Abstract
Aims
The primary aims of the study were to estimate the exposure of infants to paroxetine via breast milk and to determine the maternal milk:plasma ratio (M/P) of paroxetine. Secondary aims were to compare single point and area under the curve (AUC) estimates of M/P, to assess variability of M/P in fore and hind milk, and to compare the observed M/P with that predicted by a model.
Methods
Two studies were performed. In one study, six nursing mothers who were being treated with paroxetine were studied over a 24 h dose interval at steady-state. The total amount of paroxetine in the milk was measured, which represented the ‘dose’ to the infant. The M/PAUCwas calculated and compared with a predicted value. In the second study, four nursing mothers who were being treated with paroxetine, were studied at steady-state, around a normal infant feeding time. A single plasma sample and a prefeed milk sample were taken approximately 3 h after the morning dose of paroxetine, and a postfeed milk sample taken around 1 h later. The dose received by the infant was estimated from the average milk concentrations of the pre and postfeed samples using standard assumptions, and M/P calculated directly. Plasma concentrations of paroxetine were measured in 8 of the 10 infants in the two studies.
Results
The mean dose of paroxetine received by the infants in the first study was 1.13% (range 0.5–1.7) of the weight adjusted maternal dose. The mean M/PAUC was 0.39 (range 0.32–0.51). The predicted M/P was 0.22. The mean dose of paroxetine received by the infants in the second study was 1.25% (range 0.38–2.24) of the weight adjusted maternal dose. The mean M/P was 0.96 (range 0.31–3.33) and did not differ between fore and hind milk. The drug was not detected in the plasma of seven of the infants studied and was detected but not quantifiable (<4 μg l−1) in one infant. No adverse effects were observed in any of the infants.
Conclusions
Measured M/P and estimated infant dose were similar in the two studies, although the range was wider for the single point study. Paroxetine can be considered ‘safe’ during breast feeding because the dose transferred to the infant is well below the recommended safety limit of 10% of the weight adjusted maternal dose, concentrations in the infants were generally undetectable, and no adverse effects were reported.
Keywords: human, infant dose, milk, paroxetine
Introduction
It is important to know the extent of transfer of drugs into human milk in order to assess the safety of breast feeding during maternal ingestion of drugs. Depression in the mother is common in the peripartal period, and pharmacological treatment is often necessary. Breast feeding is important for the early bonding of mother and infant, and the risks of maternal drugs must be balanced against the advantages of this. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine, paroxetine and sertraline are being used in postpartum depression, despite the lack of information on their distribution into milk, and their safety.
Fluoxetine has been studied in some detail, with reports of infant dose as a percentage of maternal dose corrected for body weight of approximately 6.3–13.9% [1]. Given that a drug is usually not considered safe during breast feeding if this percentage is 10% or greater, it would seem unwise to recommend fluoxetine. Further, there have been cases of suspected adverse reactions in infants receiving fluoxetine via milk [2, 3]. In one of these cases the infant developed symptoms of ‘colic’, which resembled a mild serotonin syndrome, that diminished on dechallenge and reappeared on rechallenge. The major symptoms were increased crying, decreased sleep, increased vomiting and watery stools. The infant had plasma concentrations of fluoxetine and norfluoxetine above usual maternal therapeutic concentrations [3]. Data for sertraline are sparse. An M/P-value of 0.63 can be calculated from the data in one study [4] and an estimated weight adjusted infant dose of 0.5–5% from another [5].
Preliminary data for paroxetine suggests that the infant exposure is likely to be lower than that of fluoxetine. A single case report in which the concentration of paroxetine was measured in a single milk sample around 4 h after the dose suggested that the weight adjusted infant dose would be around 0.34% of the maternal dose [6]. This report concluded that more research was necessary and should ideally include measurements of maternal M/PAUC, plasma paroxetine concentrations in the infant, and observations of possible side-effects in the infant.
The M/PAUC method (study 1) involves the measurement of the area under the concentration-time curve (AUC) of both milk and plasma over a dose interval. This method provides the best time-averaged representation of concentrations in the respective phases. However, this approach is not always possible and sometimes only single point estimates are available (study 2). These provide an opportunity to look at the effect of milk lipid content by comparing concentration in fore and hind milk. They also allow most of the expression of milk to occur by the natural suckling reflex rather than the breast pump.
