Abstract
Aims
The therapeutic action of tricyclic agents may be accompanied by unwanted effects on the cardiovascular system. The evidence for the effects on vascular and nonvascular smooth muscle comes from animal studies. Whether these studies can be extrapolated to human vessels remains to be determined. Therefore, the present study was designed to investigate the influence of amitriptyline, nortriptyline and sertraline on the contractile responses of human isolated mesenteric arteries to electrical field stimulation, noradrenaline and potassium chloride.
Methods
Arterial segments (lumen diameter 0.8–1.2 mm) were obtained from portions of the human omentum during the course of 41 abdominal operations (22 men and 19 women), and rings 3 mm long were mounted in organ baths for isometric recording of tension. In some artery rings the endothelium was removed mechanically.
Results
In precontracted artery rings amitriptyline, nortriptyline and sertraline (3×10−7–10−4 m) produced concentration-dependent relaxation that was independent of the presence or absence of vascular endothelium. Incubation with indomethacin (3×10−6 m) reduced the pD2 values thus indicating the participation of dilating prostanoid substances in this response. Amitriptyline and nortriptyline inhibited both the neurogenic-and noradrenaline-induced contractions. In contrast, only the highest concentration of sertraline reduced the adrenergic responses. Amitriptyline, nortriptyline and sertraline inhibited contractions elicited by KCl and produced rightward shifts of the concentration-response curve to CaCl2 following incubation in calcium-free solution.
Conclusions
These results indicate that amitriptyline and nortriptyline could act as adrenoceptor antagonists and direct inhibitors of smooth muscle contraction of human mesenteric arteries, whereas sertraline might principally exert its action only as direct inhibitor of smooth muscle contraction. This relaxant mechanism involves an interference with the entry of calcium.
Keywords: amitriptyline, endothelium, human mesenteric artery, nortriptyline, sertraline, smooth muscle
Introduction
The efficacy of tricyclic agents as antidepressants has been known for many years. In addition, some antidepressants, particularly tricyclic antidepressants or selective serotonin reuptake inhibitors, have been used successfully and safely in the treatment of premature ejaculation in patients for whom psychological treatment had failed [1–3]. However, the therapeutic action may be accompained by unwanted effects on the cardiovascular system [4, 5]. The most common manifestations are postural hypotension, sinus tachycardia, and ECG changes [6, 7]. Alterations in cardiac conduction probably result from the inhibitory action of antidepressants on Na+ channels in cardiac tissues in a fashion similar to the action of local anaesthetics [8, 9]. The relaxation of vascular and nonvascular smooth muscle may be caused by the antagonistic action of antidepressants on muscarinic and α-adrenoceptors [10–12]. Interference with calcium entry may be another factor responsible for the inhibitory effects of tricyclic antidepressants on smooth muscle contraction [10, 13].
Whether pharmacologic studies of antidepressants in various animal species can be extrapolated to human vessels remains to be determined. No conclusive data are available concerning the possible alteration by antidepressants of the vascular neuroeffector system in human vessels nor is it known whether their effects are related to an unbalanced production or activity of endothelial relaxing factors, particularly nitric oxide and prostacyclin. Accordingly, the present investigation was undertaken to evaluate the effects of amitriptyline, nortriptyline and sertraline on constrictor responses obtained by noradrenaline, potassium chloride, and stimulation of perivascular nerves in human mesenteric arteries. We examined three compounds which have been in clinical use as antidepressants: amitriptyline, a secondary amine tricyclic, which mainly blocks the reuptake of noradrenaline with moderate effects on reuptake of serotonin; nortriptyline, a tertiary-amine tricyclic, selective inhibitor of noradrenaline reuptake; and sertraline, a selective inhibitor of serotonin reuptake. Observations were made in the presence and absence of endothelium and after exposure to NG-nitro-l-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase, or indomethacin, an inhibitor of cyclo-oxygenase.
Methods
Arterial segments were taken from portions of the human omentum during the course of abdominal operations (41 patients, 22 men and 19 women, aged 35–80 years). The study was approved by the Ethics Commitee of our institution, and informed consent was obtained from each patient before the study. The arteries were immediately placed in chilled Krebs-Henseleit solution, and rings 3 mm long were cut for isometric recording of tension. The outside diameter of the rings was measured with an ocular micrometer within a Wild M8 zoom microscope (Heerbrugg, Switzerland) and ranged from 0.8 to 1.2 mm.
