Abstract
Background and purpose:
Simvastatin, a cholesterol-lowering agent, can protect against endothelial dysfunction. However, the effects of simvastatin treatment on the restoration of blood flow to ischemic myocardium are not known. This study sought to assess such effects of simvastatin on an experimental model of myocardial no-reflow and to explore possible mechanisms.
Experimental approach:
Coronary ligation area and area of no-reflow were determined by myocardial contrast echocardiography in vivo and by histology in mini-pigs randomized into 7 study groups: controls, pretreated with simvastatin for 2 days, treated with 5-hydroxydecanoate (5-HD, the selective mitochondrial KATP channel blocker), treated with simvastatin+5-HD, treated with HMR 1883 (the selective sarcolemmal KATP channel blocker), treated with simvastatin+HMR 1883 and a sham-operated group. The myocardial no-reflow model was induced with 3 h occlusion of the left anterior descending coronary artery followed by 2 h reperfusion.
Key results:
Compared with the control group, simvastatin significantly increased coronary blood flow, decreased the area of no-reflow assessed echocardiographically and reduced the necrotic area, by histology. There was no significant difference in these outcomes between simvastatin and simvastatin+HMR 1883 groups. In contrast, 5-HD abolished the effect of simvastatin.
Conclusions and implications:
Simvastatin can reduce the area and myocardial no-reflow after ischaemia and reperfusion. This beneficial effect is due to its activation of mitochondrial KATP channels.
Keywords: simvastatin, acute myocardial infarction, no-reflow reperfusion
Introduction
The main goal of reperfusion therapy for acute myocardial infarction (AMI) is to restore both epicardial and microvascular blood flow to the ischaemic myocardium. Primary percutaneous coronary intervention, the preferred treatment for AMI, can achieve normal epicardial coronary flow. Studies have shown, however, that despite complete restoration of epicardial vessel blood flow, myocardial tissue perfusion evaluated with myocardial contrast echocardiography (MCE) remains incomplete, known as the slow flow or ‘no-reflow' phenomenon (Ito et al., 1996a, 1996b; van't Hof et al., 1997). This phenomenon occurs in 37% of patients with a first anterior AMI after receiving coronary reflow (Ito et al., 1996a, 1996b). No-reflow has been associated with severe myocardial injury, progressive left ventricular (LV) remodelling, congestive heart failure and poor prognosis (Wu et al., 1998; Swinburn et al., 2001; Hombach et al., 2005). Therefore, reduction in the extent of no-reflow is now accepted as a target of reperfusion therapy for AMI (Gersh, 1999).
The mechanism responsible for the no-reflow phenomenon is uncertain and is likely to be multifactorial. Although it has been suggested that plugging of capillaries by leukocytes and platelet aggregates contributes to no-reflow (Engler et al., 1983; Endoh et al., 1993), these blood cell elements are not necessary for the development of this phenomenon, because no-reflow has also been observed in hearts perfused with buffer (Humphrey et al., 1980; Manciet et al., 1994). As shown in animal models of coronary artery occlusion and reperfusion, localized endothelial swelling and protrusions are predominantly confined to the capillary bed (Kloner et al., 1974), indicating that endothelial dysfunction plays an important role in tissue-level perfusion. Particularly, the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are known to protect against endothelial dysfunction under normocholesterolemic conditions (Kaesemeyer et al., 1999; Lefer et al., 1999; Wassmann et al., 2001) and we hypothesized that they may also be effective in preventing from myocardial no-reflow. Furthermore, myocardial no-reflow has always been associated with microvascular structural disruption (structural no-reflow), which caused vascular leakiness. Vascular endothelial (VE)-cadherin, a specific endothelial junction, is an important determinant of vascular structural integrity (Dejana, 1996; Corada et al., 1999).
