Abstract
Background and purpose:
Mexiletine (Mex), an orally effective antiarrhythmic agent used to treat ventricular arrhythmias, has also been found to be effective for myotonia and neuropathic pain. It is extensively metabolized in humans but little information exists about the pharmacodynamic properties of its metabolites.
Experimental approach:
To determine their contribution to the clinical activity of Mex, p-hydroxy-mexiletine (PHM), hydroxy-methyl-mexiletine (HMM), N-hydroxy-mexiletine (NHM) (phase I reaction products) and N-carbonyloxy β-D-glucuronide (NMG) (phase II reaction product) were tested on sodium currents (INa) of frog skeletal muscle fibres. Sodium currents were elicited with depolarizing pulses from different holding potentials (HP=−140, −100, −70 mV) and stimulation frequencies (0.25, 0.5, 1, 2, 5, 10 Hz) using the vaseline-gap voltage-clamp method.
Key results:
All the hydroxylated derivatives blocked the sodium channel in a voltage- and use-dependent manner. The PHM, HMM and NHM metabolites were up to 10-fold less effective than the parent compound. However, HMM showed a greater use-dependent behaviour (10 Hz), compared to Mex and the other metabolites. Similar to Mex, these products behaved as inactivating channel blockers. Conjugation with glucuronic acid (NMG) resulted in almost complete abolition of the pharmacological activity of the parent compound.
Conclusions and Implications:
Thus, although less potent, the phase I metabolites tested demonstrated similar pharmacological behaviour to Mex and might contribute to its clinical profile.
Keywords: skeletal muscle, sodium channel, antiarrhythmics, mexiletine, inactivated channel blockers, metabolism, metabolites, pharmacokinetic, pharmacodynamic, use-dependent block
Introduction
Mexiletine (Mex), an orally effective antiarrhythmic agent with use-dependent sodium channel-blocking properties, is used for acute and chronic treatment of ventricular arrhythmia (Campbell et al., 1978b; Roden, 2001). Also, it is among the few drugs used to reduce or prevent myotonia (Cannon, 1996; Ptacek, 1998; Lehmann-Horn and Jurkat-Rott, 1999), to treat long QT-3 syndrome (Schwarts et al., 1995) and to alleviate neuropathic pain (Chabal et al., 1992). Mex is of particular value because of its relatively long half-life (t1/2 9–12 h) in normal subjects and its suitability for either parenteral or oral administration (Gillis and Kates, 1984). A characteristic of Mex therapy is the large interindividual variations in serum levels after a given dose, which often require monitoring of the plasma concentration and an adjustment of the dose to ensure efficacy and reduce the side effects of the drug at cardiac and central nervous system levels (Campbell et al., 1978b; Labbe and Turgeon, 1999). The predominant factor influencing plasma concentrations of Mex is the ability of the liver to process the drug. The importance of metabolism is emphasized by reports that less than 25% of a dose is recovered unchanged in human urine over 3 days (Beckett and Chidomere, 1977b; Prescott et al., 1977). The metabolic degradation proceeds via various pathways including carbon and nitrogen oxidation, deamination, methylation and reduction reactions that are mediated by hepatic microsomal cytochrome P450 (CYP) enzymes (Beckett and Chidomere, 1977a, 1977b; Labbe et al., 2003). In particular, hydroxy-methyl-mexiletine (HMM) and the p-hydroxy-mexiletine (PHM), formed by hydroxylation of the aliphatic or aromatic moieties of the drug, respectively, and their corresponding alcohols represent up to 20% of the total metabolites of Mex eliminated in urine (Beckett and Chidomere, 1977a). Metabolites, as well as Mex, undergo a further conjugation with glucuronic acid during the phase II metabolism. Among the glucuronide derivatives, the major one, directly derived from Mex, is N-carbonyloxy-β-D-glucuronide metabolite (Senda et al., 2003). In addition, Mex metabolism may occur through stereoselective pathways both in vitro (Vandamme et al., 1993), and in vivo (Fieger and Wainer, 1993; Knoche et al., 1996). In fact Mex, clinically administered as a racemic mixture, possesses a chiral centre and pharmacodynamic and pharmacokinetic differences have been described for the two enantiomers (Grech-Bélanger et al., 1986; Hill et al., 1988; Turgeon et al., 1991; De Luca et al., 1995).
Although it has, anecdotically, been reported that Mex metabolites are without biological activity (Labbe and Turgeon, 1999), no experimental evidence is available to support this view. Thus, evaluation of the pharmacodynamic properties of Mex metabolites is important, especially as the available data on the pharmacokinetic properties of the most abundant metabolites suggest that they might significantly contribute to the clinical effects of Mex. In fact, Paczkowski et al. (1990) found that the values of the elimination rate constant (λm), elimination half-life (t1/2) and mean residence time obtained for Mex and its metabolites, HMM and PHM, following a single oral administration in healthy subjects, were not significantly different. In addition, the peak serum concentration (Cmax), after 4 h, was similar for Mex and HMM, being 0.68±0.11 and 0.51±0.09 μg ml−1, respectively (Paczkowski et al., 1990). Furthermore, it has been reported that the excretion rate of Mex varies between healthy individual subjects, sometimes being even higher than that of its metabolites (Beckett and Chidomere, 1977b). In a preliminary report, we have shown that the PHM metabolite maintains the ability to block INa on skeletal muscle, in a use-dependent manner (Catalano et al., 2004).
These observations led us to investigate in more detail the pharmacological activity of the Mex metabolites, in vitro on INa of skeletal muscle fibres in order to verify their possible involvement in the clinical activity of Mex. Thus, in the present work, the effects of the major metabolites, as racemates and enantiomers, PHM, HMM, N-hydroxy-mexiletine (NHM) (phase I reaction products) and N-carbonyloxy β-D-glucuronide (NMG) (phase II reaction products) were evaluated on INa of native frog skeletal muscle fibres by means of voltage-clamp recordings (De Luca et al., 2000, 2003). Taken together, the present results indicate that all the tested metabolites of phase I, although less potent than the parent compound, behaved as use-dependent and inactivating channel blockers, and might partially contribute to the therapeutic efficacy and/or to the long duration of action of Mex.
