Abstract
Background and purpose:
The antiepileptic drug valproic acid, a histone deacetylase (HDAC) inhibitor, is currently being tested as an anticancer agent. However, HDAC inhibitors may interact with anticancer drugs through induction of P-glycoprotein (P-gp, MDR1) expression. In this study we assessed whether valproic acid induces P-gp function in tumour cells. We also investigated effects of valproic acid on the mRNA for P-gp and the cytochrome P450, CYP3A, in rat livers.
>Experimental approach:
Effects of valproic acid on P-gp were assessed in three tumour cell lines, SW620, KG1a and H4IIE. Accumulation of acetylated histone H3 in rats' livers treated for two or seven days with valproic acid was evaluated using a specific antibody. Hepatic expression of the P-gp genes, mdr1a, mdr1b and mdr2, was determined by real-time polymerase chain reaction. The effects of valproic acid on CYP3A were assessed by Northern blot analysis and CYP3A activity assays.
Key results:
Valproic acid (0.5–2.0 mM) induced P-gp expression and function up to 4-fold in vitro. The effect of a series of valproic acid derivatives on P-gp expression in SW620 and KG1a cells correlated with their HDAC inhibition potencies. Treatment of rats with 1 mmol kg−1 valproic acid for two and seven days increased hepatic histone acetylation (1.3- and 3.5-fold, respectively) and the expression of mdr1a and mdr2 (2.2–4.1-fold). Valpromide (0.5–2.0 mM) did not increase histone acetylation or P-gp expression in rat livers, but induced CYP3A expression.
Conclusions:
Valproic acid increased P-gp expression and function in human tumour cell lines and in rat liver. The clinical significance of this increase merits further investigation.
Keywords: valproic acid, P-glycoprotein, multidrug resistance protein 1, histone deacetylase, CYP3A4
Introduction
P-glycoprotein (P-gp) is the most important efflux transporter currently identified with respect to clinical practice and medicine (Gottesman et al., 2002; Giacomini and Sugiyama, 2006). It was initially discovered by its ability to confer multidrug resistance (MDR) on mammalian tumour cells (Juliano and Ling, 1976). However, P-gp is also expressed in tissues involved in detoxification of drugs and other xenobiotics and in blood–tissue barriers, such as the intestinal epithelia, bile canalicular membranes of hepatocytes, epithelial cells in renal tubules and the blood–brain barrier (Thiebaut et al., 1987; Cordon-Cardo et al., 1989). P-gp consists of two proteins: MDR1 (MDR1 in humans, mdr1a and 1b in rats and mice) and MDR2 (MDR2 in humans and mdr2 in rodents). Of these genes, MDR1, mdr1a and mdr1b expression leads to drug resistance, whereas MDR2 and mdr2 encode a phosphatidylcholine transporter in biliary canaliculi (Gottesman et al., 2002).
P-gp expression can be activated by a variety of xenobiotics, including dexamethasone (Figure 1) and paclitaxel. Many of these compounds target nuclear receptors, such as the steroid xenobiotic receptor and co-activate the expression of drug-metabolizing enzymes, such as the cytochrome P450 (CYP), CYP3A4 (Synold et al., 2001; Yokogawa et al., 2002; Wagner et al., 2005). Superimposed upon this regulation is a dynamic chromatin ultrastructure, which regulates the accessibility of transcription factors to their DNA targets. Hypomethylated DNA and hypermethylated, deacetylated histones are associated with transcriptional permissive state of genes, mostly those involved in the regulation of the cell cycle, differentiation and apoptosis (Acharya et al., 2005; Drummond et al., 2005), as well as the MDR1 gene (Scotto, 2003). Thus, the inhibitors of histone deacetylase (HDAC), butyric acid (Figure 1), trichostatin A and depsipeptide have been shown to induce P-gp in various human cancer cell lines and depsipeptide is a P-gp inducer in peripheral blood mononuclear cells from patients with acute myeloid leukaemia (Mickley et al., 1989; Frommel et al., 1993; Jin and Scotto, 1998; Scotto, 2003; Baker and El-Osta, 2004; Odenike et al., 2004; Piekarz et al., 2004; Tabe et al., 2005; Xiao et al., 2005a, 2005b). Because HDAC inhibitors are being investigated as anticancer agents, these findings aroused a concern about potential drug interactions of HDAC inhibitors with other anticancer drugs, which are also substrates for P-gp (Tabe et al., 2005; Xiao et al., 2005a).
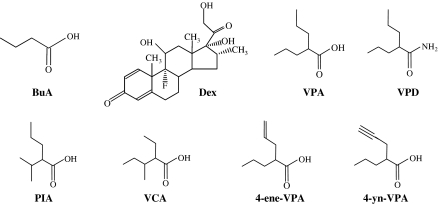
Figure 1.
Chemical structures of valproic acid (VPA) and its derivatives and analogues – valpromide (VPD), propylisopropylacetic acid (PIA), valnoctic acid (VCA), the valproic acid metabolite, 4-ene-VPA, and a synthetic analogue, 4-yn-VPA. Also shown for comparison are butyric acid (BuA), an HDAC inhibitor and dexamethasone (Dex), a P-gp inducer.
Recently, it has been shown that therapeutic concentrations of the established broad-spectrum antiepileptic drug valproic acid (Figure 1), but not valpromide (Figure 1), the primary amide of valproic acid, inhibit HDAC in a variety of human cell lines and in rodents (Göttlicher et al., 2001; Phiel et al., 2001; Ferrante et al., 2003; Gurvich et al., 2004; Eyal et al., 2005). Valproic acid is currently in clinical trials as a monotherapy or in combination with other anticancer compounds for the treatment of solid and haematopoietic malignancies (Blaheta et al., 2005; Raffoux et al., 2005; Yang et al., 2005).
In this study, we characterized the effect of valproic acid and its derivatives on P-gp expression in tumour cell lines and in rat liver, in order to assess if valproic acid has the potential to induce resistance to drugs that are substrates for P-gp. Because valproic acid could also coregulate the expression of CYP3A, we investigated the effects of valproic acid on the mRNA levels and function of this enzyme.
Materials and methods
Materials
Cells and cell culture
As models for P-gp-expressing cells, we used the human colon adenocarcinoma cell line SW620, acute myelogenous leukaemia KG1a cells and H4IIE rat hepatoma cells (Mickley et al., 1989; Mehta et al., 1994; Bailly et al., 1995). Specifically, SW620 cells respond to various HDAC inhibitors (Mickley et al., 1989; Frommel et al., 1993; Jin and Scotto, 1998; Synold et al., 2001). The cell lines were from the American Type Culture Collection (Manassas, VA, USA).
SW620 cells were cultured under standard cell culture conditions with medium Roswell Park Memorial Institute (RPMI) 1640. H4IIE and KG1a cells were cultured in minimum essential medium Eagle's alpha. Culture media were supplemented with 10% foetal calf serum, 100 U ml−1 penicillin/streptomycin and 2 mM glutamine. For H4IIE cells, medium was further supplemented with 10% calf serum. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2:95% O2 and incubated with the tested compounds or the vehicle in culture medium. Control cells were treated with an equivalent concentration of dimethyl sulphoxide (DMSO).
