Abstract
Aims
Chinese herbal treatments are being promoted as a treatment for eczema. The aim of this study was to systematically review the evidence for or against this notion.
Methods
Extensive literature searches were carried out to identify all randomised clinical trials on the subject. Data were extracted from these in a predefined standardized fashion.
Results
Only two randomized clinical trials were located. Both imply that a complex mixture of Chinese herbs is more effective than placebo in treating eczema. Yet several caveats exist, most importantly the lack of independent replication. Adverse effects have also been reported.
Conclusions
At present it is unclear whether Chinese herbal treatments of eczema do more good than harm.
Keywords: alternative medicine, complementary medicine, eczema, systematic review, traditional Chinese medicine
Introduction
Since articles first appeared in the British press some 10 years ago (e.g. Chinatown Cure. Sunday Mirror May 15, 1988), there has been considerable interest in traditional Chinese herbal remedies as a method of treating severe atopic eczema. Several papers either explicitly or implicitly support the notion that a decoction of Chinese herbs is an effective oral treatment for atopic eczema [e.g. 1–4]. No systematic review or meta-analysis is presently available to summarize the data upon which this notion is based. We report the findings of a systematic review of the current literature on this topic.
Methods
The following electronic databases were searched from their inception to March 1998: Medline, Cochrane Library, Embase, Biosis Previews, Science Citation Index and Healthstar. The search terms used to locate all articles relating to the skin condition under investigation were: ‘eczema’, ‘skin diseases, eczematous’ and ‘dermatitis, atopic’. All articles concerning the use of traditional herbal remedies were identified by the search words: ‘drugs, Chinese herbal’, ‘medicine, herbal’ and ‘medicine, oriental traditional’. All search terms were employed both as Medical Subject Headings and as text words. In addition, our own extensive database was searched and several other experts in the field were asked to contribute further references. Finally, the only British manufacturer of a commercial product (Zemaphyte®, Phytopharm plc, Cambs., UK) was approached and invited to contribute any published or unpublished material on the treatment of eczema with Chinese herbs. The bibliographies of all articles located by these various strategies were carefully searched for further relevant articles.
No restrictions were applied as to the language of publication. However, only clinical trials carried out on human patients, which incorporated a patient control group, were selected for inclusion in the systematic review. Trials without patient control groups and studies without clinical endpoints [e.g. 5–7]. were excluded. Data were extracted in a standardized, predefined fashion.
Results
Two trials [8, 9] fulfilled the above inclusion criteria. A brief description of each study is given below and further details are summarized in Table 1.
Table 1.
Trials of Chinese herbal mixture for eczema.
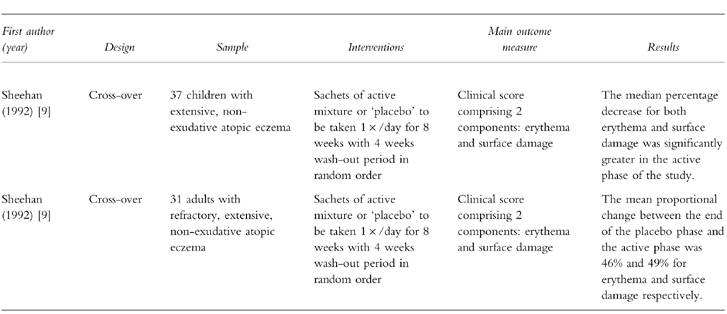
Sheehan & Atherton (1992) randomized 47 children with extensive nonexudative atopic eczema to receive either the active or a ‘placebo’ plant mixture in a randomised, double-blind, cross-over trial [8]. The sachets of active mixture comprised 10 different plants traditionally used in Chinese medicine for the treatment of eczema (Ledeboureilla seseloides, Potentilla chinensis, Aebia clematidis, Rehmannia glutinosa, Paeonia lactiflora, Lophatherum gracile, Dictamnus dasycarpus, Tribulus terrestris, Glycyrrhiza uralensis and Schizonepeta tenuifolia). The placebo sachets were said to contain ‘inert’ plant materials having a similar appearance, taste and smell as the ingredients of the active treatment (Humulus lupulus, Hordeum distichon, Hordeum distichon ustum, Baker's bran, sucrose, Salvia spp., Thymus vulgaris, Rosmarinus officianalis, Mentha piperita and clove oil. Glycyrrhiza uralensis was also present in the same quantities as in the active formulation).