Methods
Fibre preparation and voltage-clamp apparatus
All experiments were performed in vitro on native muscle fibres obtained by microsurgery from the semitendinosus muscle of Rana esculenta bathed in normal physiological solution (in mM: NaCl 115, KCl 2.5, CaCl2 1.8, Na2HPO4·12H2O 2.15, NaH2PO4·H2O 0.85; pH=7.3). The cut-end fibre was bathed with a potassium-free internal solution (in mM: CsF 105, MOPS sodium salt 5, MgSO4 2, ethyleneglycol-bis(aminoethylether)-tetraacetic acid 5, Na2ATP 0.55; the pH was set to 7.2 with NaOH) and mounted on a three-vaseline gap voltage-clamp chamber, as described previously (De Luca et al., 1995, 2003). Four KCl/agar bridge electrodes connected the recording chamber to the voltage-clamp amplifier based on methods described by Hille and Campbell (1976). The solution in the central pool A was then replaced with the external solution (in mM: NaCl 77, KCl 2.5, Choline-Cl 38, CaCl2 1.8, Na2HPO4·12H2O 2.15, NaH2PO4·H2O 0.85; pH=7.3). The mean value of membrane area in this pool, from which sodium currents (INa) were recorded, was 3.15±0.6 × 10−4 cm2 (n=20). Particular care was taken to verify that no change occurred in the membrane area in pool A throughout the experiments. The recordings were performed at 10°C, using an amplifier connected via an A/D and D/A Digitata 1200 Interface (Molecular Devices, Sunnyvale, CA, USA) to a 486 DX2/66 personal computer. Pulse generation and data acquisition were controlled by pClamp6 software (Molecular Devices, Sunnyvale, CA, USA). The currents flowing in response to depolarizing command voltages were low-pass filtered at 10 kHz (Frequency Devices, Haverhill, MA, USA), visualized on an oscilloscope, and sampled at 20 kHz. According to standard procedures, leak and capacity currents could be subtracted by P/4 method. However, as this protocol modulates the effects of the drug by influencing the frequency of stimulation, it was rarely used. The leak currents never exceeded 1–3% of total sodium current and no fast kinetic analysis, which may be influenced by capacity current, was performed. In addition, no effect of any of the test compounds was observed on leak current. The acquired traces were analysed using Clampfit program (pClamp6 software package; Molecular Devices, Sunnyvale, CA, USA).
Solutions and drugs
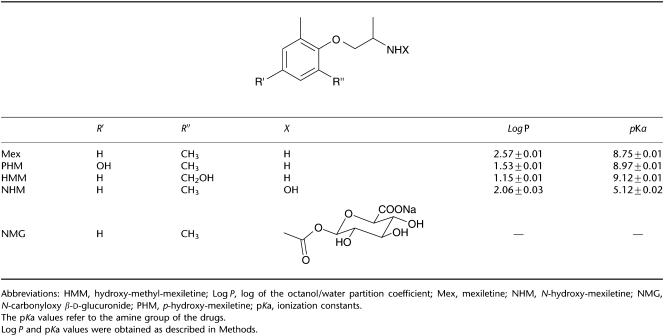
The compounds tested and shown in Table 1 were [2-(2,6-dimethylphenoxy)-1-amino-propanemethylethylamine (Mex), 1-(4-hydroxy-2,6-dimethylphenoxy)-2-propylamine (PHM), 1-[(2-hydroxymethyl) phenoxy]-2-propylamine (HMM), N-[2-(2,6-dimethylphenoxy)-1-methylethyl]hydroxylamine (NHM), N-carbonyloxy-β-D-glucuronide (NMG). The abbreviated nomenclature used throughout the text was assigned at the time the compounds were synthesized and is, therefore, arbitrary. Mex was purchased from Sigma (Milan, Italy). All the metabolites, prepared as hydrochloride or hydrobromide salts, were synthesized in our laboratories according to the procedures described in detail elsewhere (Catalano et al., 2004). All compounds were synthesized as racemates, with the exception of PHM synthesized as pure R- and S-enantiomers. Racemates of PHM metabolite, to be tested on INa max, were obtained by exactly mixing equimolar solutions of the single enantiomers. HMM was also synthesized as pure R- and S-enantiomers and NHM as pure R-enantiomer. Drugs were readily soluble in the physiological solution; fresh stock solution was daily made and diluted as required. The pH of the drug-containing solutions was carefully monitored to be within the physiological range of 7.2–7.4; however, no pH correction was necessary.
Table 1.
Chemical structures and physicochemical parameters of Mex and its metabolites
Pulse protocols description
The curves describing the voltage-dependence of the sodium current (I/V curve) were constructed with a cycle of 10 ms test pulses from the holding potential (HP) of −100 mV to increasing potentials (from −70 to +60 mV). The intervals between each test pulse were long enough (∼3 s) to allow complete recovery of sodium channel from inactivation, of particular importance during exposure to drugs (De Luca et al., 2000). The effectiveness of the clamp was estimated by the lack of shift occurring in the potential for achieving the maximal sodium current (Vmax) in controlled conditions (i.e. in the absence of any compound) for at least 15 min after solution equilibration. Similarly, the possible effect of the test compounds on the activation process was evaluated by measuring the values of both Vmax and V1/2, the potential at which one-half of the channels are activated. The activation curve was constructed from the current–voltage relationship by converting current to conductance: the current amplitude was divided by the driving force (V−VNa), where V is the potential applied to the fibre and VNa is the equilibrium electrochemical potential for sodium ions. Activation curves were fitted with the Boltzmann equation G/Gmax=1/{1+exp [(V−V1/2)/K]}, where G is conductance, Gmax is the maximal conductance, K is the slope factor and V is the voltage at which G is observed. The HP of −100 mV was chosen being close to the resting membrane potential of frog striated fibres and thus allowing the measurement of tonic block, that is, the amount of block of sodium channels on a quiescent excitable tissue, or during low-frequency stimulation. The tonic block was estimated from the I/V curve as the per cent reduction of INa at the potential of −20 mV (De Luca et al., 1997, 2003). Also, the ratios between the mean value of the tonic block of peak INa and that calculated on the area under the transients have been determined, as a greater block on the area versus peak would be predictive of an additional block by drug occurring during channel opening (De Luca et al., 1997). To evaluate the voltage-dependent effect of each drug that would reflect the binding to the channel in the resting and/or inactivated state, we used a pulse protocol of infrequent depolarizing stimulation to −20 mV for 10 ms from two different HP values: very negative (−140 mV), and depolarized, resembling a pathological condition (−70 mV) as described previously (De Luca et al., 2000, 2003). The evaluation of concentration-dependent effects of the drug, expressed as half-maximal blocking concentration (IC50), at −140 mV allowed calculation of the affinity constant for the resting state (Kr). The IC50 value calculated from the HP of −70 mV is influenced both by the higher proportion of channels entering a closed-state inactivation at this potential and by the potential ability of the drug to modify this proportion in favour of more channels being inactivated, if acting as an inactivated channel blocker. The voltage-dependent block exerted by the drugs was used to estimate the affinity constant for the inactivated state (Ki) using the above IC50 values, the relative distribution of channels in the resting and/or inactivated states at the HPs used (from the steady-state inactivation (h∞) curve), as described below.