Animals
Male Sprague–Dawley rats (Simonsen Laboratories, Gilroy, CA, USA), weighing 250–300 g were housed in a temperature- and light- (12-h light/dark cycle) controlled environment and allowed free access to food (S/L Custom Lab Diet-7) and water. Animal care and the method of killing (decapitation following an anaesthetic dose of pentobarbitone) were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Test solutions
For the in vitro experiments, valproic acid, valpromide, 4-ene-valproic acid, 4-yn-valproic acid, valnoctic acid and propylisopropylacetic acid were dissolved in DMSO. The DMSO concentration in the assays never exceeded 1% (vv−1). Sodium valproate and sodium butyrate were soluble in media.
For the animal studies, sodium valproate, valpromide and dexamethasone were suspended in 0.5% methylcellulose. Methylcellulose has been routinely used by us in pharmacokinetic and pharmacodynamic evaluation of valproic acid analogues and derivatives, including valpromide. It was selected as the suspending agent in the current study in order to simplify interpretation and comparison of our present results to previous studies (Isoherranen et al., 2003, Sobol et al., 2005). In contrast to sodium valproate, sodium butyrate was not soluble in methylcellulose and hence was dissolved in saline.
Flow cytometric detection of P-gp expression and function
P-gp expression
Cells were harvested by trypsinization, washed and resuspended in phosphate-buffered saline (PBS). Following recovery for 15 min at room temperature, cells were incubated with either the MDR1 monoclonal antibody UIC2 or the control isotype for 30 min at 4°C. Cells were washed twice with PBS/BSA (bovine serum albumin)/NaN3 and further incubated with PBS containing fluorescein-isothiocyanate (FITC)-conjugated goat–anti-mouse immunoglobulin (Ig)G (1:75) for 30 min at 4°C. The extent of specific antibody binding was described as the ratio in mean fluorescence intensities in the absence and in the presence of the primary antibody.
P-gp function
Rhodamine 123 (Rh123) effux assay was used to determine the functional effects of the test compounds on P-gp, as described previously by Frommel et al. (1993) with minor modifications. Briefly, cells were washed thoroughly with PBS after incubation with the tested compounds, incubated with drug-free media for 1 h and then preloaded with 1.2 μM Rh123 with or without one of the following P-gp inhibitors: 40 μM verapamil, 20 μM cyclosporin A (CSA) or 0.5 μM PSC-833 for 30 min at 37°C. After washing twice, cells were incubated with or without the P-gp inhibitor for 90 min in medium at 37°C. Efflux was stopped by transferring the tubes to ice. Samples were washed in ice-cold PBS and analysed by flow cytometry. Non-viable cells were excluded from analysis by propidium iodide staining. Each experiment was performed at least twice on different days. The ratio of Rh123 fluorescence retained in the cells in the absence of an inhibitor to that obtained in the presence of verapamil, CSA or PSC-833 was used as the measure of dye retention.
Treatment protocols
The doses and schedule of valproic acid, butyric acid and dexamethasone administration were selected based on the work of Tremolizzo et al. (2002), Ferrante et al. (2003) and Yokogawa et al. (2002), respectively. Owing to the adverse effects of chronic dexamethasone administration on rat liver and total body weights (Man et al., 2002), the maximal duration for its administration was 3 days. Within this period, dexamethasone has been shown to induce mdr1a mRNA expression in rat liver (Yokogawa et al., 2002). Accordingly, the dosing protocol used in this work was considered to represent an adequate stimulus for P-gp. Rats were randomly assigned to each experimental group and received valproic acid (144 mg kg−1, 1 mmol kg−1), valpromide (143 mg kg, 1 mmol kg−1), butyric acid (600 mg kg−1, 7 mmol kg−1), dexamethasone (50 mg kg−1, 0.1 mmol kg−1) or methylcellulose twice daily for 2 days. The same doses of valproic acid, valpromide and butyric acid were administered also for a period of 7 consecutive days. The parallel treatment with dexamethasone included 4 consecutive days of methylcellulose administration twice daily, followed by dexamethasone (50 mg kg−1, twice daily) for 3 days. Drugs were administered intraperitoneally in a volume of 2 μl g−1 body weight.
Two hours after the last injection, rats were killed. Their livers and brains were removed rapidly. Livers were weighed and tissue samples were either frozen on dry ice and stored at −80°C until processing or processed immediately for microsomal preparation.
Analysis of histone acetylation
Preparation of nuclear extracts
Treated and control cells were washed with PBS and harvested by scraping. They were then centrifuged at 100 g for 10 min twice and resuspended in ice-cold lysis buffer, containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethyl-sulphonic acid (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.2 M HCl, 0.5 mM dithiothreitol, 1.5 mM phenylmethylsulphonyl fluoride and 100 kIU ml−1 aprotinin. Samples were incubated on ice for 30 min. The lysates were centrifuged at 16 000 g for 30 min at 4°C. The supernatant was dialysed twice against 0.1 M acetic acid and twice against H2O, for 1 h each.
Histones were extracted from tissue samples as described by Yasmineh and Yunis (1974), with slight modifications. The tissues were homogenized in 0.32 M sucrose containing 5 mM MgCl2, 0.2 mM CaCl2, 0.5 mM dithiothreitol, 1.5 mM phenylmethylsulphonyl fluoride and 100 kIU ml−1 aprotinin (pH 7.4) and centrifuged at 1000 g for 10 min. The pellet was then suspended in 2.2 M sucrose, 5 mM MgCl2, 0.2 mM CaCl2, 0.5 mM dithiothreitol, 1.5 mM phenylmethylsulphonyl fluoride, 100 kIU ml−1 aprotinin (pH 7.4) and centrifuged at 15 000 g for 1 h. The histones present in this nuclear pellet were resuspended in 0.4 M H2SO4, placed on ice for 30 min and centrifuged at 12 000 g for 20 min. The supernatant was dialysed as described for cell lysates.
Immunodetection of acetylated histones
The concentration of each nuclear extract preparation (which contains non-histone proteins as well) was determined by the Lowry method (Lowry et al., 1951) using BSA as the standard. Samples were adjusted to equal protein concentrations, mixed with sodium dodecyl sulphate (SDS) sample buffer and separated by 10–16.5% tricine SDS–polyacrylamide gel electrophoresis. Gels were either stained with Coomassie blue and imaged with BioDocit (Upland, CA, USA) imaging system or immunoblotted with antibodies against acetyl histone H3 (1:7500) or β-actin (1:200) at 4°C overnight. The blots were then incubated with peroxidase-conjugated goat-anti-rabbit or rabbit-anti-goat IgG (1:40 000 and 1:10 000, for anti-acetyl histone H3 and anti-β-actin antibodies, respectively) for 1.5 h and developed by enhanced chemiluminesence. The ratios between the optical densities of acetylated histone-H3 and β-actin were compared among treatments.
Total RNA isolation
Total RNA was isolated from H4IIE cells or from approximately 200 mg of frozen liver tissue using the acid guanidinium thiocyanate–phenol–chloroform extraction method, modified from Chomczynski and Sacchi (1987).