In this study, we therefore used a model of AMI and reperfusion in mini-pigs, developed in our laboratory to assess the effects of the HMG-CoA reductase inhibitor, simvastatin, on myocardial no-reflow and the state of endothelial junctions. In addition, to investigate whether any beneficial effect of the statin was partly due to the activation of adenosine triphosphate-sensitive potassium (KATP) channels, the effects of a selective mitochondrial KATP channel blocker 5-hydroxydecanoate (5-HD) and a selective sarcolemmal KATP channel blocker 1-{5-[2-(5-chloro-o-anisamido)ethyl]-2-methoxyphenyl} sulphonyl-3-methylthiourea (HMR 1883) (Dhein et al., 2000; Sato et al., 2000) were also assessed in the same animal model.
Methods
Animal preparation
The animals and protocols used in the study were approved by the Institutional Animal Care and Use Committee. Mini-pigs (62 in total) of either gender, weighing 30.3±3.0 kg, were fasted overnight, sedated with 10 mg kg−1 of azaperone intramuscularly, anaesthetized with 10 mg kg−1 of thiopental intravenously and ventilated with a respirator (SV900; Siemens-Elema, Solna, Sweden). Anaesthesia was maintained with a continuous infusion of thiopental. A middle thoracotomy was performed, and the heart was suspended in a pericardial cradle. The middle and distal portion of left anterior descending coronary artery (LAD) was dissected free from surrounding tissue and was encircled by a suture. The two ends of the suture were threaded through a length of plastic tubing, forming a snare, which could be tightened to occlude the coronary artery. The right femoral artery and vein were cannulated for haemodynamic monitoring and contrast agent injection, respectively. An ultrasonic flow probe was placed proximal to the site of occlusion. The probe was connected to a flowmeter (Nihon Kohden corporation) for digital measurement of LAD flow.
Experimental protocol
Sixty-two animals were randomized into seven study groups: 10 controls, nine pretreated with simvastatin, nine treated with 5-HD (the selective mitochondrial KATP channel blocker), 10 treated with simvastatin+5-HD, eight treated with HMR 1883 (the selective sarcolemmal KATP channel blocker), eight treated with simvastatin+HMR 1883 and eight sham-operated. In the simvastatin-pretreated animals, 2 mg kg−1 day−1 of simvastatin (donated by Merck Pharmaceutical Company, Beijing, China) was given consecutively for 2 days before the experiment. This dose was based on body surface area (Freireich et al., 1966). In the 5-HD- or HMR 1883-treated animals, 5-HD (5 mg kg−1) or HMR 1883 (3 mg kg−1) was administered intravenously 30 min before coronary occlusion. In simvastatin+5-HD or simvastatin+HMR 1883-treated animals, simvastatin was given, as described above, together with 5-HD (5 mg kg−1) or HMR 1883 (3 mg kg−1) administered 30 min before coronary occlusion. The above six groups were subjected to 3 h of coronary occlusion followed by 2 h of reperfusion. In the sham-operated animals, the LAD was only encircled by a suture, but not occluded. Data were collected at baseline, at the end of 3 h of LAD occlusion and at 2 h of reperfusion.
Haemodynamic function
The mini-pigs were used in the opened-chest anaesthetized state. Fluid-filled multilumen balloon flotation and 7F or 8F pigtail catheters were inserted percutaneously under fluoroscopic guidance through the femoral vein and artery. These were used to measure cardiac output (CO), left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and the maximum change rate of LV pressure rise and fall (±dp dtmax−1). CO was measured with a flow-directed, thermodilution method with Edward's CO computer. Three consecutive readings for CO were used for the final computation. The variability of thermodilution measurements in our laboratory is ±3%. Haemodynamic data measurements were repeated at baseline, at the end of 3 h of LAD occlusion and at 2 h of reperfusion. Coronary blood flow (CBF), which reflects myocardial tissue perfusion indirectly, was also measured at baseline, immediately after release of occlusion (3 h) and at 2 h of reperfusion.
