Abstract
Background and purpose:
Thioredoxin (Trx) is an oxidoreductase that prevents free radical-induced cell death in cultured cells. Here we assessed the mechanism(s) underlying the cardioprotective effects of Trx in vivo.
Experimental approach:
The effects of myocardial ischemia (30 min) and reperfusion were measured in mice, with assays of myocardial apoptosis, superoxide production, NOx and nitrotyrosine content, and myocardial infarct size. Recombinant human Trx (rhTrx, 0.7–20 mg kg-1, i.p.) was given 10 min before reperfusion.
Key results:
Treatment with 2 mg kg-1 rhTrx significantly decreased myocardial apoptosis and reduced infarct size (P<0.01). Nitrotyrosine content of cardiomyocytes was markedly reduced in rhTrx-treated animals (P<0.01). To further identify the mechanisms by which rhTrx may exert its anti-nitrative effect, iNOS expression and production of NOx and superoxide were determined. Treatment with rhTrx had no significant effect on iNOS expression or NOx content in the ischemic/reperfused heart. However, it markedly upregulated mSOD and reduced tissue superoxide content. To further establish a causative link between the anti- peroxynitrite effect and the cardioprotective effect of rhTrx, cultured adult cardiomyocytes were incubated with SIN-1, a peroxynitrite donor, (50 μM for 3 h) resulting in a nitrotyrosine content comparable to that seen in the ischemic/reperfused heart and causing significant cardiomyocyte apoptosis (P<0.01). Treatment with rhTrx markedly decreased SIN-1 induced apoptosis (P<0.01).
Conclusions and implications:
These results demonstrate that Trx is a novel anti-apoptotic and cardioprotective molecule that exerts its cardioprotective effects by reducing ischemia/reperfusion-induced oxidative/nitrative stress.
Keywords: apoptosis, thioredoxin, protein nitration, ischemia/reperfusion
Introduction
It is well recognized that apoptosis, a gene-regulated cell suicide process, contributes significantly to cardiomyocyte loss and the development of cardiac dysfunction in a variety of pathologic conditions, including myocardial ischemia/reperfusion (MI/R) and heart failure (Cohn et al., 1997; Gill et al., 2002). The search for treatments that reduce apoptotic myocyte death may offer novel therapeutic options to reduce myocardial reperfusion injury and improve cardiac function (Ma et al., 1999; Webster and Bishopric, 2003). In a recent study (Tao et al., 2004), we have demonstrated that exogenously administered recombinant human thioredoxin (rhTrx), a 12 kDa antioxidant protein (Holmgren, 1985; Yamawaki et al., 2003), preferentially entered ischemic/reperfused cardiomyocytes and markedly reduced post-ischemic myocardial apoptosis. However, the mechanisms responsible for Trx's cardioprotective effects remain unclear.
Reactive oxygen species have long been recognized to cause oxidative protein modification and to act as the major mediator of ischemia/reperfusion injury (Dart and Sanders, 1988). However, myocardial reperfusion injury cannot be explained exclusively by oxidative stress because multiple enzymatic and non-enzymatic pathways exist that can effectively reduce oxidized molecules (Willerson, 1997). Most recent data have suggested that nitric oxide (NO)-derived reactive nitrogen intermediates (reactive nitrogen species, RNS) may contribute to pathologic tissue injury by nitrative protein modification (nitrative stress), providing potential targets for therapeutic interventions (McCann, 1997; Bauer, 2000; Brookes and Darley-Usmar, 2002; Ischiropoulos and Beckman, 2003). One of the most toxic RNS, peroxynitrite (ONOO−), is formed by NO and superoxide (O•−2) at a near diffusion-limited rate. Considerable evidence now exists that peroxynitrite plays a causative role in post-ischemic myocardial apoptosis and necrosis (Virag et al., 2003; Liang et al., 2004). Although in vitro experiments have demonstrated that Trx is a powerful antioxidative molecule that participates in the detoxification of H2O2 to H2O, whether Trx may have anti-nitrative effects, thus reducing post-ischemic myocardial apoptosis, has not been previously determined.
Therefore, the aims of the present study were (1) to determine the effect of Trx on protein nitration (nitrative stress) in ischemic/reperfused hearts, (2) to identify the mechanisms by which Trx may reduce nitrative stress (i.e., inhibiting NO or superoxide production) and (3) to establish a causative link between Trx's anti-nitrative and anti-apoptotic effects.
Figure 1.
Experimental protocol. Male adult mice were anesthetized and surgical procedure (Surgical Pro.) was performed to induce MI. After 30 min of MI, the myocardium was reperfused for 3 h (for apoptosis, Western blot and biochemical analysis) or 24 h (for infarct size). Ten minutes before reperfusion, mice were randomized to receive either vehicle (PBS, pH 7.5) or recombinant human Trx (rhTrx, 0.7–6 mg kg−1) by i.p. injection.