The use-dependent behaviour of each compound, a characteristic that strongly influences the therapeutic profile of a sodium channel blocker owing to its increased potency during high-frequency depolarization, was evaluated with 30 s trains of depolarizing 10 ms test pulses from the HP of −100 to −20 mV at frequencies of 0.5, 1, 2, 5 and 10 Hz. In the absence of drug, no frequency-dependent change in current amplitude is observed, demonstrating that no physiological inactivation accumulates in these experimental conditions. In contrast, in the presence of the drug a frequency-dependent reduction of the current is observed. The value of the current at equilibrium, normalized with respect to the current in the absence of drug, after the train at the highest frequency, 10 Hz, was used to calculate the potency of the drug for use-dependent block of the channels and compared with the tonic block. Recovery from inactivation was also estimated by evaluating the steady-state current amplitude at each frequency. In fact, for each train, the current transients recorded at the steady state were considered to be owing to the fraction of channels that had recovered from inactivation during the intervals between the pulses. The longer the interval between pulses, the greater the number of channels that recover, until an equilibrium is reached according to the time course of the process. Thus, the value of current at the steady state, at each frequency normalized with respect to the maximal steady-state current obtained at the longest interpulse interval, is used to calculate the time course of recovery from inactivation (De Luca et al., 1997).
The h∞ curves were determined by use of a recurrent protocol of pulse sequences. Each sequence consisted of a conditioning pulse to −140 mV, a prepulse of 1000 ms duration and the 10 ms test pulse to −20 mV; after a pause of 3 s, the sequence was cyclically repeated 18–20 times with the prepulse potential value increased each time by steps of 5 mV (De Luca et al., 1995, 1997).
A more detailed evaluation of absolute Ki was obtained from the voltage-dependent distribution of the channels in the resting (h) and inactivated state (1−h) according to the h∞ curve, using the equation 1/K−70=h/Kr (1−h)/Ki, where K−70 and Kr are the IC50 values obtained from dose–response curves at −140 and −70 mV, whereas h and (1−h) represent the fraction of channel present at resting and inactivated state at −70 mV, respectively (Bean et al., 1983; De Luca et al., 2003).
Data analysis and statistics
The data are expressed as mean±s.e.m. The IC50 values, in the various experimental conditions, were determined by using a non-linear least-squares fit of the concentration–response curves to the following logistic equation: Effect=−100/{1+(K/[drug])n}, where Effect is the percentage change of INa; −100 is the maximal percentage block of INa; K is IC50; n is the logistic slope factor; and [drug] is the molar concentration of the compound (De Luca et al., 1997, 2000).
The h∞ curves have been fitted with a single Boltzmann equation, and the potential at which 50% of the sodium channels were inactivated (Vh1/2) was calculated at the inflection point of the curves (De Luca et al., 1995, 2000).
Statistical significance of differences between couples mean values has been estimated by unpaired Student's t-test and considered significant for P<0.05. The statistical significance between IC50 values±s.e., obtained from the fit, was also evaluated by Student's t-test: the number of d.f. equal to the total number of preparations determining each point of the curve minus the number of means determining the curve minus two for the free parameters (De Luca et al., 2000).
The measurements of ionization constants (pKa) and lipophilicity were performed on a Sirius GLpKa analyzer for pH-metric pKa and log of the octanol/water partition coefficient (log P) (Sirius Analytical Instruments Ltd, Forest Row, UK).
Correlation analysis was evaluated by fitting the experimental data to linear regression analysis. Non-linear equation fitting and processing for data graphics were carried out by Fig P Software (Biosoft, Cambridge, UK).
Results
Tonic and use-dependent block of Na+ channels by major metabolites of Mex
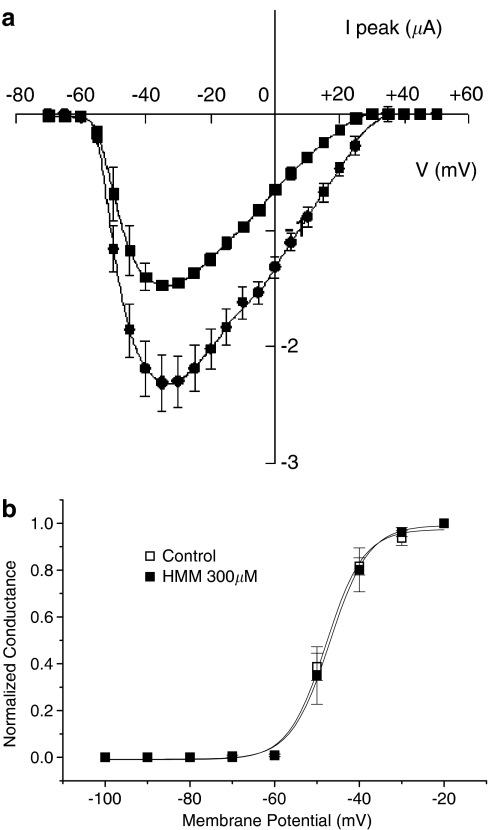
The chemical structures and physicochemical parameters of Mex and its metabolites are showed in Table 1. In particular, the introduction of a hydroxy group in the para position or on the methyl group of the aromatic ring, as in PHM and HMM, leads to the greatest decreased of the log P-values from 2.57±0.01 of Mex to 1.53±0.01 and to 1.15±0.01, respectively. The tonic block exerted by each compound, that is, the block of sodium channels on quiescent tissue, was evaluated by estimating the reduction of nearly maximal INa obtained with a depolarizing step of −20 mV from the HP of −100 mV (Figure 1), during the construction of the current–voltage relationship (I/V) curve. As already observed with Mex (De Luca et al., 1997), the metabolites did not modify the pattern of the I/V curve, as can be seen in Figure 2a, which shows the I/V curve obtained in the absence and presence of 300 μM HMM. In fact, HMM reduced the sodium current at all voltages and did not modify the voltage at which current amplitude was maximal (−35±2.9 versus −33.3±1.7 mV of control). In parallel, the voltage dependence of the activation curve was not modified by the metabolite, the V1/2 being −48.6±2 versus −49.7±1.1 mV (Figure 2b, n=3). A similar behaviour was observed with both PHM and NHM (data not shown).
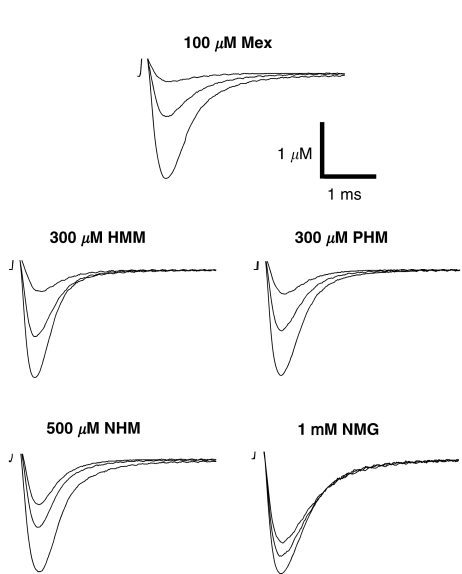
Figure 1.
Na+ current transients recorded in the absence and in the presence of 100 μM Mex, 300 μM PHM, 300 μM HMM, 500 μM NHM and 1 mM NMG. In each group of traces, the greatest one has been recorded in the absence of drug, with a depolarizing step from the HP of −100 to −20 mV for 10 ms. A similar depolarizing stimulus applied after the application of each compound allowed to estimate the tonic block exerted by the drug (middle traces). The smallest current traces correspond to the residual current at end of the 10 Hz stimulation protocol.