Two stainless steel L shaped pins 100 μm in diameter were introduced through the arterial lumen of the ring. One pin was fixed to the wall of the organ bath, while the other was connected to a strain gauge (Grass FT03). Changes in isometric force were recorded on a Macintosh computer by use of Chart v 3.4/s software and a MacLab/8e data adquisition system (ADInstruments). Each artery ring was set up in a 4 ml bath containing modified Krebs-Henseleit solution of the following mm composition: NaCl, 115; KCl, 4.6; MgCl2.6H2O, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11.1 and disodium EDTA, 0.01. The solution was equilibrated with 95% O2 and 5% CO2 to give a pH of 7.3–7.4. Temperature was held at 37° C. Calcium-free solution containing 1 mm EGTA was made by omitting CaCl2 and EDTA from the control solution. The arteries were washed out with this solution (three times) and Ca2+-free K+-depolarizing solution was prepared by omitting CaCl2 and replacing 100 mm NaCl by 100 mm KCl. To establish the resting tension for maximal force development, a series of preliminary experiments were performed on artery rings of similar length and outer diameter which were exposed repeatedly to 100 mm KCl. Basal tension was increased gradually until contractions were maximal. The optimal resting tension was 1 g. The vessels were allowed to attain a steady level of tension during a 2 h period before testing. In some experiments the endothelium was removed mechanically by inserting a roughened stainless-steel wire into the lumen and gently rolling the rings on wet filter paper. Functional integrity of the endothelium was confirmed routinely by the presence of relaxation induced by acetylcholine (10−7–10−6 m [14]) during contraction obtained with noradrenaline (1–3×10−6 m).
Electrical field stimulation was provided by a Grass S88 stimulator (Grass Instruments, Quincy, U.S.A.) via two platinum electrodes positioned on each side and parallel to the axis of the artery ring. To assess the nature of the contractile responses and avoid direct stimulation of smooth muscle, frequency-response relationships were determined on a group of arteries in the presence and absence of 10−6 m tetrodotoxin, following a procedure previously described [15, 16]. In summary, the protocol was designed to find the optimal stimulation parameters (15 V, 0.2 ms duration) for causing a contractile response that was completely eliminated by 10−6 m tetrodotoxin. Frequency-response relationships were determined using 30 s trains of pulses at 2, 4 and 8 Hz. A period of 10 min was allowed between stimulations. The stimulation sequence was repeated 15 min after the addition of tetrodotoxin (10−6 m), guanethidine (10−6 m) or prazosin (10−6 m). As a control, a second set of stimulations was recorded on artery rings in the absence of antagonists.
The effects of antidepressants on electrical stimulation-induced contractions were determined in a separate group of experiments. After an initial set of stimulations, the vessel rings were incubated with the antidepressants for 15 min and a second set of stimulations was given. As a control two consecutive sets of stimulations were given at identical intervals. Less than 10% variability in the magnitude of electrical stimulation-induced contractions was observed for a given ring during two consecutive sets of control stimulations.
To study relaxation, arterial rings were contracted with noradrenaline (1–3×10−6 m), endothelin-1 (1–3×10−9 m), or KCl (100 mm). After a stable contraction was obtained, concentration-response curves to antidepressants were determined in paired rings in the absence and presence of indomethacin (3×10−6 m), or NG-nitro-l-arginine methyl ester (L-NAME, 10−4 m).
Concentration-response curves for noradrenaline and KCl were determined in a cumulative manner. Control (in the absence of antidepressants) and experimental (in the presence of antidepressants) data were obtained from separate vascular preparations. When KCl was used, phentolamine (10−6 m) was added to the organ bath in order to prevent activation of α-adrenoceptors by noradrenaline released by neuronal depolarization. To study the effects of antidepressants on Ca2+-induced contractile responses, a group of arterial rings was incubated in Ca2+-free solution containing 100 mm KCl. After a 30 min washout period, concentration-response curves to CaCl2 (10−6–10−3 m) were determined in paired rings in the absence and in the presence of antidepressants (10−5 m). Antidepressants were added to the organ bath chambers 15 min before the initiation of cumulative concentration-response curves to agonists.
