from male rats (0.66 μM). Similarly, in vivo, significant antiarrhythmic activity was achieved with a 10-fold lower dose of 17β-estradiol in female rats than in male rats. It should be noted that the serum measurements of 17β-estradiol may not actually reflect the concentration of 17β-estradiol in the heart at the onset time of the arrhythmic episode. Previously, it has been reported that 17β-estradiol concentrations in the heart and other tissues were consistently higher than plasma concentrations after intravenous administration (Schleicher et al., 1998). Also, the blood samples were taken at the end of the experiments rather than at the time of peak arrhythmic activity. Perhaps most relevant of all is the observation that isoproterenol-stimulated ICaL in guinea-pig ventricular myocytes was much more susceptible to inhibition by 17β-estradiol than basal ICaL, with 1 nM 17β-estradiol having an effect on the isoproterenol-stimulated current (Meyer et al., 1998). In our in vivo experiments the hearts of the anaesthetized rats would have been subject to a certain degree of sympathetic tone whereas in vitro any influence of circulating catecholamines would have been removed by the cell isolation procedure. Thus, 17β-estradiol would have greater calcium channel blocking activity in vivo. These factors may explain the discrepancy between 17β-estradiol concentrations measured in vivo and those required to produce significant effects in vitro.
Sex differences in the action of 17β-estradiol
It was originally thought that the reason for the gender difference in the dose of 17β-estradiol required to exert an antiarrhythmic action in rats could be due to the fact that the female rats used in this study had their ovaries left intact. Thus, any treatment with 17β-estradiol would be supplementary to the naturally occurring 17β-estradiol already present in the female rat. However, the data presented in Figure 4 showed clearly that there was no significant difference in the serum 17β-estradiol concentrations between male and female control rats. This finding is in agreement with a previous report that plasma concentrations of 17β-estradiol were similar in male rats and female rats in di-oestrous (Shaw et al., 2001). Although serum 17β-estradiol concentrations were higher in female rats than in male rats after administration of two of the doses of 17β-estradiol, no differences between males and females were seen in the other four groups. It is therefore unlikely that the greater antiarrhythmic activity in female rats than in male rats was due to higher concentrations of 17β-estradiol and suggests that the effectiveness of 17β-estradiol differs between the sexes. The difference in the slopes of the regression lines for the correlations between the numbers of VPBs and the serum concentrations of 17β-estradiol (see Figure 5) supports this conclusion.
Comparison of the baseline data from all the cells used for the in vitro experiments revealed that the density of ICaL was lower in cells from female rats than in those from males. It is therefore possible that the inhibitory effect of 17β-estradiol was greater in the female cells because the current density was already lower. However, 17β-estradiol did not inhibit ICaL completely in either male or female cells, with the maximum effect being approximately 50% inhibition of the current in myocytes from female rats. As the effect reached a plateau before all the channels were affected it seems unlikely that the difference in ICaL density between male and female myocytes can account for the greater effect of 17β-estradiol in females.
In previous work on the action of 17β-estradiol on ICaL in cardiac myocytes, only cells from male guinea pigs were studied (Jiang et al., 1992) or the inhibition of the current was reported to be independent of sex (Meyer et al., 1998). However, Ca2+ entry into vascular smooth muscle cells was inhibited by 17β-estradiol to a much greater extent in cells from female rats than in those from male rats (Crews and Khalil, 1999). In addition, 17β-estradiol had a greater effect on calcium channel-dependent vasodilation in female rat hearts than in male rat hearts (Hugel et al., 1999).
Mechanism of action
The rapidity of the effects of 17β-estradiol on ICaL and on ischaemia-induced arrhythmias indicates that these actions are unlikely to be mediated via interaction with classical intracellular estrogen receptors (ERα or ERβ). The present study was not designed to investigate whether 17β-estradiol interacts directly with L-type Ca2+ channels or via some other estrogen ‘receptor' located on the plasma membrane which may act through a number of signal transduction pathways (Nadal et al., 2000, 2001; Kelly and Levin, 2001).