Figure 2.
(a) Typical current–voltage relationships (I/V curves) for INa recorded in the absence (filled circles) and in the presence of 300 μM HMM (filled squares). The voltage at which current amplitude was maximal (Vmax) was −33.3±1.7 mV in control and −35.0±2.9 mV in the presence of drug. Each data point shows the mean and s.e. of the mean for three fibres. (b) Steady-state activation curves constructed in the absence and in the presence of 300 μM HMM. Activation curves were fitted by Boltzmann equation, as described in Methods, with half-activation potential (Vx) value −46.9±0.4 mV in control and −44.9±0.6 mV in the presence of drug. Each data point shows the mean and s.e. of the mean for three fibres.
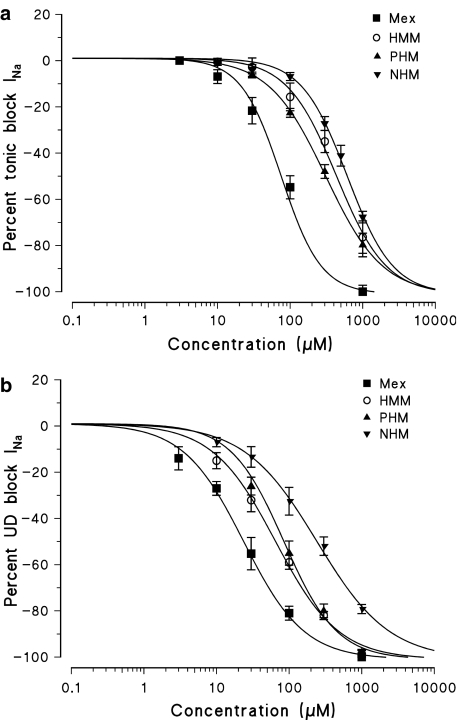
However, all the Mex metabolites were less potent than the parent compound at producing a tonic block of INa. As it can be seen in Figure 1, 300 μM of PHM and HMM produced a tonic block (48.0±2.9%, n=5 and 34.8±5.4%, n=4, respectively) lower than that observed with 100 μM of Mex (59.2±1.4%, n=5). The NHM metabolite was even less potent in producing a tonic block, causing a 26.3±7.6% (n=4) block at 500 μM. The concentration–response curves demonstrating the tonic block by metabolites were significantly shifted to the right with respect to that of Mex with the following order of potency: PHM>HMM>NHM (Figure 3a). The IC50 values showed that PHM, HMM and NHM were 4, 5.5 and 9 times less potent than Mex, respectively (Table 2). In addition, the ratios between the per cent block of INa max calculated at the peak and that calculated on the area (Peak/Area) were always close to unity, indicating that these compounds do not act through an open-channel block mechanism (data not shown) (De Luca et al., 1997).
Figure 3.
Concentration–response curves for tonic (a) and 10 Hz use-dependent block (b) of Na+ currents obtained with Mex and its metabolites PHM, HMM and NHM. Each point shows the per cent block of INa observed in the presence of each concentration of drug versus INa in the absence of the drug in the same fibre and is the mean±s.e.m. from three to seven fibres. The curves fitting the experimental points were obtained using the logistic function described in Methods.
Table 2.
Concentrations for half-maximal tonic and use-dependent block of sodium currents by Mex and its metabolites
| Compound | Tonic block IC50 (μM) | Ratio | Use-dependent block 10 Hz IC50 (μM) | Ratio | TB/UDB |
|---|---|---|---|---|---|
| Mex | 75.3±8.0 | 1 | 23.6±2.8 | 1 | 3.2 |
| PHM | 309.1±8.5 | 4 | 84.8±8.3 | 3.6 | 3.1 |
| HMM | 412.1±45.5a | 5.5 | 65.1±4.2 | 2.8 | 6.3 |
| NHM | 663.0±67.8a,b | 9 | 241.9±14.1a,b | 10 | 2.7 |
| NMG | >1000 | >10 | >500 | >20 | ∼2 |
Abbreviations: HMM, hydroxy-methyl-mexiletine; IC50, half-maximal blocking concentration; Mex, mexiletine; NHM, N-hydroxy-mexiletine; NMG, N-carbonyloxy β-D-glucuronide; PHM, p-hydroxy-mexiletine.
Concentrations able to produce half-maximal response (IC50, μM) in producing a tonic block and a use-dependent block. The ratio between IC50 values during tonic and use-dependent block (IC50 TB/IC50 UDB 10 Hz) is shown in order to allow a more easy comparison of the use-dependent behaviour of each compound. The IC50 values have been obtained during non-linear least-squares fit of the concentration–response data to the logistic equation described in Methods. The ratio between IC50 value of each compound and IC50 value of Mex, for the tonic and use-dependent block, is also shown to better evaluate the relative potency towards parent compound.
The IC50 values of each metabolite for tonic and use-dependent block were significantly different with respect to those of Mex (P<0.001).
a and b show the statistic significance by Student's t-test between the metabolites (for P<0.01 or less) as follows:
With respect to PHM.
With respect to HMM.
Use-dependent behaviour, which strongly accounts for the clinical activity of Mex, was estimated for each metabolite. For each frequency of stimulation, the HP of −100 mV was again chosen being close to the physiological resting membrane potential, but negative enough to allow almost all of the sodium channels to be in the resting state (see Figure 6 following paragraph), an important condition as the use-dependent block can be strongly enhanced by the high-affinity binding of drugs to the inactivated channels, predominating at less negative potentials. All the hydroxylated metabolites maintained a use-dependent behaviour, as a frequency-dependent increase in the potency for blocking INa max was clearly observed. The concentration–response curves of the three hydroxylated metabolites, obtained at the frequency of 10 Hz, were also shifted to the right with respect to that of Mex. However, the order of potency was different from that found for the tonic block being HMM>PHM>NHM (Figure 3b). As can be seen in Table 2, the HMM showed the greatest use-dependent behaviour, with a ratio (IC50 tonic block/IC50 10 Hz use-dependent block) of 6.3. The high use-dependent behaviour of HMM is paralleled by a high estimated pKa value (9.12±0.01) with respect to other analogues (Table 1).
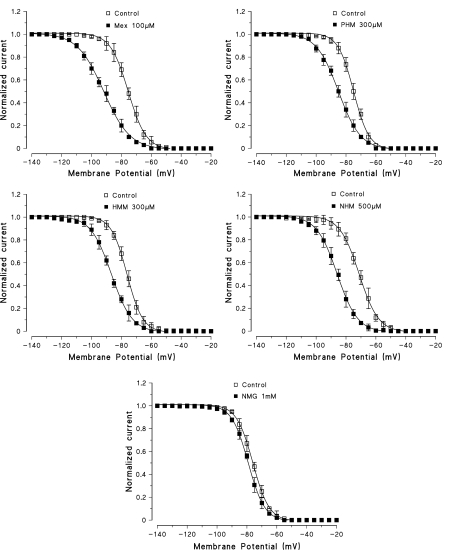
Figure 6.