MCE evaluation
Echocardiography was performed with an HP 5500 machine (Philips Ultrasound, Bothell, WA, USA). The transducer was fixed in position to obtain the same short-axis images of the left ventricle at the mid-papillary muscle level. A warm-water bath acted as an acoustic interface between the heart and the transducer. A bolus of 0.05 ml kg−1 of Sonovue (Bracco Inc., Geneva, Switzerland) was injected intravenously as a slow bolus during 30 s followed by 5 ml saline flush. Data were collected at baseline, at the end of 3 h of LAD occlusion and at 2 h of reperfusion. For each MCE, end-diastolic images were acquired at a pulsing interval of four cardiac cycles during contrast medium injection to allow complete beam replenishment and demarcation between perfused and non-perfused tissue. The myocardial ligation area (LA) and the area of no-reflow (ANR) were identified as the region of non-opacified myocardium by MCE at 3 h of LAD occlusion and at 2 h of reperfusion, respectively. LA, ANR and LV wall area were traced and measured. LA was expressed as a percentage of the LV wall area, whereas ANR was expressed as a percentage of the LA.
Histopathological evaluation
After completion of the experimental protocol, ANR was delineated by intra-atrial injection of 1 ml kg−1 of the fluorescent dye thioflavin S (Sigma Chemical Co., St Louis, MO, USA). Then, the LAD was re-occluded, and Evans blue dye was injected into the left atrium to determine LA. The animal was then killed and the heart removed. The LV was cut into 5–6 slices parallel to the atrioventricular groove. Under a UV light in a dark room, the areas not perfused by thioflavin S were identified. LA was defined as the region unstained by Evans blue, whereas ANR was defined as the non-fluorescent area within the LA. Samples were immediately taken from the myocardium in the normal, reflow or no-reflow region of two slices, washed thoroughly with saline and snap-frozen in liquid nitrogen for measurement of VE-cadherin. The other slices were incubated in a 1% solution of triphenyltetrazolium chloride for 15 min at 37°C. Regions that failed to demonstrate red staining were considered to represent the area of necrosis (NA). The outlines of the LV wall area, LA, ANR and NA were calculated. The LA was expressed as a percentage of the LV wall area, and ANR and NA were expressed as a percentage of the LA.
Measurement of total cholesterol levels in plasma
At the end of the experiment, 3 ml of blood was obtained; plasma was separated from blood cells by centrifugation and stored at −80°C. Total cholesterol levels were measured by enzyme assays with an Aeroset System (Abbott Labaratories, Wiesbaden, Germany).
Western blot analysis for VE-cadherin in myocardial tissue
Myocardial tissue samples were separately suspended in 5 ml of ice-cold lysis buffer containing (mM) Tris-HCl 20 (pH 7.4), ethylenediaminetetraacetic acid 1, NaCl 150, dithiothreitol 1, 2-mercaptoethanol 10, freshly added proteinase inhibitors and homogenized. The particulate material was discarded after centrifugation at 100 000 g at 4°C for 1 h. The clear supernatant of each tissue sample was collected and frozen at −70°C until use. Protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as a standard. Fifty micrograms of total protein solubilized for 10 min at 100°C was loaded per lane onto a 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel. Electrophoresis was performed for 1 h at 150 mA. Proteins were transferred onto Immobilon-P transfer membrane (Millipore, Bedford, MA, USA) for 1.5 h at 0.8 mA cm−2 in a 20% methanol containing cathodes buffer. The membrane was washed three times for 20 min in PBST (0.1% Tween 20, 100 mM Tris-HCl, 150 mM NaCl, pH 7.5), blocked for 1 h in 5% nonfat milk-TTBS and incubated with VE-cadherin goat polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or mouse monoclonal beta actin (from Transduction Labs, San Jose, CA, USA). The primary antibody was used in a 1:1000 dilution in PBST. After washing three times in PBST for 15 min, the membrane was incubated with a 1:3000 dilution of the appropriate secondary antibody, either a sheep anti-mouse (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) or rabbit anti-goat IgG (Jackson Immunolabs, West Grove, PI, USA) for 30 min at room temperature. To measure protein levels, the Western blots were scanned and digitized on an optical scanner (EPSON GT-8000, Seiko, Tokyo, Japan). Quantification of Western blots was performed on a computer using the Gel-Pro image analysis system. All specific values of proteins were standardized to the value of β-actin used as the internal standard to ensure that equal amount of protein is loaded.