Materials and methods
Experimental protocols (Figure 1)
Male adult mice were anesthetized with 2% isoflurane. MI was produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery. After 30 min of MI, the slipknot was released and the myocardium was reperfused for 3 h (for apoptosis) or 24 h (for infarct size). Ten minutes before reperfusion, mice were randomized to receive either vehicle (phosphate-buffered saline (PBS), pH 7.5) or rhTrx (0.7–6 mg kg−1) by intraperitoneal (i.p.) injection. Sham operated control mice (Sham MI/R) underwent the same surgical procedures except that the suture placed under the left coronary artery was not tied. At the end of the reperfusion period, the ligature around the coronary artery was retied and 2% Evan's blue dye was injected into the left ventricular cavity. The dye was circulated and uniformly distributed except in that portion of the heart previously perfused by the occluded coronary artery (area-at-risk (AAR)). The heart was quickly excised, and the ischemic/reperfused cardiac tissue (AAR, Evan's blue negative area) was isolated and processed according to the procedures described below. The experiments were performed in adherence to NIH Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal care.
Determination of myocardial apoptosis by TUNEL
To determine myocardial apoptosis in a quantitative manner, the hearts were perfused first with 0.9% NaCl for 5 min and then with 4% paraformaldehyde in PBS (pH 7.4) for 20 min. Four longitudinal sections from ischemic regions were cut and further fixed in 4% paraformaldehyde in PBS for 24 h at room temperature. Fixed tissues were embedded in a paraffin block and two slides at 4–5 μm thickness were cut from each tissue block. Immunohistochemical procedures for detecting apoptotic cardiomyocytes were performed by using an apoptosis detection kit (Boehringer Mannheim, Ridgefield, CT, USA) as described in our previous study (Liu et al., 2004). After rinsing with PBS, slides were coverslipped with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) to permit total nuclei counting.
Using a × 20 objective, the tissue slide (eight slides/heart) was digitally photographed with a QICAM-Fast Digital Camera mounted onto an Olympus BX51 Fluorescence Microscope. Total nuclei (DAPI staining) and the TdT-mediated dUTP nick end labeling (TUNEL)-positive nuclei were counted by an IP Lab Imagine Analysis Software (Version 3.5, Scanalytics, Fairfax, VA, USA) with a custom-made script (by Mr Ken Anderson, Bio Vision Technologies, North Exton, PA, USA). The index of apoptosis (number of positively stained myocytes/total number of myocytes × 100) was automatically calculated and exported to Microsoft Excel for further analysis. Eight slides from each heart were observed and the results were averaged as n=1. Assays were performed in a blinded manner.
Determination of myocardial apoptosis by caspase-3 activation
Cardiac caspase 3 activity was performed by using caspase colorimetric assay kits (Chemicon International Inc., Temecula, CA, USA) as described in our previous study (Gao et al., 2004). In brief, ischemic/reperfused myocardial tissue was homogenized in ice-cold lysis buffer for 30 s using a PRO 200 homogenizer. The homogenates were centrifuged for 5 min at 10 000 g at 4°C, supernatants were collected and protein concentrations were measured by the bicinchoninic acid method (Pierce Chemical, Rockford, IL, USA). To each well of a 96-well plate, supernatant containing 200 μg of protein was loaded and incubated with 25 μg Ac-DEVD-pNA at 37°C for 1.5 h. pNA was cleaved from DEVD and the free pNA was quantified using a SpectraMax-Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at 405 nm. The results were expressed as nmol h−1 mg−1 protein.
Determination of myocardial infarct size
At the end of the 24-h reperfusion period, the ligature around the coronary artery was retied and 0.5 ml of 2% Evan's blue dye was injected into the left ventricular cavity. The dye was circulated and uniformly distributed except in that portion of the heart previously perfused by the occluded coronary artery area-at-risk (AAR). The heart was excised, frozen in −20°C and sliced into 1 mm thick sections perpendicular to the long axis of the heart. Slices were incubated individually using a 24-well culture plate in 1% TTC in phosphate buffer at pH 7.4 at 37°C for 10 min, and photographed with a digital camera. The Evan's blue-stained area (area-not-at-risk; ANAR), the TTC-stained area (red staining, ischemic but viable tissue) and the TTC stain-negative area (infarct myocardium) were digitally measured using an IP Lab Imagine Analysis Software (Version 3.6, Scanalytics, Fairfax, VA, USA) with a custom-made script (by Mr Ken Anderson, Bio Vision Technologies, North Exton, PA, USA). The myocardial infarct size was expressed as a percentage of infarct area over AAR.
Determination of total NOx content in cardiac tissue
Cardiac tissue samples from AAR were rinsed, homogenized in deionized water (1:10, wt vol−1) and centrifuged at 14 000 g for 10 min. The tissue NO and its in vivo metabolic products (NO2 and NO3) in the supernatant, collectively known as NOx, were determined using a chemiluminescence NO detector (SIEVER 280i NO Analyzer) as described in our previous study (Gao et al., 2002).