Steady-state inactivation curves constructed in the absence and in the presence of Mex and of its metabolites. At each membrane potential is shown the current amplitude normalized to the INa max value obtained at −140 mV. Each point is the mean±s.e.m. from three to five fibres. Curves were fitted by a single Boltzmann distribution as described in Methods that allowed to calculate the Vh1/2. Owing to certain variability between controls, the effect of each metabolite, in terms of Vh1/2 and its shift, has been evaluated with respect to the control h∞ curve recorded in the same fibre. The calculated Vh1/2 for the curves shown in the figure are the following: Control, −75.4±0.1 mV and Mex 100 μM, −91.8±0.2 mV; Control, −74.9±0.2 mV and PHM 300 μM, −85.1±0.2 mV; Control, −76.3±0.2 mV and HMM 300 μM, −86.7±0.2 mV; Control −71.2±0.2 mV and NHM 500 μM, −85.9±0.2 mV; Control, −76.0±0.1 mV and NMG 1 mM, −79.1±0.1 mV. The values of Vh1/2 in the absence and presence of each compound are as mean±s.e.m. from three to five fibres. The mean values of K, as estimate of the slope factor of the curves shown in the figure and derived from the fit, are as follows: Control, 0.18±0.002 and Mex 100 μM, 0.12±0.002 (n=4; P<0.001); Control, 0.21±0.02 and PHM 300 μM, 0.19±0.02 (n=5; NS); Control, 0.2±0.01 and HMM 300 μM, 0.11±0.01 (n=3; P<0.001); Control, 0.19±0.004 and NHM 500 μM, 0.18±0.01 (n=4; NS); Control, 0.21±0.01 and NMG 1 mM, 0.19±0.003 (n=3; NS).
Interestingly, the NHM metabolite maintained a use-dependent behaviour almost comparable to that of Mex and PHM (around 3), although it was still the less potent, in absolute terms, of the analogues.
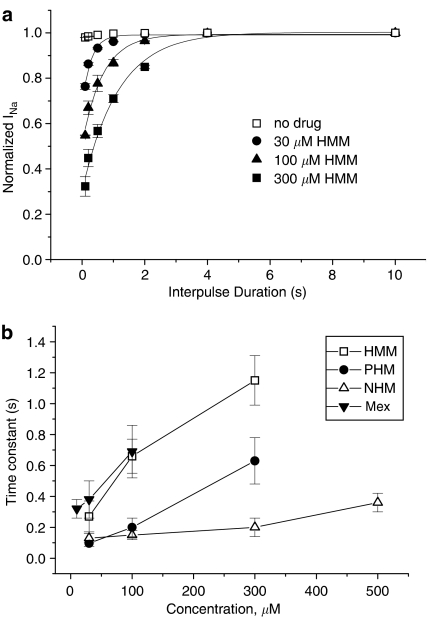
As already mentioned, the use-dependent behaviour is a complex dynamic process involving the kinetics of drug binding to and unbinding from the channel in relation to both state-dependent drug affinity and physicochemical properties. To gain insight into this dynamic process, the recovery from inactivation of drug-bound channels was determined (see Methods). This process occurred with a monoexponential time course for all the compounds tested and was clearly concentration dependent; the process took longer at higher concentrations, evident from the time constant values shown in Figure 4. As can be seen, HMM that is characterized by the highest use-dependent behaviour also exhibited the slowest time constants. For instance at 300 μM, the concentration close to IC50 for tonic block, HMM had a time constant (τ) of 1.15±0.16 s, significantly longer than τ of an equieffective concentration of PHM (0.63±0.15 s). Interestingly, Mex and PHM showed similar use-dependent behaviour and had similar τ values at equieffective concentrations. In contrast, NHM had faster kinetics than the other compounds, which is possibly related to its different pKa value (Table 1). This, along with the resultant lower affinity, probably accounts for the less striking concentration dependence of its τ (Figure 4b).
Figure 4.
(a) Recovery from inactivation measured as residual current at the end of trains of pulses at decreasing frequency from 10 to 0.25 Hz, in the absence and presence of 30, 100 and 300 μM HMM. Each point has been obtained by normalizing the residual current at the end of each train (equilibrium) to the INa max obtained at the longest interpulse used (0.25 Hz). The values, expressed as mean±s.e.m. from three to seven fibres, have been plotted against the duration of the interval between pulses and fitted to a single exponential function. (b) Time constant values for recovery from inactivation of Mex and its metabolites. The time constant values have been obtained from the fit described in panel a and have been plotted against the concentrations of each compound.
The profile of the metabolites was also accompanied by a certain degree of stereoselectivity. The PHM produced a weak stereoselective tonic and use-dependent block, the R-enantiomer being 1.3 times more potent than the S- one. The HMM metabolite produced a significant stereoselective tonic block, with the calculated IC50 values being 492.4±7.2 and 282.1±23.2 μM for R- and S-HMM, respectively (P<0.01). However, when the stimulation frequency was increased the stereoselectivity of HMM was attenuated, as previously observed with Mex and as expected by the long-time constant for recovery from inactivated sodium channel (De Luca et al., 1997). In fact, the eudismic ratio [IC50 distomer/IC50 eutomer] of HMM decreased from 1.75 for tonic block to 1.3 at 10 Hz. The NHM metabolite was devoid of stereoselectivity for both tonic and use-dependent block (data not shown).
Under the present experimental conditions, the NMG metabolite exhibited a very low potency in producing both tonic and use-dependent block, at all frequencies applied, with respect to hydroxylated metabolites. In fact, the IC50 values of this product for 10 Hz use-dependent block was greater then 500 μM (see Table 2).