Statistical methods
Data are expressed as mean (s.e.m.). Data from all stages were compared by repeated measures analysis of variance followed by the Student–Newman–Keuls test for multiple comparison. Cholesterol level, LA, ANR and NA were compared among groups by one-way analysis of variance followed by Student–Newman–Keuls test for multiple comparisons. A value of P<0.05 (two-sided) was considered statistically significant.
Results
Six mini-pigs (one receiving simvastatin, one receiving 5-HD, two receiving simvastatin+5-HD and two controls) died of ventricular fibrillation during the ischaemia period and they were excluded. Therefore, eight animals were evaluated in each group.
Plasma cholesterol
Cholesterol levels did not differ significantly between the five treatment and control groups (in mg dl−1: control: 79±4; simvastatin: 75±6; 5-HD: 77±5; HMR 1883: 77±3; simvastatin+5-HD: 76±4; and simvastatin+HMR 1883: 75±5) (P>0.05).
No-reflow and infarct size
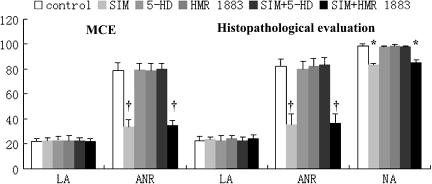
In the control group, the coronary LA as assessed by MCE in vivo was the same as that measured ex vivo by histology (Figure 1). The ANR was also similar (78.5 and 82.3% by the two methods, respectively), with the final NA comprising 99% of the LA. None of the five treatments affected the size of the LA, which was the same as in control (untreated) hearts. However, as also shown in Figure 1, the ANR was markedly reduced (about 50%) in animals receiving simvastatin only and in those given simvastatin+HMR 1883. This effect of simvastatin was abolished by 5-HD, although neither 5HD nor HMR 1883 given alone changed the size of the ANR.
Figure 1.
The variation of ANR (expressed as % LA) and NA (expressed as % LA). *P<0.05, †P<0.01 vs control group. Mean value±s.e.m.; n=8. LA, ANR, NA, MCE, 5-HD and SIM represent ligation area, the area of no-reflow, myocardial contrast echocardiography, 5-hydroxydecanoate and simvastatin, respectively. Simvastatin reduced ANR and NA, whereas 5-HD blocked the effect of simvastatin.
CBF
In the control group, CBF was significantly reduced to about half of the baseline value immediately after release of occlusion (3 h) and at 2 h of reperfusion (Figure 2). In the simvastatin group, CBF was still significantly reduced at these two time points compared with the baseline, but was higher than the CBF in the control group. Addition of HMR 1883 to simvastatin treatment did not modify the effect of simvastatin but addition of 5-HD abolished the effect of simvastatin on CBF (Figure 2).
Figure 2.
Effects of simvastatin on CBF (expressed as ml min−1). *P<0.01 vs baseline; †P<0.05 vs control group. Mean value±s.e.m.; n=8. CBF, 5-HD and SIM represent coronary blood flow, 5-hydroxydecanoate and simvastatin, respectively. Simvastatin increased CBF whereas 5-HD abolished the effect of simvastatin.
Haemodynamic function
Haemodynamic parameters did not differ significantly among the seven groups at baseline. In the control group, LVSP, ±dp dtmax−1 and CO fell, whereas LVEDP increased at the end of 3 h of LAD occlusion (P<0.01) with ±dp dtmax−1 falling further at 2 h of reperfusion. Compared with the values in the control group, ±dp dtmax−1 CO and LVEDP recovered significantly at 2 h of reperfusion in the simvastatin-pretreated group. The haemodynamic parameters in the simvastatin group were not significantly different from those in the simvastatin+HMR 1883 group. In contrast, 5-HD abolished the effects of simvastatin on ±dp dtmax−1, CO and LVEDP (Table 1).
Table 1.