Estrogens have many actions that could influence arrhythmias induced by myocardial ischaemia. For example, the vasodilator action of 17β-estradiol has been well documented (Kitazawa et al., 1997; Teoh et al., 1999) but the lack of effect on arterial blood pressure in the present study (see Table 1) suggests that this is unlikely to be of relevance here. The present data do indicate, however, that blockade of L-type Ca2+ channels by 17β-estradiol could account for the observed antiarrhythmic actions. Ca2+ channels blockers have been shown to be antiarrhythmic in models of myocardial ischaemia (Coker and Parratt, 1983; Curtis and Walker, 1988) including the anaesthetized rat (Curtis et al., 1984).
Limitations of the studies
In the above experiments, we have only examined the effects of acute administration of pharmacological doses of 17β-estradiol to intact rats. Further studies on the effects of chronic administration of estradiol to ovariectomized female rats are necessary to determine whether physiological concentrations of estradiol have similar actions. Although clear effects on ICaL were seen, estradiol may also have actions on other ion channels that could influence ischaemia-induced arrhythmias. Effects on the delayed rectifier K+ current (IKr) are unlikely to be relevant as rats do not express functional IKr and IKr blockers do not alter ischaemia-induced arrhythmias in rats (Rees and Curtis, 1993) but actions on other ionic currents cannot be excluded.
Conclusions
The experiments described above demonstrate clearly that 17β-estradiol has dose-dependent antiarrhythmic actions in anaesthetized rats subject to coronary artery occlusion, with greater efficacy in females than in males. Complementary studies in isolated ventricular myocytes indicate that gender-selective, concentration-dependent inhibition of ICaL is sufficient to account for the reduction in ischaemia-induced arrhythmias.
Acknowledgments
This work was funded by the British Heart Foundation (FS/99060).
Abbreviations
- ICaL
L-type calcium current
- IV
current–voltage
- VPBs
ventricular premature beats
- VT
ventricular tachycardia
- VF
ventricular fibrillation
Conflict of interest
The authors state no conflict of interest.
References
- Barnes CS, Coker SJ. Failure of nitric-oxide donors to alter arrhythmias induced by acute myocardial-ischaemia or reperfusion in anaesthetized rats. Br J Pharmacol. 1995;114:349–356. doi: 10.1111/j.1476-5381.1995.tb13233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Foreman MI, Kane KA, McDonald FM, Parratt JR. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J Pharmacol Methods. 1980;3:357–368. doi: 10.1016/0160-5402(80)90077-7. [DOI] [PubMed] [Google Scholar]
- Coker SJ, Parratt JR. Nifedipine reduces arrhythmias but does not alter prostanoid release during coronary-artery occlusion and reperfusion in anaesthetized greyhounds. J Cardiovasc Pharmacol. 1983;5:406–417. doi: 10.1097/00005344-198305000-00010. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26:707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, MacLeod BA, Walker MJ. Antiarrhythmic actions of verapamil against ischaemic arrhythmias in the rat. Br J Pharmacol. 1984;83:373–385. doi: 10.1111/j.1476-5381.1984.tb16497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Walker MJ. The mechanism of action of calcium antagonists on arrhythmias in early myocardial ischaemia: studies with nifedipine and DHM9. Br J Pharmacol. 1988;94:1275–1286. doi: 10.1111/j.1476-5381.1988.tb11648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE, Harrison SM, Boyett MR, Orchard CH. Ca2+ and Na+ in rat myocytes showing different force-frequency relationships. Am J Physiol Cell Physiol. 1991;261:C739–C750. doi: 10.1152/ajpcell.1991.261.5.C739. [DOI] [PubMed] [Google Scholar]