Quantitation of tissue nitrotyrosine content
Nitrotyrosine content in the ischemic/reperfused cardiac tissue, a footprint of in vivo ONOO− formation and a reliable index for nitrative stress (Aulak et al., 2004; Kuhn et al., 2004), was determined using an enzyme-linked immunosorbant assay method described in our previous publication (Ma et al., 2001). The results were presented as nanomole of nitrotyrosine per gram protein.
Quantification of superoxide production
Superoxide production in ischemic/reperfused heart tissue was measured by lucigenin-enhanced chemiluminescence as described previously (Lund et al., 2000). Superoxide production was expressed as relative light units (RLU) per second per milligram heart weight (RLU mg−1 s−1).
Immunoblotting
Protein from tissue homogenate was separated on SDS-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membranes, and Western blotted with monoclonal antibody against iNOS, magnesium superoxide dismutase (Transduction Laboratories, San Jose, CA, USA) or gp91phox (a major component of nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) Oxidase, Santa Cruz). Nitrocellulose membranes were then incubated with horseradish peroxide-conjugated anti-mouse IgG antibody (1:2000, Cell Signaling, Danvers, MA, USA) for 1 h and the blot was developed with a Supersignal chemiluminescence detection kit (Pierce, Rockford, IL, USA). The immunoblotting was visualized with a Kodak Image Station 400 and the blot densities were analyzed with Kodak 1D software.
Adult mouse cardiomyocyte culture
The hearts from 2- to 3-month-old male C57B16/J mice were removed and perfused at 37°C for ∼3 min with a Ca2+-free bicarbonate-based buffer. The enzymatic digestion was initiated by adding collagenase type B/D and protease type XIV to the perfusion solution. When the hearts became swollen and hard after ∼3 min of digestion, 50 μM Ca2+ was added to the enzyme solution. Approximately 7 min later, the left ventricle was removed, cut into several chunks and further digested in a shaker for 10 min at 37°C in the same enzyme solution. The supernatant containing the dispersed myocytes was filtered into a sterilized tube and centrifuged at 800 g for 1 min. The cell pellet was then resuspended in bicarbonate-based buffer containing 125 μM Ca2+. After the myocytes were pelleted by gravity for ∼10 min, the supernatant was aspirated and the myocytes were resuspended in bicarbonate-based buffer containing 250 μM Ca2+. Myocytes were plated at 0.5–1 × 104 cells cm−2 in the culture dishes precoated with mouse laminin. After 1 h of culture in a 5% CO2 incubator at 37°C, the medium was changed to fetal bovine serum-free minimum essential medium.
Statistical analysis
All values in the text and figures are presented as means±s.d. of n independent experiments. All data were subjected to analysis of variance followed by Bonferoni correction for post hoc t-test. Probabilities of 0.05 or less were considered to be statistically significant.
Results
Thioredoxin treatment attenuated post-ischemic myocardial apoptosis and reduced infarct size
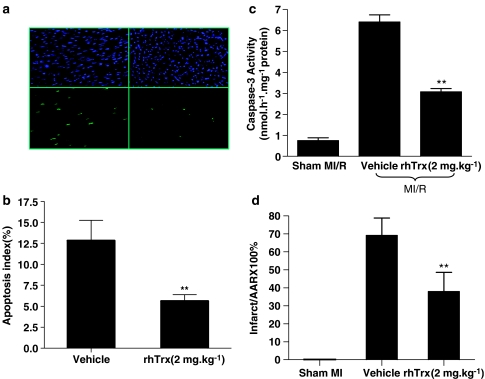
In myocardial tissue from sham MI/R hearts, <0.1% TUNEL-positive staining cells were detected, indicating that the surgical procedure did not induce detectable cardiomyocyte apoptosis. In contrast, TUNEL-positive nuclei were prevalent in tissues from ischemic-reperfused hearts, which were significantly reduced by rhTrx treatment (2 mg kg−1; Figure 2a and b). To provide more evidence in a quantitative and specific manner that rhTrx reduces post-ischemic myocardial apoptosis, myocardial caspase-3 activation, a final common pathway in caspase-dependent apoptosis, was determined. As summarized in Figure 2c, ischemia/reperfusion-induced caspase-3 activation was significantly reduced in rhTrx-treated hearts.
Figure 2.