It is possible to predict the likely M/P, and the potential dose exposure of infants, with reasonable accuracy based on the drug's protein binding characteristics, octanol:water partition coefficient and pKa [7]. The predicted M/P of paroxetine (a base, pKa −9.9, octanol water partition coefficient −3.38, protein binding in plasma −95%) based on the log phase regression model of Atkinson & Begg [7] is 0.22. With this M/P, the estimated weight adjusted dose the infant would receive during breast feeding is approximately 0.5%. Although this is consistent with the case report [6], a single case report and a theoretical prediction are an insufficient basis for sound clinical decision making. A more complete study was necessary.
The aims of this study were:
to estimate the infant ‘dose’ received via milk compared with the maternal dose.
to determine the milk to plasma area under the curve ratio (M/PAUC) of paroxetine at steady state.
to compare single point and AUC estimates of M/P.
to assess variability of M/P between fore and hind milk.
to compare the M/PAUC with that predicted by a theoretical model.
to measure the concentration of paroxetine in the infant's plasma.
Methods
Patients and sampling
Study 1
The patients were six women who were taking paroxetine for at least 2 weeks at the same dosing schedule, and who were established on breast feeding. Paroxetine was prescribed for the treatment of a major depressive episode. The decision to start the women on paroxetine was made by a team who were independent of the study. The women all had a good supply of milk and were able to express milk easily. The infants were required to be able to bottle feed so that they could continue to feed during the trial period. The study was approved by the Canterbury Ethics committee of the Southern Regional Health Authority.
The patients were admitted to the Department of Medicine Research Unit for the 24 h period of the study. An indwelling cannula was placed in a forearm vein prior to the morning dose of paroxetine. A predose blood sample was taken for the measurement of the plasma paroxetine concentration. The patient fed her baby at a time as close as possible to the time of drug administration. The paroxetine was then administered to the mother in the standard dose that she had been taking and at approximately the time she normally took her dose (usually between 08.00 and 09.00 h).
Blood (5 ml) was collected into EDTA tubes at 1, 2, 4, 6, 8, 12 and 24 h after the administration of paroxetine. The exact times of sampling were recorded. The 24 h sample was taken just prior to the next dose. The blood was centrifuged at 2000 g for 10 min and plasma separated and stored at −80° C until assayed. Milk was collected from both breasts until empty via an electric pump whenever the mother felt her breasts were full, or when the baby required to be fed, or at the following intervals, 0–4 h, 4–8 h, 8–12 h, 12–16 h, 16–20 h, 20–24 h. The total volume of milk was recorded on each occasion and an aliquot (10 ml) taken for subsequent analysis of paroxetine. The pH and temperature of the milk were recorded. The samples of milk were stored at −80° C until assayed. The remainder of the milk was available for feeding the infant.
A single sample of blood was taken from the infant where possible for measurement of a plasma paroxetine concentration at a time point as close as possible to that of the mother's drug administration. This sample was taken by a member of the paediatric department who was skilled in taking blood from infants. The mother's informed consent for this procedure was recorded. The mothers were asked in general terms about any side-effects they had observed in their infant that they related to the administration of paroxetine.
Study 2
Four breast feeding women being treated for postnatal depression, and their infants, were recruited from medical practitioner referrals. The mother/baby pairs were studied around a normal infant feeding time during the morning, after ingestion of the regular daily dose of paroxetine (usually between 08.00 and 09.00 h). A blood sample (5 ml) and a prefeed milk (fore milk) sample (10–15 ml) were taken from the mother at approximately the same time, and a postfeed (hind milk) sample was also taken. Blood and milk samples were treated as in Study 1, except that blood was collected into heparinized tubes. A blood sample was also obtained from the infants soon after the maternal dosing. The mothers were asked about possible side-effects in the infant as in study 1. The study was approved by the Ethics Committee of the King Edward Memorial Hospital for Women and all patients gave written informed consent.