The following drugs were used: amitriptyline hydrochloride, nortriptyline hydrocloride, tetrodotoxin, guanethidine sulphate, noradrenaline hydrochloride, indomethacin, NG-nitro-l-arginine methyl ester, acetylcholine chloride, endothelin-1, prazosin hydrochlorde (Sigma Chemical Co, St Louis, MO), sertraline hydrochloride (Pfizer, Sandwich, Kent). All drugs were dissolved in Krebs solution except indomethacin, which was dissolved initially in ethanol, and sertraline, which was dissolved in dimethyl sulphoxide. These two substances were further diluted in Krebs solution to the proper final concentration. Drugs were added to the organ bath in volumes of less than 70 μl. Stock solutions of the drugs were freshly prepared every day and kept on ice throughout the experiment.
The data are expresed as mean±95% confidence interval. Contractions are reported as a percentage of response to KCl (100 mm). Relaxation was expressed as a percentage of the agonist-induced contraction. pD2 values (negative logarithm of the molar concentration at which half-maximum response occurs) were determined from individual concentration-response curves by nonlinear regression analysis. The pD2 values were compared by an unpaired t-test and an analysis of variance with Scheffe's test as a posthoc test. The number of rings taken from each patient varied from eight to 16. Concentration-response curves of the tested agonists or frequency-response relationships were performed in the presence and absence of antidepressants in rings obtained from the same patient; the responses obtained in each patient were averaged to yield a single value. Therefore, all n values are presented as the number of patients from whom the blood vessel were obtained. For electrical stimulation experiments, in which the same arteries were stimulated in the absence and presence of antidepressant agents, a paired t-test was used. Statistically significance was accepted at P < 0.05.
Results
Effects of antidepressants in precontracted artery rings
Arteries exposed to antidepressants (3×10−7–10−4 m) did not show significant changes in resting tension as compared with arteries incubated in Krebs-Henseleit solution (n = 4 for each compound; data not shown). To determine whether antidepressants induce relaxation in precontracted arteries, steady contractions were induced by noradrenaline (1–3×10−6 m), endothelin-1 (1–3× 10−9 m) or KCl (100 mm), and cumulative concentrations of antidepressants were added. Amitriptyline, nortriptyline and sertraline (3×10−7–10−4 m) induced concentration-dependent relaxation (Figure 1). Maximal relaxation (>90%) was achieved at concentrations of 10−4 m. pD2 values were not significantly different when noradrenaline, endothelin-1 or KCl were used to induce contraction (results not shown). Figure 1 also shows that no differences in relaxation capacity were observed between arteries with and without endothelium. This rules out the possible involvement of endothelium-derived relaxing factors in the action of these substances.
Figure 1.
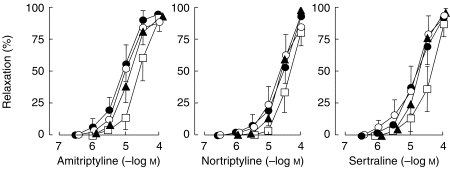
Relaxation to antidepressant drugs of human mesenteric artery rings previously contracted with noradrenaline (1–3×10−6 m) in the presence (•, n = 9), and absence (○, n = 9) of endothelium, and in the presence of L-NAME (10−4 m, n = 5, ▴) or indomethacin (3×10−6 m, n = 5, □). Values are means±95% confidence interval.
In endothelium-intact and in endothelium-denuded arteries treatment with L-NAME (10−4 m), an inhibitor of nitric oxide synthase, had no effect on the relaxation induced by the antidepressants. However, the presence of indomethacin (3×10−6 m) reduced significantly pD2 values (5.12±0.20 vs 4.67±0.21 for amitriptyline P < 0.05; 4.81±0.22 vs 4.40±0.30 for nortriptyline, P < 0.05; 4.90±0.20 vs 4.50±0.16 for sertraline, P < 0.05) without changing maximal responses, thus suggesting the intervention of dilating prostaglandins (Figure 1).