Quantitative detection of mdr1a, mdr1b, mdr2 and GAPDH mRNA by real-time RT-PCR
Twenty micrograms of total RNA from each sample (concentration of total RNA was determined from the absorbance at 260 nm) was subjected to electrophoresis in 1% denaturing agarose gel containing formaldehyde. Sample integrity was determined by visualization of sharp 18S and 28S bands with ethidium bromide. First-strand cDNA was synthesized using the Superscript II reverse transcriptase with random primers, according to the manufacturer's protocol.
Real-time polymerase chain reaction (PCR) for mRNA of mdr1a, mdr1b, mdr2 and glyceraldehyde phosphate dehydrogenase (GAPDH) was performed using the LightCycler system and software version 3.5.3 (RocheApplied Science, Indianapolis, IN, USA). The sequences of the selected gene-specific primer pairs are shown in Table 1. Amplification mixtures contained 2 μl of DNA template, 1 μl of 5 μM primers, 1.6 μl of 1.25 mM deoxynucleoside triphosphates (dNTPs), 1 μl of PCR buffer, containing MgCl2 (final concentration 20 mM), 1 μl of Taq polymerase, 1 μl of SYBR green and 2.4 μl PCR-grade H2O per sample. PCR cycling conditions were 95°C for 2 min, followed by 45 cycles of denaturation at 95°C for 0 s (rapid heat transfer across the glass PCR capillary)/annealing at 60°C (for GAPDH, mdr1a and mdr1b) or 65°C (for mdr2) for 0 s/extension at 72°C for 15 s. Separate calibration (standard) curves for mdr1a, mdr1b, mdr2 and GAPDH were constructed using serial dilutions of DNA fragments. The standard curve samples were included in each PCR. Negative controls for contamination by extraneous RNA (replacement of RNA with water) were run with each set of reactions. Melting curves, performed after each amplification, produced a single prominent product.
Table 1.
Oligonucleotide real-time PCR primers for amplification of rat mdr1a, mdr1b and GAPDH for real-time PCR
| Gene | Primers | |
|---|---|---|
| mdr1a | Forward | AGGCAATGGCAACATTTTTTGGTGG |
| Reverse | GATAAGCAGAAAAGCTGCACCCATG | |
| mdr1b | Forward | AGTCCATAACGACATTTTCAGCCGG |
| Reverse | GACCAACAGGTAGGCAATACCTATG | |
| mdr2 | Forward | GCAAAGCTTGATGAAAAGGCTGCTGGAGGTG |
| Reverse | GCTCTAGATAGGCCCAAGAAGACCAGCG | |
| GAPDH | Forward | CAGTATGATTCTACCCACGG |
| Reverse | CAGATCCACAACGGATACAT |
The mRNA level of mdr1a, mdr1b and mdr2 was expressed as a ratio to that of GAPDH.
Northern blot analysis
Total RNA was transferred to a Nytran membrane (Schleicher and Schuell) by downward alkaline transfer, crosslinked with UV light and hybridized with cDNA probes labelled with [α-32P]dCTP by random primed synthesis (Amersham Multiprime, Amersham Biosciences, Pittsburgh, PA, USA) using the manufacture's specifications. Rat cDNA probes for the assessed mRNAs were used as described previously (Lamb et al., 2001).
Hybridized blots were washed twice for 30 min at 42°C in 2 × saline-sodium citrate (SSC), 0.1% SDS, twice for 30 min at 42°C in 0.1 × SSC, 0.1% SDS and once for 45 min at 54°C in 0.1 × SSC, 0.1% SDS. Autoradiographic film was exposed for 6–72 h at −70°C with an intensifying screen and the intensity of the developed band was determined by scanning densitometry using Molecular Analyst (Bio-Rad, Hercules, CA, USA) software. To remove any effects of gel loading and transfer variations, all mRNA bands were normalized to a housekeeping gene, cyclophilin mRNA, in the same sample.
Microsomal preparation and enzyme assays
Microsomal and cytosolic fractions from saline-infused livers were prepared according to the procedure described by Franklin and Estabrook (1971). Protein concentrations were determined by the method of Lowry et al. (1951).
For CYP3A activity, liver microsomes (0.2 mg protein ml−1) from control and treated rats were incubated with 200 μM testosterone in phosphate buffer (pH 7.4), for 15 min at 37°C. The internal standard, 11β-hydroxytestosterone, was added at the termination of the incubation. Testosterone and its 6β-hydroxylated metabolite were extracted with dichloromethane and analysed by high-pressure liquid chromatography using CH3CN:0.1% trifluoroacetic acid in H2O with UV detection at 236 nm.
Statistical analysis
Five or seven rats were included in each 2- or 7-day treatment group, respectively. Experiments in cell culture were repeated at least three times. The P-values were calculated by analysis of variance with Dunn's multiple comparison test for post hoc pairwise comparison with the control value. Statistical analyses were performed with GraphPad InStat, version 3.01 (GraphPad Software, San Diego, CA, USA). Data are expressed as mean±s.e.m. A P-value of <0.05 was considered significant.
Drugs and reagents
Valproic acid was a gift from Teva (Petach Tikva, Israel). Culture media, foetal calf serum, medium supplements and antibiotics were purchased from Biological Industries (Beith Haemek, Israel). PSC 833 was kindly provided by Professor W Stein (The Hebrew University of Jerusalem, Israel). Valproic acid derivatives and constitutional isomers were synthesized as described previously in the following references: 4-ene-valproic acid (Hauck and Nau, 1989), 4-yn-valproic acid (Hauck and Nau, 1992), valnoctic acid (Radatz et al., 1998) and propylisopropylacetic acid (Bojic et al., 1996). The monoclonal antibody UIC2 and the control isotype were purchased from Immunotech (Marseille, France), antibodies against acetyl histone H3 were from Upstate Biotechnology (Lake Placid, NY, USA), antibodies against β-actin from Santa Cruz Biotechnology (Santa Cruz, CA, USA), horseradish peroxidase-conjugated goat-anti-rabbit and rabbit-anti-goat antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA, USA), FITC-conjugated goat-anti-mouse antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) and aprotinin from Kamada (Rehovot, Israel). Components for first-strand cDNA synthesis (SuperScript II reverse transcriptase, 5 × buffer, dithiothreitol, random primers, oligo-dTs, dNTPs and Taq polymerase) were purchased from Invitrogen (Carlsbad, CA, USA), 10 × PCR buffer and SYBR green were from Idaho Technology (Salt Lake City, UT, USA). Nytran membranes were purchased from ISC Bioexpress (Kaysville, UT, USA), and the Multiprime DNA labelling kit from Amersham Pharmacia Biotech (Little Chalfont, UK). [α-32P]dCTP was purchased from DuPont NEN (Boston, MA, USA). All other reagents were from Sigma-Aldrich (St Louis, MO, USA).