In vivo administration of rhTrx (2 mg kg−1) reduced post-ischemic cardiac injury. (a) Representative photomicrographs of in situ detection of DNA fragments in heart tissue from mice subjected to 30 min of ischemia and 3 h of reperfusion receiving vehicle or rhTrx. Total nuclei were labeled with DAPI, and apoptotic nuclei were detected by TUNEL staining. (b) Summary of percent of TUNEL-positive myocytes. **P<0.01 vs MI/R+vehicle. N=5–6 hearts/group. (c) Effect of rhTrx treatment on myocardial caspase 3 activity after 30 min of ischemia and 3 h of reperfusion. **P<0.01 vs MI/R+vehicle. N=12–14 hearts/group. (d) Effect of rhTrx treatment on myocardial infarct size after 30 min of ischemia and 24 h of reperfusion. **P<0.01 vs MI/R+vehicle. N=10–12 hearts/group. For colour see online version.
To determine whether an early anti-apoptotic effect of rhTrx (i.e., 3 h after reperfusion) may translate into clinically meaningful infarct reduction at a later time point, myocardial infarct size was determined 24 h after reperfusion. As illustrated in Figure 2d, treatment with rhTrx significantly reduced myocardial infarct size.
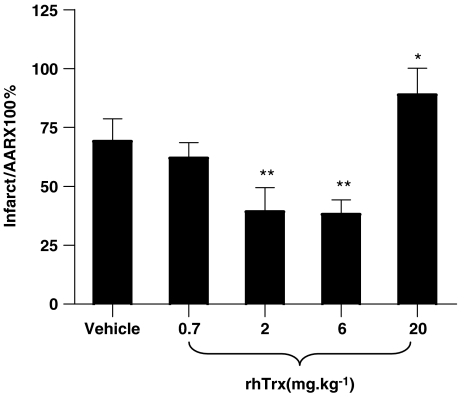
The results presented above clearly demonstrated that treatment with rhTrx at 2 mg kg−1, an effective dose in reducing brain damage following transient focal cerebral ischemia in mice (Hattori et al., 2004), significantly reduced cardiomyocyte apoptosis and limited infarct size. To further determine whether the optimal dose (i.e., 2 mg kg−1) determined in a transient focal cerebral ischemia model is also the best therapeutic dose in limiting myocardial infarct after ischemia and reperfusion, another three groups of mice were treated with different doses of rhTrx 10 min before reperfusion. As summarized in Figure 3, treatment with rhTrx reduced myocardial infarct size in a dose-dependent manner in the range of 0.7–6 mg kg−1. However, when the dose of rhTrx was increased to 20 mg kg−1, a significantly increased myocardial infarct size was observed. These results demonstrated that rhTrx exerts the best cardioprotective effect with a therapeutic dose of 2–6 mg kg−1 in mice.
Figure 3.
Dose-dependent effect of rhTrx on myocardial infarct size after 30 min of ischemia and 24 h of reperfusion. *P<0.05, **P<0.01 vs vehicle group. N=6–12/group.
Thioredoxin treatment markedly reduced nitrotyrosine content in ischemic/reperfused hearts
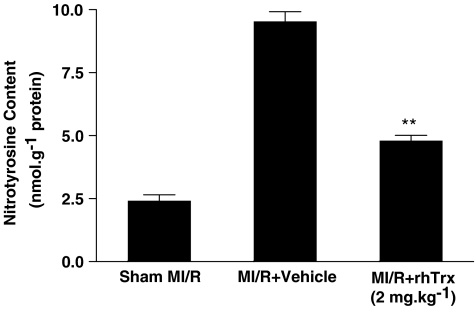
Recent studies have demonstrated that most, if not all, superoxide-initiated tissue injury is caused by ONOO−, rather than O2•− itself or H2O2/HO as previously thought (Beckman and Koppenol, 1996). Therefore, the protective effect of Trx against ischemic/reperfusion injury may not be explained by its previously reported H2O2 detoxification property. To explore the potential mechanism by which Trx may reduce post-ischemic injury, we determined the effect of rhTrx on tissue content of nitrotyrosine, a well-accepted indirect measurement of in vivo ONOO− production and a reliable index for nitrative stress. As summarized in Figure 4, 30 min of ischemia followed by 3 h of reperfusion resulted in a greater than threefold increase in nitrotyrosine content. Treatment with rhTrx before reperfusion significantly reduced nitrotyrosine content, suggesting that rhTrx exerted its cardioprotective effects, at least in part, by its anti-nitrative activity.
Figure 4.
Effect of rhTrx (2 mg kg−1) treatment on myocardial nitrotyrosine content after 30 min of ischemia and 3 h of reperfusion. **P<0.01 vs MI/R+vehicle. N=10–12 hearts/group.