Effects of antidepressants on electrical stimulation-induced responses
Electrical stimulation induced frequency-dependent increases in tension that were abolished by tetrodotoxin (10−6 m), guanethidine (10−6 m) and prazosin (10−6 m), thus indicating that the effect was due to the release of noradrenaline acting on α1-adrenoceptors (results not shown). Low concentrations of nortriptyline and sertraline (10−7 m) did not induce changes in the contractile response to electrical stimulation whereas the same concentration of amitriptyline significantly diminished the contractile response to electrical stimulation (Figure 2). At higher concentrations (10−6–10−5 m) both amitriptyline and nortriptyline reduced neurogenic contractions. However, the same concentrations of sertraline reduced but not significantly (P >0.05) the contractile response to electrical stimulation.
Figure 2.

Neurogenic contractions in human mesenteric arteries under control conditions (▪) and after incubation with various concentrations of amitriptyline, nortriptyline and sertraline (□ 10−7 m,  10−6 m,
10−6 m,  10−5 m). Values are means±95% confidence interval; n, number of patients. *Significant difference from control value, P < 0.05.
10−5 m). Values are means±95% confidence interval; n, number of patients. *Significant difference from control value, P < 0.05.
Effects of antidepressants on noradrenaline-and KCl-induced contractions
Cumulative application of noradrenaline produced concentration-dependent increases in tension with a pD2 of 5.89±0.43 and a maximal response of 115% of that induced by 100 m m KCl (Figure 3). Treatment with amitriptyline and nortriptyline (10−6 m) displaced the control curve of noradrenaline to the right in a parallel manner but differences in the maximal tensions developed were not significant (Figure 3a). At 10−6 m sertraline did not modify the concentration response curve to noradrenaline. However, at 10−5 m all three agents decreased the pD2 values and significantly reduced the maximal responses (Figure 3b).
Figure 3.

Concentration-response curves for noradrenaline in the absence (•, n = 8) and presence of amitriptyline (○, n = 6), nortriptyline (▪, n = 6) and sertraline (▵, n = 6) at concentration of 10−6 m (a) and 10−5 m (b). Values are means±95% confidence interval.
Addition of KCl caused a concentration-dependent contraction with the maximal response at 100 m m. The three antidepressants (10−5 m) depressed the KCl concentration-response curve (Figure 4a). Maximal contractions to KCl were reduced to approximately 60% by the three antidepressants.
Figure 4.

Concentration-response curves to KCl (a) and CaCl2 (b) in the absence (•, n = 7 for a and n = 8 for b) and presence of 10−5 m of amitriptyline (○, n = 5 for a and n = 7 for b), nortriptyline (▪, n = 5 for a and n = 6 for b) and sertraline (▵, n = 5 for a and n = 6 for b). Values are means±95% confidence interval.
Antidepressants and calcium
In arteries incubated in Ca2+-free solution containing 100 mm KCl, the addition of CaCl2 (3×10−6–10−2m) elicited a concentration-dependent contractile response (pD2 = 3.80±0.19, n = 8) (Figure 4b). The presence of antidepressants (10−5 m) shifted the concentration-response curve to CaCl2 to the right and significantly decreased the pD2 values (amitriptyline, 3.06±0.12, n = 7; nortriptyline, 3.01±0.37, n = 6; sertraline, 2.97±0.23, n = 6; P < 0.05). Differences in the maximal tensions developed were also reduced.
Discussion
The present results show that amitriptyline, nortriptyline and sertraline are effective in relaxing the vascular smooth muscle of human arteries precontracted with noradrenaline or KCl. In addition, amitriptyline and nortriptyline are potent inhibitors of neurogenic-induced contractions.