Results
Effect of valproic acid on P-gp expression and function
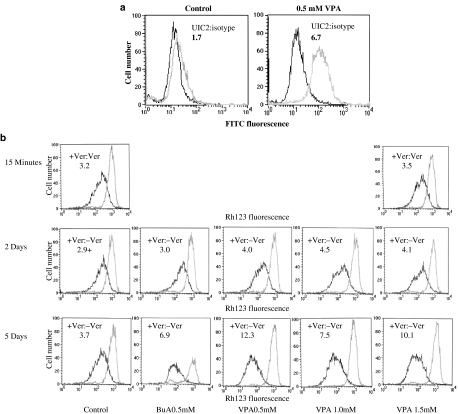
We have compared P-gp function in SW620 colon carcinoma and KG1a lymphoma cells treated with valproic acid to those of cells treated with the vehicle. Using an antibody specific to the human MDR1, we demonstrated that valproic acid induced a fourfold increase of MDR expression in SW620 cells (Figure 2a). A 2.2- to 4-fold induction of P-gp function in KG1a was observed 5 days after the onset of incubation of KG1a with 1 mM valproic acid (Figure 2b), using verapamil as the P-gp inhibitor. The effect of valproic acid on P-gp function was time dependent. It was not demonstrated following 15 min of incubation and was more pronounced after 5 days of incubation, then after 2 days (Figure 2b). Similar results were obtained using the P-gp-specific inhibitor PSC-833 (data not shown). We also investigated whether P-gp expression was reversed by removal of valproic acid. KG1a cells were cultured for 5 days with valproic acid and then washed and incubated without valproic acid for additional 3 days. Withdrawal of valproic acid from treated cells resulted in a return of P-gp function to baseline levels (data not shown). These results demonstrate that P-gp overexpression is dependent on the presence of valproic acid.
Figure 2.
Effect of valproic acid on P-gp function in SW620 and KG1a cells. The abscissa is a logarithmic scale of fluorescence intensity. The ordinate shows the relative frequency of cells in a given fluorescence intensity channel. (a) Indirect immunofluorescence staining of P-gp in SW620 cells. Cells incubated for 5 days with medium containing valproic acid or the vehicle only were washed and incubated with UIC2 anti-P-gp antibody (bright line) or the isotype control (dark line). The ratio of mean fluorescence intensities in the absence and in the presence of the primary antibody is presented for each treatment. (b) Rh123 efflux from KG1a cells treated with butyric acid (BuA, 0.5 mM) or valproic acid (VPA, 0.5–1.5 mM). Cells were treated with the test compounds for the indicated periods, washed with PBS and incubated with Rh123 with or without verapamil (Ver), then washed again and Rh123 efflux established, in the absence (dark line) or presence (bright line) of verapamil. The ratio of Rh123 fluorescence retained in the cells in the absence of verapamil to that obtained in the presence of verapamil is presented for each treatment as a numerical value within each histogram. Higher values indicate increased P-gp expression or function, for Figure 2a and b, respectively. Similar results were obtained in two additional experiments.
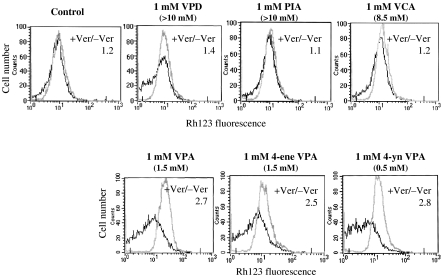
In order to assess whether P-gp induction is shared by valproic acid analogues and derivatives, which also inhibit HDAC, we compared the effect of these compounds, each possessing various potencies as HDAC inhibitors (Eyal et al., 2005), on P-gp function in SW620 cells (Figure 3). The tested drug concentrations were selected according to the previous cytotoxicity data for the tested compounds in these cells (Eyal et al., 2005). The effects of 4-ene- valproic acid and 4-yn valproic acid, which are established HDAC inhibitors with IC50 values for inhibition of HDAC from HeLA nuclear extracts of 1.5 and 0.5 mM, respectively, compared to 1.5 mM of valproic acid (Göttlicher et al., 2001; Eyal et al., 2005, unpublished data), were similar to that of valproic acid. On the other hand, valnoctic acid (IC50=8.5 mM) and propylisopropylacetic acid (IC50>10 mM), which are both less potent than valproic acid as HDAC inhibitors (Eyal et al., 2005), did not induce P-gp function. These results suggest a correlation between HDACs inhibition and P-gp expression by valproic acid analogues and derivatives.
Figure 3.
Effect of valproic acid derivatives and constitutional isomers (see Figure 1) on P-gp function in SW620 cells. Cells were treated with the test compounds for 5 days. Rh123 efflux was assessed as described in Figure 2, in the absence (dark line) or presence (bright line) of verapamil (Ver). The ratio of Rh123 fluorescence retained in the cells in the absence of verapamil to that obtained in the presence of verapamil is presented for each treatment as a numerical value within each histogram. IC50 values for inhibition of HDAC from HeLa nuclear extracts by each compound are given in brackets.
Quantitative PCR analysis was used to evaluate alterations in mdr1a and mdr1b gene expression in the rat hepatoma cell line H4IIE treated with valproic acid. Figure 4 shows that, at concentrations associated with induction of the MDR1 protein expression and activity, valproic acid increased the mRNA levels of mdr1a and mdr1b twofold and more than threefold, respectively. Valpromide did not affect mdr1 mRNA levels in these cells.
Figure 4.
Quantitative PCR determination of mdr1a and mdr1b mRNA copy numbers in control and valproic acid-treated H4IIE cells. Cells were incubated for 36 h in the presence of 1 mM valproic acid, 1 mM valpromide or the vehicle only. The relative copy number of mdr1a and mdr1b was determined by quantitative real-time PCR and normalized by the amount of GAPDH. Results are expressed as fold increase in the copy number, compared to control (mean±s.e.m., N=3).
Effect of valproic acid on histone acetylation in rat liver
To correlate the effects of the tested compounds on levels of the mRNA for hepatic transporter and metabolic enzymes with histone acetylation status in vivo, nuclear extracts were prepared from the livers of control and treated animals and the levels of acetylated histone H3 were evaluated using a specific antibody (Figure 5a). Representative experiments demonstrating the effects of the tested compounds on histone H3 in rat liver are shown in Figure 5b. Valproic acid induced significant histone hyperacetylation in rat liver after 7 treatment days (3.5±1.0-fold of control, P<0.05) (Figure 5a). At this time, butyric acid increased histone H3 acetylation by 3.3±0.4-fold, as compared to control (P<0.05). Valpromide, in contrast to valproic acid, did not induce histone H3 hyperacetylation.
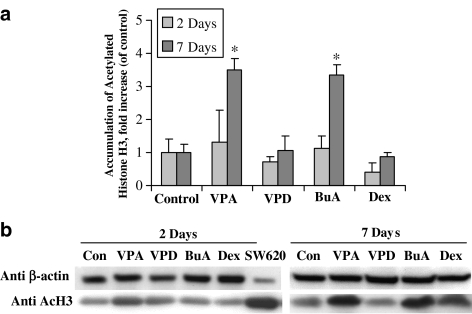
Figure 5.