Analysis of paroxetine by h.p.l.c
The same h.p.l.c. analytical method was used for both studies, but was performed in the respective institutions (Christchurch Hospital, study 1, The Western Australian Centre for Pathology and Medical Research, study 2). Plasma (0.5 ml) was mixed with internal standard, amitriptyline 100 μl (165 μg l−1) then made alkaline with 250 μl of 2% Na2B4O7(pH 9.2). Paroxetine and amitriptyline were extracted by vortexing for 1 min with 5 ml of 1% isoamylalcohol in hexane. Separation of phases was achieved by centrifugation at 2000 g for 10 min and the organic phase was transferred to a clean tube and back extracted with 100 μl 0.05 m HCl by vortexing for 1 min. The organic phase was discarded following centrifugation and 80 μl of the acid phase was injected onto the h.p.l.c. column. Paroxetine concentrations in unknown samples were interpolated from a plasma paroxetine:amitriptyline peak height ratio vs paroxetine concentration standard curve (200, 100, 50, 25 and 10 μg l−1). Paroxetine concentrations in milk were determined by dividing milk samples into six (0.5 ml) aliquots, five of which were spiked with aqueous paroxetine standards giving final paroxetine concentrations equivalent to the plasma standard curve values. Milk samples were then assayed as for plasma samples. Paroxetine concentrations in milk were calculated by dividing the y intercept of a peak height ratio (paroxetine:amitriptyline) vs paroxetine concentration curve by the slope of the regression line for each milk sample. This procedure avoids variable recovery of paroxetine and/or the internal standard that may arise because of variable milk composition.
Analyses were carried out using a RP Select B 250×4.6 mm i.d. Lichrospher® column (Merck) and a mobile phase of 35% acetonitrile and 65% water containing 0.01% NaCl w/v and 0.01% H3PO4 v/v (pH 2.5). The mobile phase flow rate was 1.5 ml min−1 and compounds were detected by their u.v. absorbance at 210 nm. Under these conditions the approximate retention times for paroxetine and amitriptyline were 12.4 and 15.8 min, respectively. The plasma standard curves were linear over the range 10–200 μg l−1 with correlation coefficients (r2) ranging from 0.977 to 1.00. The intraday coefficients of variation (CVs) for plasma were 3.3% and 4.8% at 150 and 20 μg l−1, respectively. The interday CVs for the plasma assay were 3.9% and 5.5% at 150 and 20 μg l−1. The intraday CVs for milk were 4.8% and 8.9% at 150 and 20 μg l−1 respectively. The interday CVs for milk were 8.6% and 12.2% at 150 and 20 μg l−1. Quality control samples were assayed with each analytical run and calculated concentrations were within ± 15% of spiked values. The limit of detection (3×baseline noise) for paroxetine using this method was 2 μg l−1 and the limit of quantification was 4 μg l−1.
Data analysis
Study 1 Pharmacokinetic analysis
The plasma AUC(0,24h) was calculated using the trapezoidal rule for the ascending part of the curve and the log trapezoidal rule for the descending part of the curve. The time to peak concentration (tmax) and the concentration at maximum (Cmax) were read directly from the data. The milk AUC was calculated using a linear ‘rectangular’ method that involved multiplying the concentration measured in the aliquot from each time collection by the time period from the last milk collection to the current milk collection. Successive AUCs were summed to provide the AUC(0,24h). Cmax and tmax were determined as for plasma.
M/P ratio and infant dose
M/PAUC was calculated from the milk and plasma AUC(0,24h) data. The absolute dose received by the infant was calculated in two ways. The amount (μg) excreted in each time period was calculated as concentration×volume of milk and cumulated over the 24 h of the study. In this way the ‘maximum theoretical daily dose’ that the infant would receive was derived. Where the milk dose was not recorded over the full 24 h period, the daily dose was adjusted to be representative of the full 24 h period. The dose was also calculated as the product of the average maternal plasma paroxetine concentration, the M/PAUC and an assumed milk intake of 0.15 l kg−1 day−1. The infant dose was standardized to the infant's body weight and expressed as a percentage of the maternal weight-adjusted dose.