One of the possible mechanisms triggered by antidepressants to induce relaxation of the mesenteric vasculature may be derived from blockade of α1-adrenoceptors that mediate contractions [10, 13, 17]. Amitriptyline and nortriptyline (10−6 m) antagonized the noradrenaline concentration-response curve in a competitive fashion whereas at higher concentration (10−5 m) the antagonism was noncompetitive. These results are in agreement with the noncompetitive inhibition of the noradrenaline contractions by protriptyline, amitriptyline and xylamine observed in rat aortic smooth muscle [13]. Direct evidence for antagonism by antidepressants of α-adrenoceptor comes from radioligand binding studies at the human brain [18]. In addition, amitriptyline and nortriptyline reduced the contractile response to electrical stimulation in a concentration-dependent manner. The tertiary-amine amitriptyline was more potent than the secondary-amine nortriptyline. On the other hand, sertraline, a selective inhibitor of serotonin reuptake, did not modify the contractile response to electrical stimulation and the noradrenaline-induced contraction was reduced only when high concentrations (10−5 m) of sertraline were used. These results suggest that sertraline has no appreciable effects on noradrenergic responses of human mesenteric arteries and confirm results from in vitro radioligand receptor binding studies indicating that sertraline has no significant affinity for α-adrenoceptors [19, 20].
The three antidepressants studied also relaxed vessels contracted with endothelin-1 and KCl which indicate the existence of a nonspecific mechanisms other than α-adrenoceptor blockade. Thus the relaxant effects of antidepressants are not restricted to events triggered by α-adrenergic receptors but seem to reflect a general modification of the contractile function of vascular smooth muscle. One of these mechanisms may derive from interference with the entry of calcium. Contractions to KCl, which are mediated by voltage-dependent Ca2+ channels [21], diminished in the presence of antidepressants. In addition, the three antidepressants reduced contractions elicited by CaCl2 in KCl-depolarized arteries. These results indicate that antidepressants could inhibit Ca2+ entry through the depolarized cell membrane in human mesenteric arteries and thus support an earlier report of antagonism between Ca2+ and desipramine in the perfused rat renal artery [10]. Interference with Ca2+ influx has been proposed for the inhibitory effects of tricyclic agents on contractions in rat aortic [13] and vas deferens [22] smooth muscle.
Because antidepressants cause Ca2+ release from inositol 1,4,5-triphosphate-sensitive Ca2+ stores [23, 24], it is possible that the molecular target of antidepressants may be the second messenger systems. The net result of the activation of the secondary messenger systems is to increase the activity of the various protein kinases that phosphorylate membrane-bound proteins to induce the physiologic response [25]. Sertraline and imipramine both selectively enhance cyclic AMP-dependent protein kinase activity in the frontal cortex of the rats [26] and protein kinase C activation participates in the regulation of smooth muscle contraction. Since protein kinase C participation was not studied in this work, a contribution of intracellular Ca2+ cannot be excluded. Under the present experimental conditions, the effect of antidepressants on the release of intracellular Ca2+ appears to be masked by a marked effect on extracellular Ca2+ influx and thus vasodilation is observed. However, the increase in intracellular Ca2+ by protein kinase C is closely associated with prostacyclin production in human endotelial cells [27] and aortic smooth muscle cells [28]. The attenuation by indomethacin of the relaxant effects of antidepressants is consistent with the release of prostacyclin from the artery wall.
Since removal of the endothelial layer did not modify the relaxant response of antidepressants, it appears that endothelium-relaxing factors or hyperpolarizing factors do not modulate this response. The fact that maximal contractions to KCl did not differ between rubbed and unrubbed artery rings suggests that a change in the mechanical properties of the vessel wall due to endothelium removal does not play an important role in the reactivity of rubbed arteries. Furthermore, the response does not involve the intervention of the l-arginine-nitric oxide pathway because L-NAME, an inhibitor of nitric oxide synthase [29], did not modify the antidepressants-induced relaxation.
In the absence of cardiac disease, the principal vascular problem associated with tricyclic agents is postural hypotension due to α1-adrenergic receptor antagonism. The recommended therapeutic plasma concentrations for antidepressant treatment with amitriptyline (0.1–1×10−6 m) and nortriptyline (0.2–0.6×10−6 m) are within the range of the concentrations tested in the present experiments [30, 31]. However, the concentrations of sertraline used in our experiments are 10–100 fold higher than the anticipated plasma concentrations in patients being treated for depression with this compound [20]. Due to the low α-adrenergic antagonistic action of sertraline as well as the lack of effects on adrenergic neurotransmission observed in our experiments, we can speculate that sertraline has a low risk of inducing postural hypotension except at toxic plasma concentrations.