Effect of valproic acid on histone H3 acetylation in rat liver. Rats were treated with valproic acid (VPA; 144 mg kg−1 b.i.d.), valpromide (VPD; 143 mg kg−1 b.i.d.), butyric acid (BuA; 600 mg kg−1 b.i.d.), dexamethasone (Dex; 50 mg kg−1 b.i.d.) or methylcellulose (control). Acetylated histone H3 from liver homogenates was stained with a specific antibody. Autoradiographs were analysed by densitometry, and the optic densities (OD) values for acetylated histone H3 were normalized by the OD of β-actin (the loading control). (a) Effects of the tested compounds on acetylated histone H3 in rat liver. (b) Representative immunoblots of liver extracts for one animal from each treatment – butyric acid (BuA), dexamethasone (Dex); valproic acid (VPA) and valpromide (VPD). SW620, nuclear extract from SW620 cells, treated for 24 h with 2 mM valproic acid before lysis. Values (mean±s.e.m.) are expressed as fold increase, compared to control run in parallel. *Significantly different from control, P<0.05; n=5 or n=7 in the 2- or 7-day treatment groups, respectively.
Expression of P-gp genes in the livers of control and treated rats
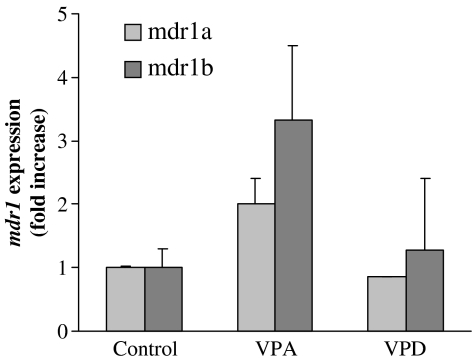
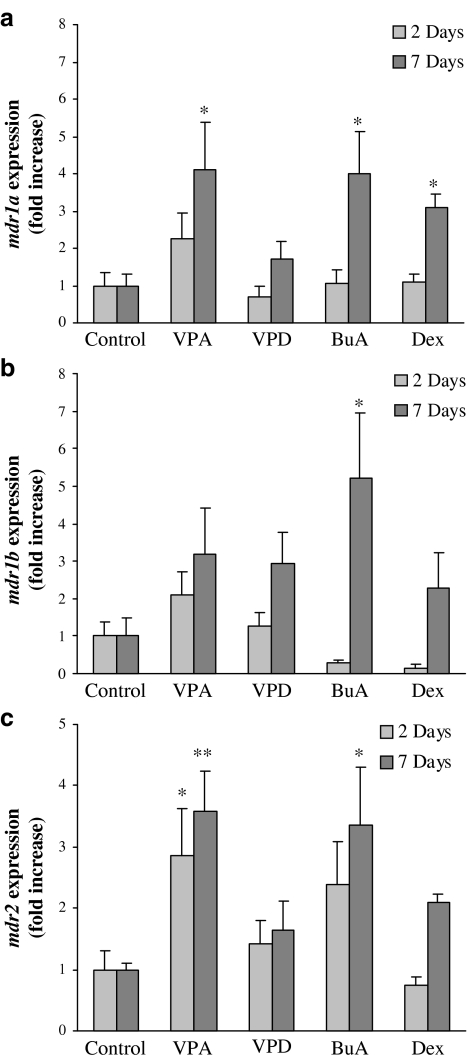
Real-time PCR was used to determine the relative levels of mdr1a, mdr1b and mdr2 mRNA in the livers from treated animals, compared with controls. The mRNA content of the mdr1a and mdr1b and mdr2 genes PCR products was normalized to GAPDH. As shown in Figure 6a, valproic acid significantly increased mdr1a mRNA levels in the liver after 2 treatment days, and the magnitude of this effect was higher after 7 treatment days (2.2±0.7- and 4.1±1.3-fold increase, respectively, compared with control, P<0.05). Unlike valproic acid, valpromide did not induce mdr1a expression. The effect of both valproic acid and valpromide on mdr1b expression was not statistically significant and its extent was lesser compared to mdr1a (Figure 6b). Valproic acid also induced mdr2 expression (28±0.8- and 3.6±0.7-fold) after 2 and 7 days, respectively (Figure 6c).
Figure 6.
Quantitative PCR determination of mdr1a (a), mdr1b (b) and mdr2 (c) mRNA copy numbers in the livers of control and treated rats. Rats were treated with valproic acid (VPA; 144 mg kg−1 b.i.d.), valpromide (VPD; 143 mg kg−1 b.i.d.), butyric acid (BuA; 600 mg kg−1 b.i.d.), dexamethasone (Dex; 50 mg kg−1 b.i.d.) or methylcellulose (control). The relative copy number of mdr1a, mdr1b and mdr2 was determined by quantitative real-time PCR and normalized by the amount of GAPDH. Results are expressed as fold increase in the copy number, compared to control (mean±s.e.m.). *Significantly different from control, P<0.05. **Significantly different from control, P<0.01. n=5 or n=7 in the 2- or 7-day treatment groups, respectively.
Effects of valproic acid on expression and function of hepatic drug-metabolizing enzymes in control and treated rats
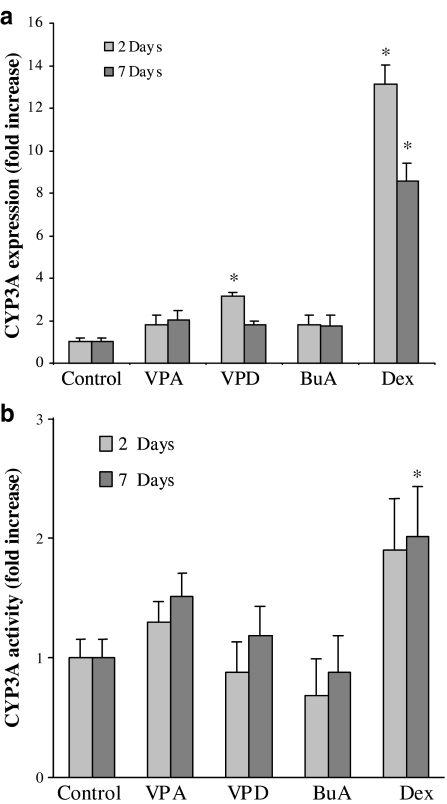
In order to assess whether valproic acid coregulates P-gp and CYP isozymes in vivo in rat liver, the effect of valproic acid on the levels of CYP3A mRNA was assessed following 2 and 7 days of treatment. Although valproic acid did not affect CYP3A expression in rats, valpromide produced a significant increase of CYP3A mRNA levels after 2 days of treatment, but not after 7 days (Figure 7a). However, the effect of valpromide on CYP3A mRNA did not translate into an increase in CYP3A activity in microsomes prepared from the livers of treated rats (Figure 7b).
Figure 7.
Hepatic enzyme expression and activity in the livers of control and treated rats. (a) Relative amount of CYP3A mRNA. The amount of CYP3A was determined by Northern blot analysis and normalized by the amount of cyclophyllin. Results are expressed as fold increase in the copy number, compared to control (mean±s.e.m.). (b) Microsomal CYP3A activity. Testosterone was used as the substrate for CYP3A. *Significantly different from control, P<0.05; n=5 or n=7 in the 2- or 7-day treatment groups, respectively.