Voltage-dependent block and effect of the major metabolites of Mex on steady-state inactivation of Na+ channels
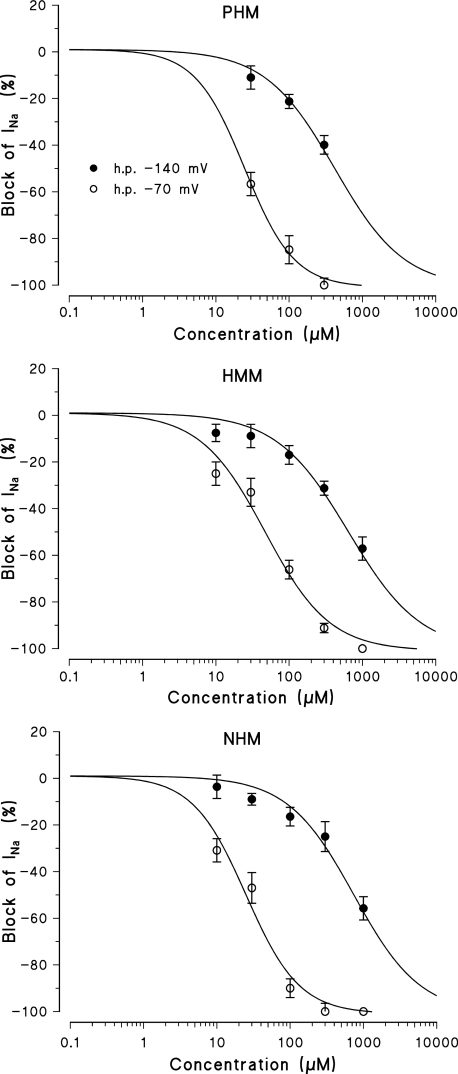
In view of the different use-dependent behaviours of the various hydroxylated metabolites, we investigated their channel state-dependent affinity further. For each hydroxylated metabolite, we constructed concentration–response curves for the block produced at two different HP, −140 and −70 mV (Figure 5), in conditions of low-frequency stimulation. Table 3 shows that the scale of the potency of the metabolites for binding the resting channels (Kr), evaluated at −140 mV of HP, was the same as that found for tonic block at −100 mV. A great increase in potency, up to 30 times, was observed when the HP was held at −70 mV (K−70). However, a different order of potency was again found being PHM=NHM>HMM. From the values of Kr and K−70, their affinity for the inactivated state was calculated (as described in detail in Methods). For all compounds, the Ki values calculated were markedly lower than the K−70, given the contribution of the resting-state binding at −70 mV (Table 3). As expected for inactivating sodium channel blockers, all the derivatives tested shifted the steady-state channel availability (h∞ curves) towards more negative potentials. For each compound, the shift of the h∞ curves was clearly concentration-dependent and related to the potency in blocking INa max. Mex produced a greater shift of the h∞ curves (taken as ΔVh1/2) than its metabolites. Among the hydroxylated metabolites, the NHM, which was less potent at blocking sodium channels, was also less effective in shifting the h∞ curves towards more negative potentials. For example, 500 μM of NHM produced a shift in the h∞ curves (14.7±1.4 mV, n=4) similar to 300 μM of PHM and HMM (10.2±1.1, n=6 and 10.4±1.4, n=5, respectively). The shift of the h∞ curves, produced by 1 mM of NMG was very small (3.1±0.5 mV, n=3) (Figure 6).
Figure 5.
Concentration–response curves obtained with Mex and its metabolites PHM, HMM and NHM, constructed at the HPs values of −140 and at −70 mV. The curves fitting the experimental points were obtained using the logistic function described in Methods and allowed the calculation of the IC50 values (in Table 3). Each value is the mean±s.e.m. from four to seven fibres of the percentage block of INa in the presence of each concentration of drugs versus INa in the absence of the drug in the same fibre.
Table 3.
Voltage-dependent block of sodium currents by Mex and its metabolites
| Drug |
Voltage-dependent block |
Inactivated-state block | |
|---|---|---|---|
| HP −140 mV IC50 (μM); Kr | HP −70 mV IC50 (μM); K−70 | Calculated Ki (μM) | |
| Mex | 147.9±20.0 | 1.9±0.2 | 1.3 |
| PHM | 418.6±65.4 | 24.7±1.9 | 17.6 |
| HMM | 645.0±98.1a | 47.0±6.4a | 36.4 |
| NHM | 740.6±113.5a | 25.0±3.4b | 13.2 |
Abbreviations: HMM, hydroxy-methyl-mexiletine; HP, holding potentials; IC50, half-maximal blocking concentration; Ki, affinity constant for the inactivated state; Kr, affinity constant for the resting state; Mex, mexiletine; NHM, N-hydroxy-mexiletine; NMG, N-carbonyloxy β-D-glucuronide; PHM, p-hydroxy-mexiletine.
The columns from left to right are as follows: drug used; IC50 values a two different HP values: of −140 and −70 mV; inactivated-state block refers to the affinity constants for inactivated sodium channels (Ki) according to the equation in Methods.The IC50 values of each metabolite were significantly different with respect to those of Mex (P<0.001).
a and b show the statistic significance by Student's t-test between the metabolites (for P<0.05 or less) as follows:
With respect to PHM.
With respect to HMM.
For HMM and PHM, as well as for Mex, the shift was accompanied by a decrease in the slope factor (see legend of Figure 6), indicative of a difference in voltage dependence of inactivation. It is worth noting that the change in slope was observed with those drugs having the longest time constant for recovery from inactivation and the greatest use dependence. Thus, we cannot exclude the possibility that this phenomenon could be owing to a dynamic interaction of drug coming off and going on during the conditioning pulse, although the latter should be long enough to minimize these events.
All these observations suggest that, in our system, the inactivated channel block by the phase I metabolites tested has a dominant role, but is not the only determinant, in this use-dependent phenomenon.
Discussion
In the present study, we investigated the pharmacological effects of the metabolites of Mex, as relatively little information exists about their pharmacodynamic properties. In fact, some of the Mex metabolites have a pharmacokinetic profile comparable to that of parent compound (Paczkowski et al., 1990) and may therefore significantly contribute, if still active, to the clinical efficacy of Mex.
Progress in the clinical pharmacology of antiarrhythmic agents is the recognition that the therapeutic profiles of several drugs are influenced by pharmacologically active metabolites produced during hepatic biotransformation. N-acetyl-procainamide is the best-known example of an active metabolic product, which strongly influences the pharmacological profile of its parent drug to treat ventricular arrhythmias (Roden et al., 1980).
The main finding of the present work is that, despite the differences in potency, the hydroxylated metabolites of Mex tested are able to act as inactivating sodium channel blockers in skeletal muscle.
The therapeutic profile of local anaesthetic (LA)-like drugs is defined by their potency, which is an indicator of their affinity for the receptor site on the sodium channel, and by their use-dependent behaviour, which is indicative of their effect on conditions involving pathological hyperexcitability, such as myotonic syndromes and periodic paralyses (Catterall, 1987). Lipophilicity can contribute to drug potency, firstly, by facilitating simple drug diffusion through the membrane, and secondly, by enhancing the drug binding to hydrophobic pockets in the channel protein (Catterall, 1987; Courtney 1990; Sheldon et al., 1991; De Luca et al., 2000).