Thioredoxin inhibits nitrative stress by blocking superoxide, but not NO, production in vivo
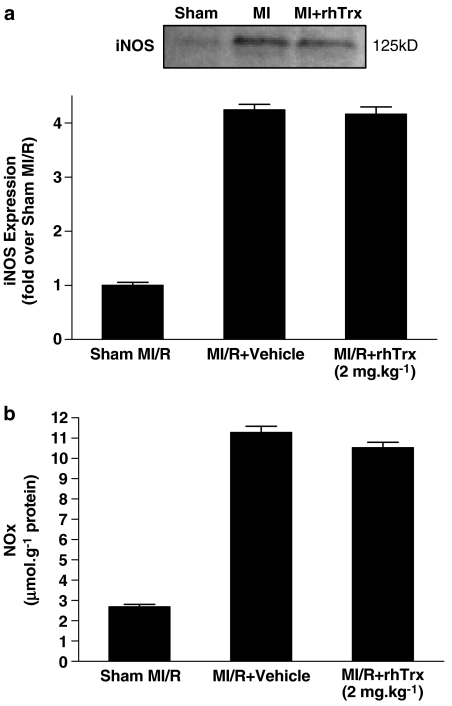
Having demonstrated that treatment with rhTrx markedly attenuated nitrative stress in the ischemic/reperfused heart, we further investigated the mechanisms by which rhTrx may reduce nitrative stress. Consistent with previous reports from other investigators and our own group, significant iNOS expression was detected in ischemic/reperfused cardiac tissue and total NO production as determined by tissue NOx content was markedly increased. Surprisingly, treatment with rhTrx neither inhibited iNOS expression nor total NO production in the ischemic/reperfused heart, suggesting that rhTrx reduced nitrative stress via mechanisms other than direct inhibition of NO production (Figure 5a and b). However, superoxide content in the ischemic/reperfused cardiac tissue was markedly reduced in the rhTrx-treated group when compared with the vehicle-treated group (Figure 6b). As the production of potent nitrative species, such as ONOO−, requires simultaneous production of superoxide and NO, our results indicate that Trx reduces ONOO− formation and subsequent nitrative tissue injury likely by blocking ischemia/reperfusion-induced superoxide, but not NO, production.
Figure 5.
Effect of rhTrx (2 mg kg−1) treatment on cardiac iNOS expression (a) (ischemic/reperfused area, N=4/group) and total NO production (b) (n=8–10/group) after 30 min of ischemia and 3 h of reperfusion.
Figure 6.
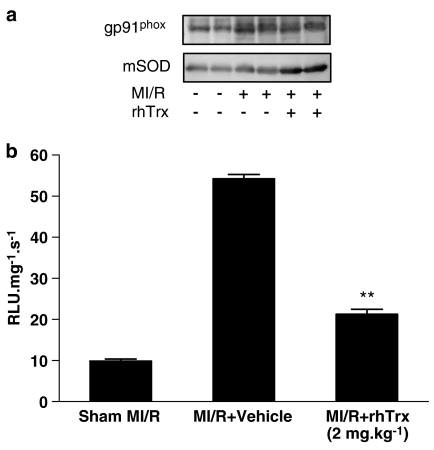
(a) Effect of rhTrx (2 mg kg−1) treatment on NADPH oxidase and mSOD expression. (b) Effect of rhTrx treatment on myocardial superoxide production (RLU) after 30 min of ischemia and 3 h of reperfusion. **P<0.01 vs MI/R+vehicle. N=6–8 hearts/group.
To further dissect the mechanisms by which Trx may reduce superoxide content in ischemic/reperfused cardiac tissue, we determined the effect of rhTrx on the expression of gp91phox, a major component of NADPH oxide that is the most important superoxide-producing enzyme in the ischemic reperfused heart, and the expression of mSOD, the single most important enzyme that competes with NO to inhibit peroxynitrite formation. Interestingly, although ischemia/reperfusion increased gp91phox expression (2.1±0.3-fold over sham MI/R, n=6, P<0.01), treatment with rhTrx failed to inhibit gp91phox expression (P>0.5). In contrast, mSOD expression was not significantly changed in ischemic/reperfused cardiac tissue when compared with sham MI/R. However, treatment with rhTrx significantly increased mSOD expression (2.3±0.2-fold over vehicle group, n=6, P<0.01) (Figure 6a).
Thioredoxin blocked ONOO−-induced cardiomyocyte apoptosis in vitro
Our in vivo experimental results demonstrated that treatment with rhTrx significantly reduced post-ischemic superoxide production and nitrotyrosine content, and decreased myocardial apoptosis. To obtain more evidence to support a causative link between Trx's anti-nitrative and anti-apoptotic effects, an additional study was performed using cultured adult cardiomyocytes. Adult mouse cardiomyocytes were treated with 50 μM SIN-1 (which releases NO and O2•− simultaneously and produces ONOO− (Holm et al., 1998; Balafanova et al., 2002) for 3 h in the presence or absence of rhTrx (500 nM) and cardiomyocyte apoptosis was determined. Consistent with previously published results, exposing cardiomyocytes to an ONOO− donor resulted in significant apoptosis as evidenced by increased TUNEL staining and caspase-3 activation. Most important, treatment with rhTrx significantly attenuated SIN-1 induced apoptosis (Figures 7 and 8), indicating that Trx is capable of blocking nitrative stress-induced cardiomyocyte apoptosis.