- Hugel S, Neubauer S, Lie SZ, Ernst R, Horn M, Schmidt HH, et al. Multiple mechanisms are involved in the acute vasodilatory effect of 17beta-estradiol in the isolated perfused rat heart. J Cardiovasc Pharmacol. 1999;33:852–858. doi: 10.1097/00005344-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Jiang C, Poole-Wilson PA, Sarrel PM, Mochizuki S, Collins P, MacLeod KT. Effect of 17 beta-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br J Pharmacol. 1992;106:739–745. doi: 10.1111/j.1476-5381.1992.tb14403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Wilson PWF, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK.Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle J Physiol 1997499497–511.Part 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. Cardioprotective effects of 17 beta-estradiol produced by activation of mitochondrial ATP-sensitive K+channels in canine hearts. J Mol Cell Cardiol. 2000;32:1147–1158. doi: 10.1006/jmcc.2000.1167. [DOI] [PubMed] [Google Scholar]
- McHugh NA, Cook SM, Schairer JL, Bidgoli MM, Merrill GF. Ischemia- and reperfusion-induced ventricular arrhythmias in dogs: effects of estrogen. Am J Physiol. 1995;268:H2569–H2573. doi: 10.1152/ajpheart.1995.268.6.H2569. [DOI] [PubMed] [Google Scholar]
- McHugh NA, Merrill GF, Powell SR. Estrogen diminishes postischemic hydroxyl radical production. Am J Physiol. 1998;274:H1950–H1954. doi: 10.1152/ajpheart.1998.274.6.H1950. [DOI] [PubMed] [Google Scholar]
- Meyer R, Linz KW, Surges R, Meinardus S, Vees J, Hoffmann A, et al. Rapid modulation of L-type calcium current by acutely applied estrogens in isolated cardiac myocytes from human, guinea-pig and rat. Exp Physiol. 1998;83:305–321. doi: 10.1113/expphysiol.1998.sp004115. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Fuentes E, Soria B. The plasma membrane estrogen receptor: nuclear or unclear. Trends Pharmacol Sci. 2001;22:597–599. doi: 10.1016/s0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96:1953–1963. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Selective IK blockade as an antiarrhythmic mechanism: effects of UK66,914 on ischaemia and reperfusion arrhythmias in rat and rabbit hearts. Br J Pharmacol. 1993;108:139–145. doi: 10.1111/j.1476-5381.1993.tb13453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano GMC, Simon T, Mercuro G, Sans S, Schenck-Gustaffson K, Stevenson JC, et al. Hormone replacement therapy: where we stand in Europe. Eur Heart J. 2001;22:439–441. doi: 10.1053/euhj.2000.2298. [DOI] [PubMed] [Google Scholar]
- Schleicher F, Tauber U, Louton T, Schunack W. Tissue distribution of sex steroids: concentration of 17beta-oestradiol and cyproterone acetate in selected organs of female Wistar rats. Pharmacol Toxicol. 1998;82:34–39. doi: 10.1111/j.1600-0773.1998.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Shaw L, Taggart M, Austin C. Effects of the oestrous cycle and gender on acute vasodilatory responses of isolated pressurized rat mesenteric arteries to 17β-oestradiol. Br J Pharmacol. 2001;132:1055–1062. doi: 10.1038/sj.bjp.0703908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H, Leung SWS, Man RYK. Short-term exposure to physiological levels of 17[beta]-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- Tsai C-H, Su S-F, Chou T-F, Lee T-M. Differential effects of sarcolemmal and mitochondrial KATP channels activated by 17β-estradiol on reperfusion arrhythmias and infarct sizes in canine hearts. J Pharmacol Exp Ther. 2002;301:234–240. doi: 10.1124/jpet.301.1.234. [DOI] [PubMed] [Google Scholar]
- Waldo AL, Wit AL. Mechanisms of cardiac arrhythmias. Lancet. 1993;341:1189–1193. doi: 10.1016/0140-6736(93)91012-b. [DOI] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth Conventions – Guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Welty FK. Women and cardiovascular risk. Am J Cardiol. 2001;88:48–52. doi: 10.1016/s0002-9149(01)01884-7. [DOI] [PubMed] [Google Scholar]
- Wenger NK. Clinical characteristics of coronary heart disease in women: emphasis on gender differences. Cardiovasc Res. 2002;53:558–567. doi: 10.1016/s0008-6363(01)00511-9. [DOI] [PubMed] [Google Scholar]