Phase I metabolic reactions convert Mex to more water-soluble metabolites, which should be eliminated much faster than the parent compound, although results from the studies available do not support their more rapid excretion (Beckett and Chidomere, 1977b). However, with respect to the role of lipophilicity in drug potency, the present data show that the hydroxylated metabolites, considered to be the major products of Mex, are less potent at inducing both tonic and resting block than Mex. A direct comparison between two metabolites with the hydrophilic group on the aryloxy moiety (HMM) or in para position of the aromatic ring (PHM) shows that PHM is about 1.3-fold more potent than HMM in producing the tonic block, the former being more lipophilic (1.53 versus 1.15). However, the NHM metabolite with a hydroxy group linked to the pharmacophore amino-terminal group was the least potent, in spite of its lipophilicity being comparable to that of Mex. In fact, no linear correlation was found between potency and log P when all the compounds were considered (r2=0.126), corroborating the idea that the degree of lipophilicity, although important, is not the sole determinant of drug potency. This latter is also determined by structural modifications at the level of chemical groups directly involved in drug binding, as extensively demonstrated by our previous structure–activity relationship studies with Mex and tocainide analogues (De Luca et al., 1997, 2000, 2003). A Phe1764 and Tyr1771, in the D4–S6 transmembrane segment of voltage-gated sodium channel α subunit, are two main aromatic amino acids lining the pore involved in drug binding. These can establish hydrophobic (π–π) and/or cation–π interactions with the pharmacophore moieties of LA-like drugs, that is, the aromatic ring and the charged tertiary amine (Ragsdale et al., 1994; Nau et al., 1999; Wang et al., 2000; Yarov-Yarovoy et al., 2001, 2002). Accordingly, the presence of a hydroxy group in the 4th position or on the methyl group in the 2nd position of the aromatic ring, as PHM and HMM, other than reducing lipophilicity, can weaken the hydrophobic π–π interaction with the aromatic residues on D4–S6, that is, by changing the electronic clouds or the steric hindrance. Similar results obtained with the β-antagonists propanolol and nadolol and with the β-agonist salbutamol suggested the presence of two hydroxy groups on the aromatic moiety of the drugs as a molecular requisite for impeding sodium channel block (Desaphy et al., 2003). In this respect, the hydroxylation of the pharmacophore amino group in NHM is very important because it significantly modifies the ionization state (pKa=5.12±0.02) and consequently weakness the π–cation interaction with the above-mentioned amino-acid residues during state-dependent block. In fact, as already stated, this metabolite shows a relatively high log P, but it is the least potent blocker of the phase I products. Furthermore, the change in the pKa can in turn contribute to the decrease in use-dependent behaviour, by affecting drug ability to gain access to and egress from the receptor through the hydrophilic pathway of the open channel (De Luca et al., 1997; Catterall, 2002; Liu et al., 2003). Accordingly, in this study, we confirmed that the high use-dependent behaviour is paralleled by a high estimated pKa value. In addition, the PHM and HMM metabolites produced a weak stereoselective block of INa thus minimizing the role of individual enantiomers in the pharmacological profile of each metabolite.
It is well known that glucuronide conjugates represent one of the major types of naturally occurring phase II metabolites of xenobiotics and endobiotics. The process underlying their formation plays a significant role in drug metabolism and modulates both the duration and intensity of the effects of many drugs. In most cases, the glucuronide, very water-soluble and totally ionized in plasma and urine, results in an inactive or poorly active compound (Lanchote et al., 1999). In the present study, glucuronidation resulted in almost complete abolition of the pharmacological activity of Mex.
Finally, our results show that the hydroxylated products of Mex tested are pharmacologically active and retain the ability to block skeletal muscle sodium channels. As their potency for both tonic and resting block is markedly decreased compared to that of parent compound, it is predicted that they have a minor residual effect on normal excitability. However, it should be noted that both PHM and, especially, HMM exhibit marked use-dependent behaviour. Accordingly, the HMM metabolite may significantly contribute to the clinical profile of Mex, as it shows the most favourable ratio between tonic and use-dependent block along with pharmacokinetic properties similar to those observed for the parent compound (Paczkowski et al., 1990). However, more detailed pharmacokinetic studies in vitro and in vivo are needed to validate the possibility that these metabolites are of therapeutic value for the treatment of chronic diseases.
In addition, it is important to realize that tissue-specific sodium channel isoforms have different intrinsic sensitivity to LA-like drugs and specifically to Mex, the cardiac isoform being more potently blocked than neuronal or skeletal muscle isoforms (Catterall, 1987; Kawagoe et al., 2002). Thus, any definitive conclusion regarding clinical or toxicological aspects of Mex metabolites requires a careful monitoring of their effect on different channel isoforms.
Acknowledgments
This work has been supported by Telethon-Italy, Project no. GGP041440. The contribution of COFIN-PRIN Project no. 2005033023_001 is also acknowledged.
Abbreviations
- h∞
steady-state availability function
- HMM
1-[(2-hydroxymethyl) phenoxy]-2-propylamine
- HP
holding potential
- INa
sodium currents
- Ki
affinity constant for the inactivated state
- Kr
affinity constant for the resting state
- LA
local anaesthetic
- log P
log of the octanol/water partition coefficient
- Mex
[2-(2,6-dimethylphenoxy)-1-amino-propanemethylethylamine
- NHM
N-[2-(2,6-dimethylphenoxy)-1-methylethyl]hydroxylamine
- NMG
N-carbonyloxy-β-D-glucuronide
- PHM
1-(4-hydroxy-2,6-dimethylphenoxy)-2-propylamine
- pKa
ionization constant
- Vh1/2
potential for half-maximal inactivation of sodium channels
Conflict of interest
The authors state no conflict of interest.
References
- Bean PB, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Phisiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett AH, Chidomere EC. The identification and analysis of mexiletine and its metabolic products in man. J Pharm Pharmacol. 1977a;29:281–285. doi: 10.1111/j.2042-7158.1977.tb11312.x. [DOI] [PubMed] [Google Scholar]
- Beckett AH, Chidomere EC. The distribution, metabolism and excretion of mexiletine in man. Postgrad Med J. 1977b;53 Suppl 1:60–66. [PubMed] [Google Scholar]
- Campbell NPS, Kelly JG, Adgey AAJ, Shanks RG. The clinical pharmacology of mexiletine. Br J Clin Pharmacol. 1978b;6:103–108. doi: 10.1111/j.1365-2125.1978.tb00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC. Ion-channel defects and aberrant excitability in myotonia and periodic paralysis. Trends Neurosci. 1996;19:3–10. doi: 10.1016/0166-2236(96)81859-5. [DOI] [PubMed] [Google Scholar]