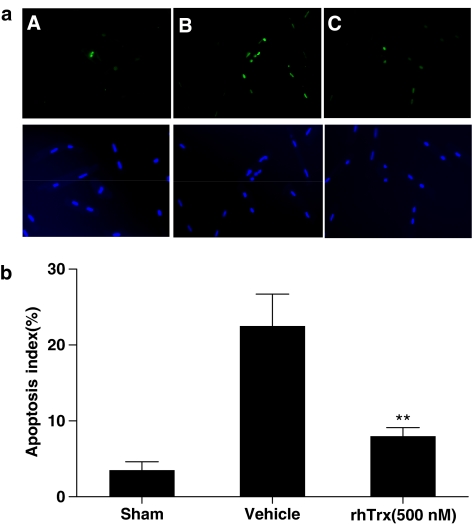
Figure 7.
(a) Representative photomicrographs of in situ detection of DNA fragments in cultured adult mouse cardiomyocytes treated with SIN-1. A: Sham control; B: SIN-1+Vehicle; C: SIN-1+rhTrx (500 nM). Total nuclei were labeled with DAPI, and apoptotic nuclei were detected by TUNEL staining. (b) Summary of TUNEL staining from five independent experiments/group. For colour see online version.
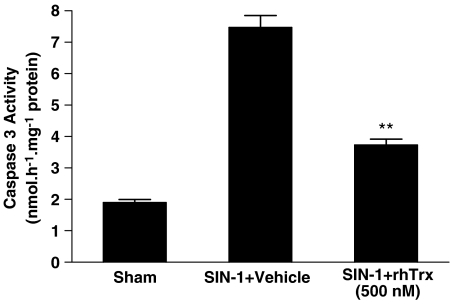
Figure 8.
Effect of rhTrx (500 nM) on SIN-1-induced caspase-3 activation in cultured cardiomyocytes. **P<0.01 vs SIN-1+vehicle. N=15–20 plates/group from at least three animals.
Discussion
We have recently reported that administration of rhTrx before reperfusion exerts significant cardioprotective effects after myocardial ischemia and reperfusion (Tao et al., 2004). However, the mechanism by which Trx may exert its anti-apoptotic effect was not directly determined. The present study provides the first direct evidence that treatment with rhTrx significantly reduces nitrotyrosine content in ischemic/reperfused cardiac tissue, suggesting that Trx may exert its anti-apoptotic effect via a novel anti-nitrative property. In this connection, we have also provided direct evidence that in vitro treatment with rhTrx blocks SIN-1 (a peroxynitrite donor)-induced cardiomyocyte apoptosis. Moreover, we have demonstrated for the first time in an in vivo animal model that treatment with thioredoxin reduces post-ischemic nitrative stress via upregulation of mSOD and inhibition of superoxide content, rather than by inhibition of NO production.
The Trx system, including Trx, Trx reductase and Trx peroxidase, has been shown to play a critical role in maintaining physiologic cardiovascular function as well as protecting cells from oxidative injury under a variety of pathologic conditions (Yamawaki et al., 2003). Our present experimental results provide direct evidence that exogenous rhTrx, when administered before reperfusion, significantly reduces myocardial apoptosis and infarct size. This result suggests that Trx may have great clinical applications in the treatment of cardiovascular diseases where apoptosis plays a pathogenic role.
It has been recognized for many years that superoxide production is markedly increased in ischemic/reperfused cardiac tissue and that subsequent formation of H2O2 and HO. has been thought to be the primary mechanism responsible for reperfusion injury (Dart and Sanders, 1988). However, antioxidant treatment has not been proven beneficial in several clinical trails (Willerson, 1997). Accumulating evidence from recent biochemical and biological studies indicates that besides oxidative stress, NO-initiated nitrative stress also plays a critical role in cell death and tissue injury associated with a variety of cardiovascular diseases (Zweier et al., 2001; Ferdinandy and Schulz, 2003). High concentrations of NO have been reported to induce apoptotic cell death in cultured cells (Chung et al., 2001). The reported mechanisms involve DNA strand breaks (Richard et al., 1995; Pieper et al., 1999), p53 accumulation (Kim et al., 1999; Chung et al., 2001), cdc42 activation (Thomas et al., 2000), p38 mitogen activated protein kinase activation (Ghatan et al., 2000) and mitochondrial permeability transition formation (Hortelano et al., 1997). However, in a recent study, we have demonstrated that in cultured cardiomyocytes, neither superoxide nor NO alone results in significant apoptosis. Specifically, exposing cardiomyocytes to high concentrations of pyrogallol (>100 μM), a superoxide donor, or SNAP (>300 μM), an NO donor, induces significant apoptosis. However, pyrogallol-induced apoptosis is blocked not only by Tiron, a cell-permeable superoxide scavenger, but also by L-NMMA, a non-selective NOS inhibitor (administered 10 min before pyrogallol treatment). On the other hand, SNAP-induced apoptosis at high concentrations is blocked not only by hemoglobin, an NO scavenger, but also by Tiron. Moreover, exposing cultured cardiomyocytes to SIN-1, an ONOO− donor, at concentrations that caused an increase in nitrotyrosine content comparable to that seen in the ischemic/reperfused heart, resulted in significant cardiomyocyte apoptosis (Ma et al., 2004). This result strongly suggests that superoxide and NO induce cardiomyocyte apoptosis by a peroxynitrite-dependent mechanism. Therefore, it is conceivable that therapeutic interventions that block excessive production of NO or superoxide, or directly inhibit ONOO−-induced nitrative stress, may exert a significant cardioprotective effect.