Study 2 M/P ratio and infant dose
M/P was calculated directly from the paired maternal plasma concentration and pre and postfeed milk samples, giving two estimates for each mother. Absolute infant dose was estimated from the product of the average concentration of paroxetine in the pre and postfeed milk samples and an assumed average milk consumption of 0.15 l kg−1 day−1. Infant intake was described as the percentage of the weight-adjusted maternal dose.
Results
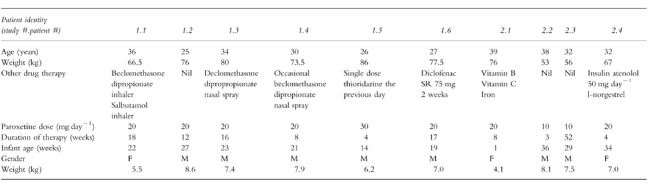
The demographic details of the mothers and their babies are shown in Table 1. In study one, one mother was taking 30 mg paroxetine while all the others were taking 20 mg day−1. The mothers had been taking paroxetine at this dose for a minimum of 4 weeks (maximum 18 weeks). The youngest infant at the time of study was 14 weeks, confirming that the milk was ‘mature’ milk. In the second study, two mothers were taking 20 mg and two were taking 10 mg paroxetine daily. The mothers had been taking paroxetine at this dose for a minimum of 3 weeks (maximum 1 year). The youngest infant was 1 week old and the oldest was 36 weeks.
Table 1.
Demographic data for patients in studies 1 and 2.
A summary of the results is presented in Table 2 and Table 3, and the plasma and milk concentration-time profiles for a typical patient (#1.6) are shown in Figure 1. The relative dose received by the infants in study one was a mean of 1.13% (range 0.5–1.7; s.d. 0.5) and the mean M/PAUC was 0.39 (range 0.32–0.51; s.d. 0.1). The tmax for paroxetine in milk (median 5.7 h) was later than that in plasma (median 2.2 h) in five of the six patients, but the difference was not statistically significant (Mann Whitney U-test, P = 0.18). The mean relative dose received by the infants in study two was 1.25% (range 0.4–2.2; sd 0.8), while the mean M/P ratio was 0.96 (range 0.31–3.33, sd 1.0). The M/P ratio was similar for the pre and postfeed milk samples.
Table 2.
Pharmacokinetic and infant dose data for study 1.

Table 3.
Pharmacokinetic and infant dose data for study 2.

Figure 1.
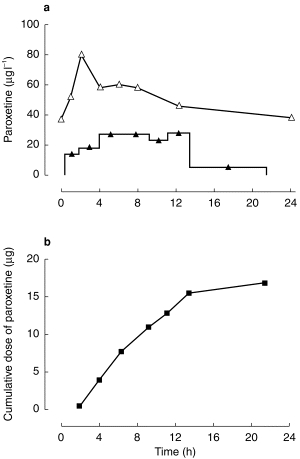
Plasma (Δ) and milk (▴) paroxetine concentrations (a) and cumulative excretion of paroxetine over 24 h (b) in patient 1.6 at steady-state on a dose of 20 mg day−1 paroxetine. ▴ concentration of paroxetine in breast milk; Δ concentration of paroxetine in plasma; ▪ cumulative dose of paroxetine in breast milk.
Plasma paroxetine concentrations were available from five of six infants in study 1 and three of four infants in study 2. Paroxetine was not detected (limit of detection 2 μg l−1) in seven of the eight infants from whom plasma samples were obtained. In one infant (#1.2) the drug was detected, but at a concentration that was below the level of quantification for the assay (4 μg l−1).
The pH of the maternal milk showed variability both within-and between-the patients in study one. The mean result of 7.24 (s.d. 0.27) is consistent with previous data [8]. The mean 24 h milk volume produced by the women in Study 1 was 766±113 ml.