- Catalano A, Carocci A, Fracchiolla G, Franchini C, Lentini G, Tortorella V, et al. Stereospecific synthesis of ‘para-hydroxymexiletine' and sodium channel blocking activity evaluation. Chirality. 2004;16:72–78. doi: 10.1002/chir.10307. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Common mode of drug action on Na+ channels: local anesthetics, antiarrhythmics and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- Catterall WA. Molecular mechanism of gating and drug block of sodium channel. Novartis Found Symp. 2002;241:206–218. [PubMed] [Google Scholar]
- Chabal C, Jacobson L, Mariano A, Chaney E, Britell CW. The use of oral mexiletine for the treatment of pain after peripheral nerve injury. Anesthesiology. 1992;76:513–517. doi: 10.1097/00000542-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Courtney KR. Sodium channel blockers: the size/solubility hypothesis revisited. Mol Pharmacol. 1990;37:855–859. [PubMed] [Google Scholar]
- De Luca A, Natuzzi F, Falcone G, Duranti A, Lentini G, Franchini C, et al. Inhibition of frog skeletal muscle sodium channels by newly synthesized chiral derivatives of mexiletine and tocainide. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;356:777–787. doi: 10.1007/pl00005118. [DOI] [PubMed] [Google Scholar]
- De Luca A, Natuzzi F, Lentini G, Franchini C, Tortorella V, Conte Camerino D. Stereoselective effects of mexiletine enantiomers on sodium currents and excitability characteristics of adult skeletal muscle fibers. Naunyn-Schmiedeberg's Arch Pharmacol. 1995;352:653–661. doi: 10.1007/BF00171325. [DOI] [PubMed] [Google Scholar]
- De Luca A, Natuzzi F, Desaphy J-F, Lentini G, Franchini C, Tortorella V, et al. Molecular determinants of mexiletine structure for potent and use-dependent block of skeletal muscle sodium channels. Mol Pharmacol. 2000;57:268–277. [PubMed] [Google Scholar]
- De Luca A, Talon S, De Bellis M, Desaphy J-F, Franchini C, Lentini G, et al. Inhibition of skeletal muscle sodium currents by mexiletine analogues: specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn-Schmiedeberg's Arch Pharmacol. 2003;367:318–327. doi: 10.1007/s00210-002-0669-0. [DOI] [PubMed] [Google Scholar]
- Desaphy J-F, Pierno S, De Luca A, Didonna MP, Conte Camerino D. Different ability of clenbuterolo and salbutamolo to block sodium channels predicts their therapeutic use in muscle excitability disorders. Mol Pharmacol. 2003;63:659–670. doi: 10.1124/mol.63.3.659. [DOI] [PubMed] [Google Scholar]
- Fieger H, Wainer IW. Direct analysis of the enantiomers of mexiletine and its metabolites in plasma and urine using an HPLC-CSP. J Pharmacol Biomed Anal. 1993;11:1173–1179. doi: 10.1016/0731-7085(93)80101-6. [DOI] [PubMed] [Google Scholar]
- Gillis AM, Kates RE. Clinical pharmacokinetics of the newer antiarrhythmic agents. Clin Pharmacokinet. 1984;9:375–403. doi: 10.2165/00003088-198409050-00001. [DOI] [PubMed] [Google Scholar]
- Grech-Bélanger O, Turgeon JA, Gilbert M. Stereoselective disposition of mexiletine in man. Br J Clin Pharmacol. 1986;21:481–487. doi: 10.1111/j.1365-2125.1986.tb02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RJ, Duff HJ, Sheldon RS. Determinants of stereospecific binding of type I antiarrhythmic drugs to cardiac sodium channels. Mol Pharmacol. 1988;34:659–663. [PubMed] [Google Scholar]
- Hille B, Campbell DT. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976;67:265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe H, Yamaoka K, Kinoshita E, Yoshinori F, Maejima H, Yuki T, et al. Molecular basis foe exaggerated sensitivity to mexiletine in the cardiac isoform of the fast Na channel. FEBS Lett. 2002;513:235–241. doi: 10.1016/s0014-5793(02)02320-7. [DOI] [PubMed] [Google Scholar]
- Knoche B, Gehrcke B, König WA, Wainer IW. Determination of the enantiomeric composition of mexiletine and its four hydroxylated metabolites in urine by enantioselective capillary gas chromatography. Chirality. 1996;8:30–34. doi: 10.1002/(SICI)1520-636X(1996)8:1<30::AID-CHIR7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Labbe L, Abolfanthi Z, Lessard E, Pakdel H, Beaune P, Turgeon J. Role of specific cytochrome P450 enzymes in the N-oxidation of the antiarrhythmic agent mexiletine. Xenobiotica. 2003;33:13–25. doi: 10.1080/0049825021000017948. [DOI] [PubMed] [Google Scholar]
- Labbe L, Turgeon J. Clinical pharmacokinetics of mexiletine. Clin Pharmacokinet. 1999;37:361–384. doi: 10.2165/00003088-199937050-00002. [DOI] [PubMed] [Google Scholar]
- Lanchote VL, Cesarino EJ, Santos VJ, Moraes-Júnior Y, Zanardi AMT, Santos SRCJ. Enantioselectivity in the metabolism of mexiletine by conjugation in female patients with the arrhythmic form of chronic Chagas heart disease. Chirality. 1999;11:29–32. doi: 10.1002/(SICI)1520-636X(1999)11:1<29::AID-CHIR5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C, Wang SY, Strichartz GR, Wang GK. Point-mutations at N434 in DI–S6 of μ1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- Paczkowski D, Sadowski Z, Filipek M, Kolinski P. Pharmacokinetics of mexiletine and its metabolites, hydroxymethylmexiletine and p-hydroxymexiletine, after single oral administration in healthy subjects. Pol J Pharmacol Pharm. 1990;42:365–375. [PubMed] [Google Scholar]
- Prescott LF, Pottage A, Clements JA. Absorption, distribution and elimination of mexiletine. Postgrad Med J. 1977;53 Suppl 1:50–55. [PubMed] [Google Scholar]
- Ptacek L. The familial periodic paralyses and nondystrophic myotonias. Am J Med. 1998;104:58–70. doi: 10.1016/s0002-9343(98)00123-5. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catteral WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Roden DM.Antiarrhythmic drugs Goodman and Gilman' s the Pharmacological Basis of Therapeutics 2001Macmillan Publishing Co.: New York; 933–970.In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG (eds) [Google Scholar]
- Roden DM, Reele SB, Higgins SB, Wilkinson GR, Smith RF, Oates JA, et al. Antiarrhythmic efficacy, pharmacokinetics and safety of N-acetylprocainamide in human subjects: comparison with procainamide. Am J Cardiol. 1980;46:463–468. doi: 10.1016/0002-9149(80)90016-8. [DOI] [PubMed] [Google Scholar]
- Schwarts PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential response to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- Senda C, Toda S, Tateishi M, Kobayashi K, Igarashi T, Chiba K. Mexiletine carbonyloxy β-D-glucuronide: a novel metabolite in human urine. Xenobiotica. 2003;33:871–884. doi: 10.1080/0049825031000140904. [DOI] [PubMed] [Google Scholar]
- Sheldon RS, Hill RJ, Touis M, Wilson LM. Aminoalkyl structural requirements for interaction of lidocaine with class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991;39:609–614. [PubMed] [Google Scholar]
- Turgeon J, Uprichard ACG, Belanger PM, Harron DW, Grech-Bélanger O. Resolution and electrophysiological effects of mexiletine enantiomers. J Pharm Pharmacol. 1991;43:630–635. doi: 10.1111/j.2042-7158.1991.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Vandamme N, Broly F, Libersa C, Courseau C, Lhermitte M. Stereoselective hydroxylation of mexiletine in human liver microsomes: implication of P450IID6. A preliminary report. J Cardiovasc Pharmacol. 1993;21:77–83. doi: 10.1097/00005344-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Wang SY, Nau C, Wang GK. Residues in Na+ channel D3–S6 segment modulate both batrachotoxin and local anesthetic affinities. Biophys J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Sheuer T, Catterall WA. Role of a amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]