In summary, our study shows that amitriptyline, nortriptyline and sertraline dilate human mesenteric arteries through different mechanisms. Amitriptyline and nortriptyline nonselectively antagonize the contractile response to both endogenous and exogenous noradrenaline. Inhibition of Ca2+ entry through voltage-dependent Ca2+ channels contributes to the relaxation. Sertraline has no effect on adrenergic contraction but inhibits Ca2+ entry. The three antidepressant drugs release prostacyclin from the vessel wall, an effect which appears to be independent of the presence or absence of endothelium and nitric oxide formation.
Acknowledgments
This work was supported by the Comision Interministerial de Ciencia y Tecnología, Ministerio de Sanidad and Generalitat Valenciana.
References
- 1.Zajecka J, Fawcett J, Schaff M, Jeffriess H, Guy C. The role of serotonin in sexual dysfunction. J Clin Psychiat. 1991;52:66–68. [PubMed] [Google Scholar]
- 2.Kara H, Aydin S, Agargün MY, Odabas Ö, Yilmaz Y. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo controlled study. J Urol. 1996;156:1631–1632. 10.1097/00005392-199611000-00023. [PubMed] [Google Scholar]
- 3.Kim SC, Seo KK. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-blind, placebo controlled study. J Urol. 1998;159:425–427. doi: 10.1016/s0022-5347(01)63940-5. [DOI] [PubMed] [Google Scholar]
- 4.Roose SP, Glassman AH, Giardina EGV. Tricyclic antidepressants in depressed patients with cardiac conduction disease. Arch Gen Psychiatry. 1987;44:273–275. doi: 10.1001/archpsyc.1987.01800150093011. [DOI] [PubMed] [Google Scholar]
- 5.Glassman AH, Roose SP, Bigger JTJ. The safety of tricyclic antidepressants in cardiac patients: risk-benefit reconsidered. JAMA. 1993;269:2673–2675. 10.1001/jama.269.20.2673. [PubMed] [Google Scholar]
- 6.Preskorn SH, Irwin HA. Toxicity of tricyclic antidepressants-kinetics, mechanism, intervention: a review. J Clin Psychiat. 1982;43:151–156. [PubMed] [Google Scholar]
- 7.Kirch W. Hemodynamic effects of extracardiac drugs. Int J Clin Pharmacol Ther. 1995;33:190–193. [PubMed] [Google Scholar]
- 8.Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C. Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284:208–214. [PubMed] [Google Scholar]
- 9.Barber MJ, Starmer CF, Grant AO. Blockade of cardiac sodium channels by amitriptyline and diphenylhydantoin: Evidence for two use-dependent binding sites. Circ Res. 1991;69:677–696. doi: 10.1161/01.res.69.3.677. [DOI] [PubMed] [Google Scholar]
- 10.Hrdina PD, Ling GM. Studies on the mechanism of the inhibitory effect of desipramine (DMI) on vascular smooth muscle contraction. J Pharmacol Exp Ther. 1970;173:407–415. [PubMed] [Google Scholar]
- 11.Doggrell SA, Vicent L. The postsynaptic effects of antidepressant drugs in the rat anococcygeus muscle. J Pharm Pharmacol. 1981;33:720–724. doi: 10.1111/j.2042-7158.1981.tb13912.x. [DOI] [PubMed] [Google Scholar]
- 12.Rehavi M, Weiss H, Nissenkorn I, Rubinstein R, Cohen S. A comparative study of the affinities of some tricyclic antidepressants for the muscarinic cholinergic receptor in human and guinea-pig bladder, ilium and brain in relation to differential drug potency. Life Sci. 1987;40:1819–1827. doi: 10.1016/0024-3205(87)90093-2. 10.1016/0024-3205(87)90093-2. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y. Inhibitory effect of noradrenaline uptake inhibitors on contractions of rat aortic smooth muscle. Br J Pharmacol. 1996;117:533–539. doi: 10.1111/j.1476-5381.1996.tb15223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Aldasoro M, Martínez C, Vila JM, Flor B, Lluch S. Endothelium-dependent component in the contractile responses of human omental arteries to adrenergic stimulation. Eur J Pharmacol. 1993;250:103–107. doi: 10.1016/0014-2999(93)90626-s. 10.1016/0014-2999(93)90626-S. [DOI] [PubMed] [Google Scholar]