Peroxynitrite is the bi-radical reaction product of NO and O2•− at a diffusion-limited rate (Ferdinandy, 2006). Peroxynitrite has been reported to increase apoptotic cell death in a variety of cell types. The proapoptotic mechanisms of ONOO− include protein and DNA oxidation (Chiarugi and Moskowitz, 2002), lipid peroxidation (Ushmorov et al., 1999), protein nitration (Francescutti et al., 1996; Gow et al., 1996; MacMillan-Crow et al., 1998), apoptosis-inducing factor release (Zhang et al., 2002) and endoplasmic reticulum stress with the subsequent release of caspase 12 (Oyadomari et al., 2001). Our present experiment demonstrated for the first time that administration of rhTrx markedly reduced cardiac nitrotyrosine content and reduced apoptosis. However, treatment with rhTrx failed to inhibit iNOS expression and NO production in ischemic/reperfused cardiac tissue, indicating that Trx blocks nitrative stress and subsequent cell death by mechanisms other than direct inhibition of NO production. In this connection, we have provided direct evidence that treatment with rhTrx caused a significant upregulation of mSOD and markedly reduced superoxide content in the ischemic/reperfused heart. As the production of potent nitrative products, such as ONOO−, requires simultaneous production of NO and superoxide, the upregulation of mSOD and inhibition of superoxide content by Trx provides a likely explanation for Trx's anti-nitrative and anti-apoptotic effects.
Another interesting finding of the present study is that the addition of rhTrx in cultured cardiomyocytes blocked SIN-1-induced cardiomyocyte apoptosis. This result provides the strongest evidence that Trx may exert its anti-apoptotic effect via a novel anti-nitrative mechanism. There are two possible explanations for this novel phenomenon. First, by its antioxidant property, Trx may scavenge SIN-1-released superoxide, thus preventing peroxynitrite formation. However, as NO reacts with superoxide at a diffusion-limited rate, it is unlikely that Trx may effectively compete with NO to react with superoxide. Another possible explanation is that exogenously administered rhTrx may compete with other endogenous anti-apoptotic molecules (such as SOD and endogenous Trx) to react with peroxynitrite, thus preventing their nitrative inactivation as previously reported (Guo et al., 2003). More experiments are needed to provide direct evidence to support this hypothesis.
In summary, we have demonstrated in the present study for the first time that Trx reduces nitrative stress by multiple mechanisms that involve blocking superoxide production (thus preventing production of potent nitrative species) and possibly by directly inhibiting peroxynitrite-induced nitrative inactivation of endogenous anti-apoptotic molecules. As recent studies have clearly demonstrated that nitrative stress plays a critical pathogenic role in many cardiovascular diseases, the novel anti-nitrative property of Trx provides a likely explanation for its cardiovascular protective effect as previously reported. Therefore, Trx may prove to have therapeutic value in cardiovascular diseases where ONOO− generation is increased and apoptotic cell death occurs.
Acknowledgments
This research was supported in part by NIH Grant 2R01HL-63828, a research award from the American Diabetes Association (7-05-RA-83) and a research award from the Commonwealth of Pennsylvania, Department of Health (to XLM).
Abbreviations
- MI/R
myocardial ischemia/reperfusion
- NO
nitric oxide
Conflict of interest
The authors state no conflict of interest.