Discussion
Inhibitors of HDAC such as butyric acid, trichostatin A and depsipeptide have been shown to induce P-gp expression and function in various human cancer cell lines and in blood cells from patients with acute myeloid leukaemia (Mickley et al., 1989; Frommel et al., 1993; Jin and Scotto, 1998; Synold et al., 2001; Scotto, 2003; Baker and El-Osta, 2004; Odenike et al., 2004; Piekarz et al., 2004; Tabe et al., 2005; Yamada et al., 2005). It has been suggested that P-gp induction by HDAC inhibitors may be associated with a low response to those anticancer drugs that are substrates for P-gp (Tabe et al., 2005; Xiao et al., 2005a). In the current study, we addressed the question whether, similarly, P-gp may be induced during the treatment with the antiepileptic and recently introduced anticancer agent, valproic acid.
In SW620 cells, a model cell line for P-gp induction by HDAC inhibitors (Mickley et al., 1989; Frommel et al., 1993), and in P-gp-expressing KG1a cells, valproic acid induced both expression and function of P-gp, as indicated by immunocytochemistry and by Rh123 efflux assays, respectively (Figure 2). This effect of valproic acid was pronounced at a concentration of valproic acid that is therapeutically effective (as an antiepileptic) and inhibitory for HDAC activity in vitro; that is, 0.5 mM (Göttlicher et al., 2001; Phiel et al., 2001; Johannessen et al., 2003).
The effect of valproic acid on P-gp was not considered to be associated with a genomic change of P-gp, as overexpression was reversible after removal of valproic acid from the culture medium (data not shown). Similarly, Mickley et al. (1989) have previously demonstrated a rapid decline in P-gp mRNA after withdrawal of butyric acid and a gradual decline in the level of the protein with a half-life of about 24 h and a return to near-normal by 96 h. It has also been shown that butyric acid and trichostatin A affect MDR1 transcription rather than the mRNA stability (Mickley et al., 1989; Frommel et al., 1993; Jin and Scotto, 1998; Synold et al., 2001; Baker et al., 2005).
The possibility of phosphorylation-induced activation of P-gp function (Lee, 2001) was also examined. Our results demonstrate time dependence of the effect of valproic acid on P-gp function, without any shift in signal of the fluorescent P-gp substrate Rh123 after 15 min of incubation of SW620 cells with valproic acid (Figure 2b). A shift appeared when the cells were incubated for 48 h with valproic acid and was more pronounced after 7 days of incubation, suggesting a transcriptional rather than post-transcriptional effect of valproic acid. The selectivity of the effect of valproic acid on MDR1 in SW620 cells was confirmed using PSC 833, a specific P-gp inhibitor.
To characterize further the relationships between in vitro inhibition of HDAC and P-gp induction and establish a relationship between HDAC inhibition and induction of P-gp function, we compared the efflux function of cells treated with valproic acid, its derivatives (valpromide, 4-ene- valproic acid and 4-yne- valproic acid; Figure 1) and constitutional isomers (propylisopropylacetic acid, valnoctic acid; Figure 1) possessing various degrees of HDAC inhibitory activity. We found that the valproic acid metabolite, 4-ene- valproic acid, and the synthetic valproic acid derivative, 4-yn- valproic acid, both established HDAC inhibitors (Göttlicher et al., 2001; S Eyal et al., 2005, unpublished data), mimic valproic acid in inducing P-gp function in SW620 cells. In contrast, the constitutional isomers of valproic acid, valnoctic acid and propylisopropylacetic acid, which are less potent HDAC inhibitors (Eyal et al., 2005), did not induce P-gp function. These results support the assumption that induction of P-gp function in vitro by valproic acid and its derivatives may be related to their activity as HDAC inhibitors.
In order to assess whether the increase in P-gp function is owing to the selection of subpopulations of cancer cells with pre-existing high MDR1 expression or is owing to MDR1 induction by valproic acid, we analysed the effect of valproic acid on P-gp mRNA in rat hepatoma cells using real-time reverse transcriptase–PCR. In hepatoma cells, valproic acid increased both mdr1a and mdr1b mRNA levels after 36 h of incubation (Figure 4), which is approximately the time to maximal induction of P-gp mRNA in several cell lines (Mickley et al., 1989). Taken together with the effect of valproic acid on P-gp expression in rat liver and the fact that valproic acid is not a substrate for P-gp in the rat (Baltes et al., 2005), this result suggests that valproic acid induces P-gp expression, rather than selecting for resistant cells.
In order to assess the effect of valproic acid on histone acetylation and P-gp mRNA levels in vivo, we measured hepatic levels of acetylated histone H3, an index of HDAC inhibition, following 2 and 7 days of drug administration to rats. The positive external standard was the nuclear extracts from valproic acid-treated SW620 cells, which expresses high levels of acetylated histone H3 following treatment with valproic acid (Eyal et al., 2005), and valpromide was used as a valproic acid derivative, which does not inhibit HDAC. The observed increase in acetylated histone levels in the livers of rats following 7 days of treatment with valproic acid suggests that this compound inhibit liver HDACs in vivo (Figure 5a and b). The dose of valproic acid, 1 mmol kg−1 (144 mg kg−1), was chosen following Tremolizzo et al. (2002), who assessed the dose–response relationships for the induction of histone hyperacetylation by valproic acid in mice tissues. This dose equals the ED50 for valproic acid in anticonvulsant rodent models (Bialer et al., 1994; Bialer et al., 2004) and produces in rats an average plasma Cmax of 247 mg l−1 (Blotnik et al., 1996), compared with 73.6–170.49 mg l−1 achieved in cancer patients (Chavez-Blanco et al., 2005). Despite the fact that, in rats, the fraction of valpromide metabolized to valproic acid is 42% (Blotnik et al., 1996), the resulting concentration of valproic acid (as a valpromide metabolite) was probably not sufficient to induce significant HDAC inhibition and histone hyperacetylation in the livers of valpromide-treated rats. It has been demonstrated that following intravenous administration of 74 mg kg−1 valproic acid or valpromide to rats, Cmax values of valproic acid in plasma were 247 mg l−1 (1.71 mM) and 78 mg l−1 (0.55 mM), respectively. The Cmax, expressed as drug amount per tissue weight, of valproic acid and valpromide in rat liver was 72 mg kg−1 and 105 mg kg−1, respectively (Blotnik et al., 1996). Assuming linear pharmacokinetics of both valproic acid and valpromide at this dose range, the administration of 143 mg kg−1 valpromide to rats would result in a hepatic Cmax of valproic acid, which is below its reported IC50 for HDAC inhibition in vitro (Göttlicher et al., 2001; Phiel et al., 2001; Gurvich et al., 2004; Eyal et al., 2005).