Discussion
The mean relative infant doses of 1.13% (range 0.5–1.7) in study 1 and 1.25% (range 0.4–2.2) in study 2 indicate that the infant exposure to paroxetine, as a percentage of the weight-adjusted maternal dose, is well within the recommended limit of 10%. The method used to calculate this dose in study 1 is arguably the most accurate way of measuring the drug dose in milk, as it involves measuring the cumulative dose in milk over the dose interval. The method used to calculate the dose in study two was based on fewer milk samples, and on an assumed milk intake of 0.15 l kg−1 day−1. This estimate therefore is likely to be less accurate. Nevertheless, the mean estimated doses from both studies were remarkably similar, although that from study two had a larger variance. In study one, the mean dose received by the infant based on the measured M/PAUC and the assumed milk intake of 0.15 l kg−1 day−1 was 1.35% (range 0.9–2.2). Thus, use of this commonly assumed milk intake value provides an acceptable estimate of infant dose for paroxetine.
The mean M/PAUC of 0.39 (range 0.32–0.51) from study 1 is very close to the value of 0.22 predicted by the model of Atkinson & Begg [7]. The M/P ratio of 0.96 (range 0.31–3.33) from study 2 is substantially higher than either of the former values but the range of values was much larger, and was influenced strongly by a high value in patient #2.4. This illustrates the greater reliability of M/PAUC by comparison with single paired M/P data. Study 1 also showed that the concentration-time profiles that were not entirely parallel, highlighting why M/P estimates based on single paired measurements are likely to be more variable and sometimes misleading. For these reasons, M/PAUC should be the preferred method of measurement of the M/P ratio whenever possible.
The very low relative dose received by the infant was confirmed by the lack of detection of the drug in the plasma of seven of the eight infants from whom blood was sampled. In one infant a low concentration between 2 and 4 μg l−1 was observed. No adverse effects were seen by the investigators, or reported by the mothers, in any the 10 infants studied.
In summary, these studies provide useful data on the distribution of paroxetine in human milk and on estimated and actual infant drug exposure via breast feeding. The data suggest that paroxetine can be considered ‘safe’ during breast feeding, at least according to the 10% rule. With this rule, infant concentrations less than 10% of the maternal concentrations are considered ‘safe’ unless the drug has particular problems pre-empting use. Clearly this is simplistic as the longterm effects of even small amounts of drugs are often not known, especially if the drug is new. However, given that many mothers who are breast feeding are currently taking paroxetine and other SSRIs, then our results provide some reassurance. This study involved 10 patients, which is quite a large number of terms of studies of drugs in human milk. However is is important to be cautious in extrapolating this data to the general population. Further, it is always wise to heed the following advice, ‘prescription of an antidepressant for a breast feeding woman is a case-specific risk-benefit decision’ [9].
Acknowledgments
The authors would like to acknowledge the financial support of Smith Kline Beecham.
References
- 1.Taddio A, Ito S, Koren G. Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol. 1996;36:42–47. doi: 10.1002/j.1552-4604.1996.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 2.Isenberg KE. Excretion of fluoxetine in human breast milk. J Clin Psychiat. 1990;51:169. [PubMed] [Google Scholar]
- 3.Lester BM, Cucca J, Andreozzi L, Flanagan P, Oh W. Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Child Adolesc Psychiat. 1993;32:1253–1255. doi: 10.1097/00004583-199311000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Altshuler LL, Burt VK, McMullen M, Hendrick V. Breastfeeding and sertraline: a 24 hour analysis. J Clin Psychiat. 1995;56:243–245. [PubMed] [Google Scholar]
- 5.Winn SS, Drawer PO, Stowe ZN, et al. Sertraline in breast milk and nursing infants. Proc 148th Ann Meeting Am Psychiat Assocn Miami, FL. 1995:73. (Abstract #NR72) [Google Scholar]
- 6.Spigset O, Carleborg L, Norstrom A, Sandlund M. Paroxetine level in breast milk. J Clin Psychiat. 1996;57:39. [PubMed] [Google Scholar]
- 7.Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151–167. doi: 10.2165/00003088-199018020-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ansell C, Moore A, Barrie H. Electrolyte and pH changes in human milk. Pediat Res. 1977;11:1177–1179. doi: 10.1203/00006450-197712000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast feeding. Am J Psychiatry. 1996;153:1132–1137. doi: 10.1176/ajp.153.9.1132. [DOI] [PubMed] [Google Scholar]