Effect of simvastatin on haemodynamic function
| Treatment group | HR (beats min−1) | LVSP (mm Hg) | LVEDP (mm Hg) | +dp dtmax−1 (mm Hg s−1) | −dp dtmax−1 (mm Hg s−1) | CO (l min−1) |
|---|---|---|---|---|---|---|
| Sham operated (n=8) | ||||||
| Baseline | 100±4 | 115±5 | 3.9±2.0 | 2900±541 | 2612±112 | 2.67±0.12 |
| Ischaemia (3 h) | 98±4 | 114±3 | 4.0±1.2 | 2883±359 | 2574±125 | 2.51±0.11 |
| Reperfusion (2 h) | 97±2 | 116±6 | 3.9±1.8 | 2891±413 | 2596±147 | 2.62±0.16 |
| Control (n=8) | ||||||
| Baseline | 99±5 | 115±4 | 4.0±1.5 | 2850±547 | 2538±207 | 2.58±0.16 |
| Ischaemia (3 h) | 97±6 | 100±4a | 7.1±2.0a | 2475±468b | 2275±191b | 1.26±0.19a |
| Reperfusion (2 h) | 98±6 | 109±3a,c | 6.1±1.6a | 2287±551a,c | 2112±242a,c | 1.34±0.15a |
| SIM (n=8) | ||||||
| Baseline | 99±6 | 116±7 | 3.9±2.6 | 2889±453 | 2568±189 | 2.49±0.17 |
| Ischaemia (3 h) | 98±5 | 101±5a | 6.7±2.3a | 2456±436a | 2164±159a | 1.22±0.16a |
| Reperfusion (2 h) | 97±5 | 111±2b,c | 4.3±1.9a,c,d | 2659±492b,c,d | 2319±183b,c,d | 1.94±0.11a,c,d |
| 5-HD (n=8) | ||||||
| Baseline | 99±5 | 116±3 | 4.1±1.7 | 2846±237 | 2599±154 | 2.66±0.11 |
| Ischaemia (3 h) | 98±6 | 101±2a | 7.3±1.3a | 2415±233b | 2235±134b | 1.25±0.12a |
| Reperfusion (2 h) | 98±7 | 110±4b | 6.4±1.5a | 2177±253a,c | 2089±121ac | 1.51±0.10a |
| HMR 1883 (n=8) | ||||||
| Baseline | 99±4 | 117±3 | 4.2±1.5 | 2863±176 | 2602±112 | 2.56±0.13 |
| Ischaemia (3 h) | 98±3 | 100±3a | 7.2±1.4a | 2442±156b | 2251±142b | 1.28±0.11a |
| Reperfusion (2 h) | 97±2 | 111±3b,c | 6.1±1.1a | 2135±199a,c | 2049±134a,c | 1.57±0.14a |
| SIM+5-HD (n=8) | ||||||
| Baseline | 98±5 | 116±4 | 3.9±1.7 | 2796±137 | 2589±208 | 2.54±0.17 |
| Ischaemia (3 h) | 97±4 | 101±2a | 6.9±1.9a | 2415±106b | 2290±189b | 1.27±0.16a |
| Reperfusion (2 h) | 99±4 | 109±3b,c | 5.91±1.1a | 2189±227a,c | 2098±111a,c | 1.39±0.18a |
| SIM+ HMR 1883 (n=8) | ||||||
| Baseline | 99±2 | 117±5 | 4.0±1.6 | 2868±134 | 2575±111 | 2.51±0.13 |
| Ischaemia (3 h) | 98±3 | 100±4a | 6.8±2.1a | 2457±136a | 2242±131a | 1.25±0.16a |
| Reperfusion (2 h) | 97±4 | 110±2b,c | 4.5±1.2a,c,d | 2691±124a,c,d | 2399±101b,c,d | 1.89±0.11a,c,d |
Abbreviations: CO, cardiac output; HR, heart rate; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; 5-HD, 5-hydroxydecanoate. Data are expressed as the mean value±s.e.m. HR, LVSP, LVEDP, ±dp dtmax−1 and CO, represent heart rate, left ventricular systolic pressure, left ventricular end-diastolic pressure, the maximal change rate of left ventricular pressure rise and fall, and cardiac output, respectively. 5-HD and SIM represent 5-hydroxydecanoate and simvastatin, respectively.