We used gene-specific primers for mdr1a, mdr1b and mdr2 and real-time PCR, to determine whether valproic acid induces P-gp expression in rats. Valproic acid and butyric acid induced expression of mdr1a, but not mdr1b, in the livers of treated rats (Figure 6). Therefore, the expression of these transporters may be at least partially differentially regulated by valproic acid, as has been previously demonstrated for dexamethasone (Salphati and Benet, 1998). In accordance with its lack of inhibition of HDAC both in vitro and in rat liver, under the current experimental conditions valpromide did not induce P-gp expression in the livers from the treated rats. However, we cannot exclude effects of valpromide on P-gp expression that are mediated by mechanisms other that HDAC inhibition (Figure 6). Similarly, alternative regulatory pathways might lead to the higher increase in mdr1b mRNA following the treatment with butyric acid, compared with valproic acid. In addition to mdr1a, valproic acid also regulates the hepatic expression of mdr2, which is involved in bile acid excretion. The implications of mdr2 induction by valproic acid are currently unknown.
To test the hypothesis that valproic acid might coordinately regulate hepatic P-gp and CYP3A, we investigated the effects of valproic acid on CYP3A mRNA levels in the livers of treated rats. CYP3A enzymes in the rat include CYP3A2, the orthologue of the human CYP3A4, which is responsible for the metabolism of about 50–70% of all pharmaceutical agents (Synold et al., 2001; Yokogawa et al., 2002). Dexamethasone, a potent inducer of both P-gp and CYP3A through activation of nuclear receptors, was used as the positive control and induced CYP3A expression in rat liver. In contrast to dexamethasone, valproic acid did not affect the expression of CYP3A in rat liver (Figure 7). This finding is consistent with the lack of effect of the HDAC inhibitor trichostatin A on CYP3A expression in human cell lines (Rodriguez-Antona et al., 2003). On the other hand, valpromide induced CYP3A expression, but not the function of these isosymes, probably by molecular mechanisms different from those activated by valproic acid (Figure 5).
In conclusion, the present study demonstrates that valproic acid induces the expression of P-gp in rat liver and activates P-gp function in human tumour cell lines. Although valpromide is the primary amide of valproic acid, the effects of these two closely related compounds on the investigated genes are probably mediated by distinct molecular mechanisms, and the free carboxylic group in the valproic acid molecule is likely to be important for valproic acid's effects on both HDAC and P-gp. Currently, most reported effects of valproic acid on the pharmacokinetics of other drugs result from inhibition of their hepatic metabolism (Patsalos and Perucca, 2003a, 2003b), and we are not aware of any clinical studies assessing valproic acid effects on P-gp substrates such as paclitaxel, doxorubicin and digoxin. Thus, the clinical significance of P-gp induction by valproic acid should be further investigated.
Acknowledgments
We gratefully acknowledge the expert technical assistance of John Constance, Neta Pessach, Jacob Shimshoni and Aviva Tinberger. We thank Professor W Stein, from the Hebrew University of Jerusalem, Israel, for supplying PSC-833. This work was supported by Contract No. NIH NO1-NS-4-2359 (to HSW) from the National Institutes of Health.
Abbreviations
- CYP
cytochrome P450
- FITC
fluorescein-isothiocyanate
- GAPDH
glyceraldehyde phosphate dehydrogenase
- HDAC
histone deacetylase
- MDR
multidrug resistance
- Mdr
multidrug resistance transporter
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- P-gp
P-glycoprotein
- SSC
saline-sodium citrate
- SDS
sodium dodecyl sulphate
Conflict of interest
The authors state no conflict of interest.
References
- Acharya MR, Sparreboom A, Venitz J, Figg WD. Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol. 2005;68:917–932. doi: 10.1124/mol.105.014167. [DOI] [PubMed] [Google Scholar]
- Bailly JD, Muller C, Jaffrezou JP, Demur C, Gassar G, Bordier C, et al. Lack of correlation between expression and function of P-glycoprotein in acute myeloid leukemia cell lines. Leukemia. 1995;9:799–807. [PubMed] [Google Scholar]
- Baker EK, El-Osta A. MDR1, chemotherapy and chromatin remodeling. Cancer Biol Ther. 2004;3:819–824. doi: 10.4161/cbt.3.9.1101. [DOI] [PubMed] [Google Scholar]
- Baker EK, Johnstone RW, Zalcberg JR, El-Osta A. Epigenetic changes to the MDR1 locus in response to chemotherapeutic drugs. Oncogene. 2005;24:8061–8075. doi: 10.1038/sj.onc.1208955. [DOI] [PubMed] [Google Scholar]
- Baltes S, Potschka H, Löscher W. Investigation of the transport of valproic acid by the multidrug transporters P-gp (ABCB1) and MRP2 (ABCC2) Epilepsia. 2005;46 Suppl 8:205–206. [Google Scholar]
- Bialer M, Haj-Yehia A, Badir K, Hadad S. Can we develop improved derivatives of valproic acid. Pharm World Sci. 1994;16:2–6. doi: 10.1007/BF01870931. [DOI] [PubMed] [Google Scholar]
- Bialer M, Twyman RE, White HS. Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav. 2004;5:866–872. doi: 10.1016/j.yebeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- Blotnik S, Bergman F, Bialer M. Disposition of valpromide, valproic acid, and valnoctamide in the brain, liver, plasma, and urine of rats. Drug Metab Dispos. 1996;24:560–564. [PubMed] [Google Scholar]
- Bojic U, Elmazar MM, Hauck RS, Nau H. Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem Res Toxicol. 1996;9:866–870. doi: 10.1021/tx950216s. [DOI] [PubMed] [Google Scholar]
- Chavez-Blanco A, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Cetina L, Candelaria M, et al. Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer. A phase I study. Mol Cancer. 2005;4:22. doi: 10.1186/1476-4598-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- Eyal S, Yagen B, Shimshoni J, Bialer M. Histone deacetylases inhibition and tumor cells cytotoxicity by CNS-active VPA constitutional isomers and derivatives. Biochem Pharmacol. 2005;69:1501–1508. doi: 10.1016/j.bcp.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MR, Estabrook RW. On the inhibitory action of mersalyl on microsomal drug oxidation: a rigid organization of the electron transport chain. Arch Biochem Biophys. 1971;143:318–329. doi: 10.1016/0003-9861(71)90213-x. [DOI] [PubMed] [Google Scholar]