- 16.Medina P, Acuña A, Martínez-León JB, et al. Arginine vasopressin enhances sympathetic constriction through the V1 vasopressin receptor in human saphenous vein. Circulation. 1998;97:865–870. doi: 10.1161/01.cir.97.9.865. [DOI] [PubMed] [Google Scholar]
- 17.McCulloch MW, Story DF. Antagonism of noradrenaline and histamine by desipramine in the isolated artery of the rabbit ear. Br J Pharmacol. 1972;46:140–150. doi: 10.1111/j.1476-5381.1972.tb06856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richelson E, Nelson A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984;230:94–102. [PubMed] [Google Scholar]
- 19.Koe BK. Preclinical pharmacology of sertraline: a potent and specific inhibitor of serotonin reuptake. J Clin Psychiat. 1990;51:13–17. [PubMed] [Google Scholar]
- 20.Murdoch D, McTavish D. Sertraline: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992;44:604–624. doi: 10.2165/00003495-199244040-00007. [DOI] [PubMed] [Google Scholar]
- 21.Van Breemen C, McNaughton E. The separation of cell membrane calcium transport from extracellular Ca2+ exchange in vascular smooth muscle. Biochem Biophys Res Commun. 1970;39:567–574. doi: 10.1016/0006-291x(70)90241-x. 10.1016/0006-291X(70)90241-X. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y. Inhibition of contractions by tricyclic antidepressants and xylamine in rat vas deferens. Eur J Pharmacol. 1997;327:41–47. doi: 10.1016/s0014-2999(97)89676-8. 10.1016/S0014-2999(97)89676-8. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda H, Nishida A, Saito H, Shimizu M, Yamawaki S. Imipramine stimulates phospholipase C activity in rat brain. Neurochem Int. 1994;25:567–571. doi: 10.1016/0197-0186(94)90155-4. 10.1016/0197-0186(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu M, Nishida A, Yamawaki S. Forskolin and phorbol myristate acetate inhibit intracellular Ca2+ mobilization induced by amitriptiline and bradykinin in rat frontocortical neurons. J Neurochem. 1993;61:1748–1754. doi: 10.1111/j.1471-4159.1993.tb09812.x. [DOI] [PubMed] [Google Scholar]
- 25.Nestler EJ, Walaas SJ, Greengard P. Neuronal phosphoproteins: physiological and clinical implications. Science. 1984;225:1357–1364. doi: 10.1126/science.6474180. [DOI] [PubMed] [Google Scholar]
- 26.Tadokoro C, Kiuchi Y, Oguchi K, Kamijima K. Effects of imipramine and sertraline on protein kinase activity in rat frontal cortex. Eur J Pharmacol. 1998;19:51–54. doi: 10.1016/s0014-2999(97)01530-6. 10.1016/S0014-2999(97)01530-6. [DOI] [PubMed] [Google Scholar]
- 27.Griesmacher A, Wiegel G, David M, Horvarth G, Mueller M. Functional implication of cAMP and Ca2+ on prostaglandin I2 and thromboxane A2 synthesis by human endothelial cells. Arterioscler-Thromb. 1992;12:512–518. doi: 10.1161/01.atv.12.4.512. [DOI] [PubMed] [Google Scholar]
- 28.Erbrich A, Church D, Vallotton M, Lang U. Regulation of prostacyclin production by [Ca2+]i and protein kinase C in aortic smooth muscle cells. Am J Physiol. 1992;263:E800–E806. doi: 10.1152/ajpendo.1992.263.4.E800. [DOI] [PubMed] [Google Scholar]
- 29.Rees DD, Palmer RMJ, Schultz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeVane CL, Jarecke CR. Cyclic antidepressants. In: Evans EE, Schentag JJ, Jusko WJ, Relling MV, editors. Applied Pharmacokinetics. 3. Vancouver: Applied Therapeutics, Inc.; 1992. pp. 1–47. [Google Scholar]
- 31.Doogan DP, Caillard V. Sertraline: a new antidepressant. Drugs. 1988;44:604–624. [PubMed] [Google Scholar]