P<0.01 vs baseline
P<0.05
P<0.05 vs ischaemia 3 h
P<0.05 vs control.
VE-cadherin in the myocardium
In control and the five treated groups, VE-cadherin level in the reflow and no-reflow myocardium was significantly lower than that in normal myocardium (P<0.01). In the simvastatin group, VE-cadherin level in the reflow and no-reflow myocardium was significantly higher than that in the control group (P<0.01). VE-cadherin level in the simvastatin group was not significantly different from that in the simvastatin+HMR 1883 group (P>0.05). In contrast, 5-HD abrogated the effect of simvastatin on VE-cadherin (Figure 3).
Figure 3.
Effects of ischaemia and treatments on levels of VE-cadherin (expressed as % actin). *P<0.01 vs normal region, †P<0.01 vs reflow region, ‡P<0.01 vs control group. Mean value±s.e.m.; n=8. 5-HD and SIM represent 5-hydroxydecanoate and simvastatin, respectively. VE-cadherin level in the reflow and no-reflow myocardium was significantly lower than that in normal myocardium. Simvastatin maintained VE-cadherin level, whereas 5-HD abolished the effect of simvastatin.
Discussion
The HMG-CoA reductase inhibitors (statins) are potent inhibitors of cholesterol biosynthesis and are widely recommended for treating hypercholesterolemia and for primary and secondary prevention of cardiovascular diseases. The statins reduce mortality and morbidity associated with cardiovascular disease beyond their cholesterol-lowering effect, especially the incidence of myocardial infarction and stroke (Scandinavian, 1994; Ridker et al., 1998; Williams et al., 1998). However, whether this benefit is partly owing to its effect on tissue-level perfusion is unknown.
The present study showed that the HMG-CoA reductase inhibitor, simvastatin, decreased the ANR and improved CBF. Our data established directly that simvastatin exerts a favourable effect on microvascular perfusion after restoration of flow in epicardial vessels. The proposed mechanism of the no-reflow phenomenon is multifactorial. Animal and post-mortem histologic studies have demonstrated varying degrees of small-vessel vasospasm, endothelial gap and bleb formation, neutrophil plugging of capillaries as well as microvascular compression from myocytes, interstitial oedema and haemorrhage after recanalization (Gavin et al., 1983; Manciet et al., 1994). Our study provides one possible mechanism for the beneficial effect of simvastatin on myocardial no-reflow, namely an effect on KATP channels. These ion channels exist in high density in the sarcolemmal membrane as well as in the mitochondrial membrane. The KATP channel is a weakly inward-rectifying K+ channel that is inhibited by intracellular ATP and activated by intracellular nucleoside diphosphates. A study has shown that KATP channel opening may be an important mechanism of protection against myocardial no-reflow (Gande et al., 2002). The present study demonstrated that 5-HD (a specific mitochondrial KATP channel blocker) but not HMR 1883 (a selective sarcolemmal KATP channel blocker) abolished the beneficial effect of simvastatin on myocardial no-reflow, indicating that such effect was achieved via the selective activation of mitochondrial KATP channels rather than sarcolemmal KATP channels. As activation of the KATP channel is a crucial step in mediating ischaemic preconditioning (Bernardo et al., 1999; O'Rourke, 2000), simvastatin may trigger ischaemic preconditioning.
Myocardial no-reflow has been classified into two different forms: structural and functional no-reflow (Galiuto, 2004; Reffemann and Kloner, 2004). In structural no-reflow, microvessels confined within necrotic myocardium exhibit irreversible structural disintegration; in functional no-reflow, patency of anatomically intact microvessels is compromised because of spasm or microembolization. Our findings showed that myocardial VE-cadherin level was significantly decreased in the reflow and no-reflow myocardium compared to that in the non-ischaemic myocardium, reflecting the fact that microvascular structural integrity was damaged by ischaemia and reperfusion. The data from the study also demonstrated that simvastatin can maintain VE-cadherin level, indicating simvastatin can preserve endothelial junctions and attenuate structural no-reflow.