References
- Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–H38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, et al. Nitric oxide (NO) induces nitration of protein kinase cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon–RACK2 Interactions. A novel mechanism of NO-triggered activation of PKCepsilon. J Biol Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–4139. [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol Cell Physiol. 271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Brookes P, Darley-Usmar VM. Hypothesis: the mitochondrial NO(*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: an alternative function for cytochrome C oxidase. Free Radic Biol Med. 2002;32:370–374. doi: 10.1016/s0891-5849(01)00805-x. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Moskowitz MA. Cell biology: PARP-1 – a perpetrator of apoptotic cell death. Science. 2002;297:200–201. doi: 10.1126/science.1074592. [DOI] [PubMed] [Google Scholar]
- Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, et al. Report of the national heart, lung, and blood institute special emphasis panel on heart failure research. Circulation. 1997;95:766–770. doi: 10.1161/01.cir.95.4.766. [DOI] [PubMed] [Google Scholar]
- Dart RC, Sanders AB. Oxygen free radicals and myocardial reperfusion injury. Ann Emerg Med. 1988;17:53–58. doi: 10.1016/s0196-0644(88)80504-3. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P. Peroxynitrite: just an oxidative//nitrosative stressor or a physiological regulator as well. Br J Pharmacol. 2006;148:1–3. doi: 10.1038/sj.bjp.0706693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescutti D, Baldwin J, Lee L, Mutus B. Peroxynitrite modification of glutathione reductase: modeling studies and kinetic evidence suggest the modification of tyrosines at the glutathione disulfide binding site. Protein Eng. 1996;9:189–194. doi: 10.1093/protein/9.2.189. [DOI] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, et al. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia–reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- Gao F, Tao L, Yan W, Gao E, Liu HR, Lopez BL, et al. Early anti-apoptosis treatment reduces myocardial infarct size after a prolonged reperfusion. Apoptosis. 2004;9:553–559. doi: 10.1023/B:APPT.0000038035.75845.ab. [DOI] [PubMed] [Google Scholar]
- Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, et al. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease – a novel therapeutic target. FASEB J. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, et al. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- Holm P, Kankaanranta H, Metsa-Ketela T, Moilanen E. Radical releasing properties of nitric oxide donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur J Pharmacol. 1998;346:97–102. doi: 10.1016/s0014-2999(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin SA, Marzo I, et al. Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. FEBS Lett. 1997;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association. J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons. J Neurochem. 2004;89:529–536. doi: 10.1111/j.1471-4159.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- Liang F, Gao E, Tao L, Liu H, Qu Y, Christopher TA, et al. Critical timing of L-arginine treatment in post-ischemic myocardial apoptosis-role of NOS isoforms. Cardiovasc Res. 2004;62:568–577. doi: 10.1016/j.cardiores.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Liu HR, Tao L, Gao E, Lopez BL, Christopher TA, Willette RN, et al. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004;62:135–144. doi: 10.1016/j.cardiores.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Lund DD, Faraci FM, Miller FJ, Jr, Heistad DD. Gene transfer of endothelial nitric oxide synthase improves relaxation of carotid arteries from diabetic rabbits. Circulation. 2000;101:1027–1033. doi: 10.1161/01.cir.101.9.1027. [DOI] [PubMed] [Google Scholar]
- Ma XL, Gao F, Nelson AH, Lopez BL, Christopher TA, Yue TL, et al. Oxidative inactivation of nitric oxide and endothelial dysfunction in stroke-prone spontaneous hypertensive rats. J Pharmacol Exp Ther. 2001;298:879–885. [PubMed] [Google Scholar]
- Ma XL, Hu A, Liu HR, Tao L, Christopher TA, Lopez BL. Peroxynitrite is responsible for high concentrations of NO-induced myocardial apoptosis. Nitric Oxide. 2004;11:120–121. [Google Scholar]
- Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Mccann SM. The nitric oxide hypothesis of brain aging. Exp Gerontol. 1997;32:431–440. doi: 10.1016/s0531-5565(96)00154-4. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Verma A, Zhang J, Snyder SH. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999;20:171–181. doi: 10.1016/s0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- Richard V, Blanc T, Kaeffer N, Tron C, Thuillez C. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: role of nitric oxide. Br J Pharmacol. 1995;115:1532–1538. doi: 10.1111/j.1476-5381.1995.tb16647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, et al. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc Natl Acad Sci USA. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Giesler T, White E. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene. 2000;19:5259–5269. doi: 10.1038/sj.onc.1203895. [DOI] [PubMed] [Google Scholar]
- Ushmorov A, Ratter F, Lehmann V, Droge W, Schirrmacher V, Umansky V. Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome C release. Blood. 1999;93:2342–2352. [PubMed] [Google Scholar]
- Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- Webster KA, Bishopric NH. Apoptosis inhibitors for heart disease. Circulation. 2003;108:2954–2956. doi: 10.1161/01.CIR.0000097188.26010.E8. [DOI] [PubMed] [Google Scholar]
- Willerson JT. Pharmacologic approaches to reperfusion injury. Adv Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–1033. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen J, Graham SH, Du L, Kochanek PM, Draviam R, et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J Neurochem. 2002;82:181–191. doi: 10.1046/j.1471-4159.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Fertmann J, Wei G. Nitric oxide and peroxynitrite in postischemic myocardium. Antioxid Redox Signal. 2001;3:11–22. doi: 10.1089/152308601750100443. [DOI] [PubMed] [Google Scholar]