- Frommel TO, Coon JS, Tsuruo T, Roninson IB. Variable effects of sodium butyrate on the expression and function of the MDR1 (P-glycoprotein) gene in colon carcinoma cell lines. Int J Cancer. 1993;55:297–302. doi: 10.1002/ijc.2910550221. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Sugiyama Y.Membrane transporters and drug response Goodman & Gilman's The Pharmacological Basis of Therapeutics 2006McGraw-Hill: New York; 41–70.In: Brunton LL, Lazo JS, Parker KL (ed) [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Göttlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- Hauck RS, Nau H. Asymmetric synthesis and enantioselective teratogenicity of 2-n-propyl-4-pentenoic acid (4-en-VPA), an active metabolite of the anticonvulsant drug, valproic acid. Toxicol Lett. 1989;49:41–48. doi: 10.1016/0378-4274(89)90099-4. [DOI] [PubMed] [Google Scholar]
- Hauck RS, Nau H. The enantiomers of the valproic acid analogue 2-n-propyl-4-pentynoic acid (4-yn-VPA): asymmetric synthesis and highly stereoselective teratogenicity in mice. Pharm Res. 1992;9:850–855. doi: 10.1023/a:1015832411981. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, White HS, Klein BD, Roeder M, Woodhead JH, Schurig V, et al. Pharmacokinetic–pharmacodynamic relationships of (2S,3S)-valnoctamide and its stereoisomer (2R,3S)-valnoctamide in rodent models of epilepsy. Pharm Res. 2003;20:1293–1301. doi: 10.1023/a:1025069519218. [DOI] [PubMed] [Google Scholar]
- Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen SI, Battino D, Berry DJ, Bialer M, Kramer G, Tomson T, et al. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit. 2003;25:347–363. doi: 10.1097/00007691-200306000-00016. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Lamb JG, Sorensen JS, Dearing MD. Comparison of detoxification enzyme mRNAs in woodrats (Neotoma lepida) and laboratory rats. J Chem Ecol. 2001;27:845–857. doi: 10.1023/a:1010366322299. [DOI] [PubMed] [Google Scholar]
- Lee CH. Induction of P-glycoprotein mRNA transcripts by cycloheximide in animal tissues: evidence that class I Pgp is transcriptionally regulated whereas class II Pgp is post-transcriptionally regulated. Mol Cell Biochem. 2001;216:103–110. doi: 10.1023/a:1011086716568. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Man WJ, White IR, Bryant D. Protein expression analysis of drug-mediated hepatotoxicity in the Sprague–Dawley rat. Proteomics. 2002;2:1577–1585. doi: 10.1002/1615-9861(200211)2:11<1577::AID-PROT1577>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mehta R, Davis HG, Laver GW, Rowsell PR, Bondy GS.Glutathione S-transferases and P-glycoprotein in normal rat hepatocytes and hepatoma cells: analysis using flow cytometry Cancer Lett 199484163–172.(Erratum in: Cancer Lett89:231) [DOI] [PubMed] [Google Scholar]
- Mickley LA, Bates SE, Richert ND, Currier S, Tanaka S, Foss F, et al. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989;264:18031–18040. [PubMed] [Google Scholar]
- Odenike OM, Alkan S, Sher D, Godwin JE, Huo D Myers M, Brandt SJ, et al. The histone deacetylase inhibitor depsipeptide has differential activity in specific cytogenetic subsets of acute myeloid leukemia (AML) [abstract] Blood. 2004;104:79. [Google Scholar]
- Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003a;2:473–481. doi: 10.1016/s1474-4422(03)00483-6. [DOI] [PubMed] [Google Scholar]
- Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol. 2003b;2:347–356. doi: 10.1016/s1474-4422(03)00409-5. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Robey RW, Zhan Z, Kayastha G, Sayah A, Abdeldaim AH, et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood. 2004;103:4636–4643. doi: 10.1182/blood-2003-09-3068. [DOI] [PubMed] [Google Scholar]
- Radatz M, Ehlers K, Yagen B, Bialer M, Nau H. Valnoctamide, valpromide and valnoctic acid are much less teratogenic in mice than valproic acid. Epilepsy Res. 1998;30:41–48. doi: 10.1016/s0920-1211(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Raffoux E, Chaibi P, Dombret H, Degos L. Valproic acid and all-trans retinoic acid for the treatment of elderly patients with acute myeloid leukemia. Haematologica. 2005;90:986–988. [PubMed] [Google Scholar]
- Rodriguez-Antona C, Bort R, Jover R, Tindberg N, Ingelman-Sundberg M, Gomez-Lechon MJ, et al. Transcriptional regulation of human CYP3A4 basal expression by CCAAT enhancer-binding protein alpha and hepatocyte nuclear factor-3 gamma. Mol Pharmacol. 2003;63:1180–1189. doi: 10.1124/mol.63.5.1180. [DOI] [PubMed] [Google Scholar]
- Salphati L, Benet LZ. Modulation of P-glycoprotein expression by cytochrome P450 3A inducers in male and female rat livers. Biochem Pharmacol. 1998;55:387–395. doi: 10.1016/s0006-2952(97)00436-x. [DOI] [PubMed] [Google Scholar]
- Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- Sobol E, Yagen B, Winkler I, Britzi M, Gibson D, Bialer M. Pharmacokinetics and metabolism of a new potent antiepileptic drug, 2,2,3,3-tetramethycyclopropanecarbonylurea, in rats. Drug Metab Dispos. 2005;33:1538–1546. doi: 10.1124/dmd.105.005637. [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Konopleva M, Contractor R, Munsell M, Schober WD, Jin L, et al. Upregulation of MDR1 and induction of doxorubicin resistance by histone deacetylase inhibitor depsipeptide (FK228) and ATRA in acute promyelocytic leukemia cells. Blood. 2005;107:1546–1554. doi: 10.1182/blood-2004-10-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, et al. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci USA. 2002;99:17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- Xiao JJ, Foraker AB, Swaan PW, Liu S, Huang Y, Dai Z, et al. Efflux of depsipeptide FK228 ( FR901228, NSC-630176) is mediated by P-glycoprotein and multidrug resistance-associated protein 1. J Pharmacol Exp Ther. 2005a;313:268–276. doi: 10.1124/jpet.104.072033. [DOI] [PubMed] [Google Scholar]
- Xiao JJ, Huang Y, Dai Z, Sadee W, Chen J, Liu S, et al. Chemoresistance to depsipeptide FK228 [(E)-(1S,4S,10S,21R)-7-[(Z)-ethylidene]-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8,7,6]-tricos-16-ene-3,6,9,22-pentanone] is mediated by reversible MDR1 induction in human cancer cell lines. J Pharmacol Exp Ther. 2005b;314:467–475. doi: 10.1124/jpet.105.083956. [DOI] [PubMed] [Google Scholar]
- Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leukemia Res. 2005;29:739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Yasmineh WG, Yunis JJ. Isolation of mammalian heterochromatin and euchromatin. Methods Cell Biol. 1974;8:151–177. doi: 10.1016/s0091-679x(08)60450-1. [DOI] [PubMed] [Google Scholar]
- Yokogawa K, Shimada T, Higashi Y, Itoh Y, Masue T, Ishizaki J, et al. Modulation of mdr1a and CYP3A gene expression in the intestine and liver as possible cause of changes in the cyclosporin A disposition kinetics by dexamethasone. Biochem Pharmacol. 2002;63:777–783. doi: 10.1016/s0006-2952(01)00911-x. [DOI] [PubMed] [Google Scholar]
- Yamada H, Arakawa Y, Saito S, Agawa M, Kano Y, Horiguchi-Yamada J. Depsipeptide-resistant KU812 cells show reversible P-glycoprotein expression, hyper-acetylated histones, and modulated gene expression profile. Leukemia Res. 2005;30:723–734. doi: 10.1016/j.leukres.2005.09.014. [DOI] [PubMed] [Google Scholar]