We found that the NA comprised 99% of the LA in our model of AMI and reperfusion. Simvastatin decreased the NA by about 20%, an effect comparable with that of Wolfrum et al. (2004) and Wayman et al. (2003). The mechanism of simvastatin in reducing infarct size is not well defined and may be explained by at least three mechanisms: preventing myocardial no-reflow, activating the phosphatidylinositide 3-kinase/Akt pathway and modulating nitric oxide synthase expression. The data from this study also showed that simvastatin improved ventricular function, which is in agreement with the reports of Jones et al.(2001, 2002). The beneficial effect of simvastatin on ventricular function was due not only to decreased myocardial necrosis but also to preservation of microvascular integrity and improved myocardial tissue perfusion during AMI and reperfusion.
This study has several limitations. One of particular relevance to the clinical importance of our findings is that our results were observed in a short-term experimental setting and no long-term data are available. Because we assessed infarct size after only 2 h of reperfusion, the ultimate infarct size may be larger. However, as all animals were evaluated at the same point, the comparison between treatments is still valid.
A significant body of clinical research and experimental study within the past decade established important prognostic implications of the occurrence and extent of no-reflow for recovery of regional myocardial function and clinical outcome. There is also evidence that HMG-CoA reductase inhibitors have a favourable impact on clinical outcome. We believe that the beneficial effects of HMG-CoA reductase inhibitors on clinical outcome are partly due to the reduction of myocardial no-reflow. Therefore, patients under treatment with HMG-CoA reductase inhibitors for indications such as high cholesterol may also benefit from an attenuation of myocardial no-reflow and consequently show an improved outcome after suffering from an AMI.
In conclusion, the present study demonstrated that pretreatment with simvastatin markedly attenuated myocardial no-reflow, and 5-HD but not HMR 1883 abolished the effect of simvastatin. These results suggested that this beneficial effect of simvastatin was in part due to the activation of mitochondrial KATP channels.
Acknowledgments
The present work was supported by a grant-in-aid (30572439) from the National Natural Science Foundation of China.
Abbreviations
- AMI
acute myocardial infarction
- ANR
area of no-reflow
- ±dp dtmax−1
maximum change rate of left ventricular pressure rise and fall
- CBF
coronary blood flow
- CO
cardiac output
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- 5-HD
5-hydroxydecanoate
- HMR 1883
1-{5-[2-(5-chloro-o-anisamido)ethyl]-2-methoxyphenyl}sulphonyl-3-methylthiourea
- KATP
adenosine triphosphate-sensitive potassium channel
- LA
ligation area
- LAD
left anterior descending coronary artery
- LV
left ventricle
- MCE
myocardial contrast echocardiography
- NA
necrosis area
Conflict of interest
The authors state no conflict of interest.
References
- Bernardo NL, D'Angelo M, Okubo S, Joy A, Kukreja RC. Delayed ischaemic preconditioning is mediated by opening of ATP-sensitive potassium channels in the rabbit heart. Am J Physiol. 1999;276:H1323–H1330. doi: 10.1152/ajpheart.1999.276.4.H1323. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1996;98:1949–1953. doi: 10.1172/JCI118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein S, Pejman P, Krusemann K. Effects of the KATP blockers glibenclamide and HMR 1883 on cardiac electrophysiology during ischaemia and reperfusion. Eur J Pharmacol. 2000;398:273–284. doi: 10.1016/s0014-2999(00)00322-8. [DOI] [PubMed] [Google Scholar]
- Endoh A, Miura T, Iimura O. Does delayed no-reflow phenomenon cause myocardial necrosis. J Cardiovasc Pathol. 1993;2:225–230. [Google Scholar]
- Engler RL, Schmid-Schönbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischaemia and reperfusion in the dog. Am J Pathol. 1983;111:98–111. [PMC free article] [PubMed] [Google Scholar]
- Freireich EJ, Gehan DP, Rall LH, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- Gavin JB, Thomson RW, Humphrey SM, Herdson PB. Changes in vascular morphology associated with the no-reflow phenomenon in ischaemic myocardium. Virchows Arch. 1983;399:325–332. doi: 10.1007/BF00612950. [DOI] [PubMed] [Google Scholar]
- Gersh BJ. Optimal management of acute myocardial infarction at the down of the next millennium. Am Heart J. 1999;138:188–202. doi: 10.1016/s0002-8703(99)70342-x. [DOI] [PubMed] [Google Scholar]
- Gande S, Miura T, Miki T, Ichikawa Y, Shimamoto K. K (ATP) channel opening is an endogenous mechanism of protection against the no-reflow phenomenon but its function is compromised by hypercholesterolemia. J Am Coll Cardiol. 2002;40:1339–1346. doi: 10.1016/s0735-1097(02)02156-3. [DOI] [PubMed] [Google Scholar]
- Galiuto L. Optimal therapeutic strategies in the setting of post-infarc no reflow: the need for a pathogenetic classification. Heart. 2004;90:123–125. doi: 10.1136/hrt.2003.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey SM, Gavin JB, Herdson PB. The relationship of ischaemic contracture to vascular reperfusion in the isolated rat heart. J Mol Cell Cardiol. 1980;12:1397–1406. doi: 10.1016/0022-2828(80)90124-8. [DOI] [PubMed] [Google Scholar]
- Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the ‘no-reflow' phenomenon: a predictor of complications and left ventricular remodeling in perfused anterior wall myocardial infarction. Circulation. 1996a;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamura A, Iwakura K, Masuyama T, Hori M, Takiuchi S, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996b;93:1993–1999. doi: 10.1161/01.cir.93.11.1993. [DOI] [PubMed] [Google Scholar]
- Jones S, Gibson M, Rimmer D, Gipson TM, Sharp BR, Lefer DJ. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J Am Coll Cardiol. 2002;40:1172–1178. doi: 10.1016/s0735-1097(02)02115-0. [DOI] [PubMed] [Google Scholar]
- Jones SP, Trocha SD, Lefer DJ. Pretreatment with simvastatin attenuates myocardial dysfunction after ischaemia and chronic reperfusion. Arterioscler Thromb Vasc Biol. 2001;21:2059–2064. doi: 10.1161/hq1201.099509. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999;33:234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Ganote CE, Jennings RB. The ‘no-reflow' phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer AM, Campbell B, Shin YK, Caldwell RW. Simvastatin preserves the ischaemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–184. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- Manciet LH, Poole DC, McDonagh PF, Coprland JG, Mathieu-Costello O. Microvascular compression during myocardial ischaemia: mechanistic basis for no-reflow phenomenon. Am J Physiol. 1994;266:H1541–H1550. doi: 10.1152/ajpheart.1994.266.4.H1541. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Myocardial K(ATP) channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- Reffemann T, Kloner RA. Effects of adenosine and verapamil on anatomic no-reflow in a rabbit model of coronary artery occlusion and reperfusion. J Cardiovasc Pharmacol. 2004;43:580–588. doi: 10.1097/00005344-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4,444 patients with coronary heart disease the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- Sato T, Sasaki N, Seharaseyon J, O'Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial, but not sarcolemmal KATP channels in ischaemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol. 2001;38:19–25. doi: 10.1016/s0735-1097(01)01317-1. [DOI] [PubMed] [Google Scholar]
- van't Hof AW, Liem A, de Boer MJ, Zijlstra F. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle Myocardial Infarction Study Group. Lancet. 1997;350:615–619. doi: 10.1016/s0140-6736(96)07120-6. [DOI] [PubMed] [Google Scholar]
- Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- Williams JK, Sukhova GK, Herrington DM, Libby P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol. 1998;31:684–691. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- Wayman NS, Ellis BL, Thiemermann C. Simvastatin reduces infarct size in a model of acute myocardial ischaemia and reperfusion in the rat. Med Sci Monit. 2003;9:155–159. [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, et al. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase Akt pathway. J Cardiovasc Pharmacol. 2004;44:348–355. doi: 10.1097/01.fjc.0000137162.14735.30. [DOI] [PubMed] [Google Scholar]