Ten children were excluded from the final analysis because of noncompliance or protocol violations. Means of a clinical score, which comprised erythema and surface damage components, were calculated at weeks 4 and 8 of each treatment phase. The median percentage decrease in erythema scores was 51.0 (95% CI 34.5, 72.6) during the active phase and 6.1 (95% CI −25.2, 30.7) during placebo phase. The median percentage decrease in surface damage scores during the active phase was 63.1 (95% CI 34.5, 72.6) compared with 6.2 (95% CI −25.2, 30.7) during the placebo phase (95% CI for the difference 19.2, 97.9). There was no evidence of carry-over effects between the two phases of the study.
The same research group recruited 40 adult patients to take part in a further trial [9], the methodological details of which are similar to those of the previous study. Thirty-one patients completed this study. Based on logarithmic values, the mean proportional change between the end of the placebo phase and the end of the active phase for erythema was 46% (95% CI 25.2, 67). The mean proportional change for surface damage between the end of the placebo phase and the end of the active phase was 49% (95% CI 27, 71). In general, itching and sleep patterns were also improved. Adverse effects were mild with slight abdominal distension and headaches being reported for two patients.
Discussion
The most surprising finding of this systematic review is the extreme paucity of controlled clinical trials on the subject. This is in stark contrast to the intense coverage of the topic often aimed at the lay-reader which could lead to the impression that the efficacy of this treatment is well documented.
Both studies are open to criticism. The authors mention a power calculation based upon data gathered from a pilot investigation, according to which 50 patients would have been needed to achieve ‘adequate power’ to determine the treatment effect. Thus the sample size, particularly in the study of adult patients, would appear to be inadequate in both trials. Furthermore, the authors do not discuss the pros and cons of an intention to treat analysis and do not include such an assessment. In addition, it is also conceivable that a degree of ‘unblinding’ in the cross-over studies may have taken place due to the unpleasant taste of the active mixture (which must be difficult to exactly replicate without using the same constituents). Also, in the absence of laboratory evidence, it is questionable whether the herbs used as ‘controls’ were truly ‘inert’ for the target condition, eczema.
For both trials described above, follow-up reports have been published. When the 17 adult patients who continued to take the herbal mixture were re-examined 1 year later, 12 had a greater than 90% reduction in the clinical score and the remaining 5 had greater than 60% reduction in clinical scores compared with baseline values [10]. This was significantly better than those 11 patients who choose not to carry on taking the medication (P = 0.005 and P = 0.002 for erythema and surface damage, respectively). Similarly, the 23 children from the above-described trial who opted to continue with the Chinese herbs showed better results after 1 year follow-up than those who discontinued taking the Chinese herbal mixture [11]. These findings are encouraging but, due to the possibility of selection bias, not ultimately compelling.
Although none of the subjects involved in these trials experienced signs of short-term toxicity, the authors of these studies state that no patient should receive this type of therapy without prior routine checks of haematological, renal and hepatic function. Serious adverse effects have been reported recently by independent investigators [e.g. 12–14].
It is concluded that evidence of the efficacy of Chinese herbal therapy for eczema, although encouraging, is not based on a sufficiently large number of rigorous clinical trials. Furthermore there is some doubt about the safety of this treatment. More studies are required to establish the effectiveness, safety, cost-effectiveness and mechanism of action of this mode of treatment.
Acknowledgments
N. C. Armstrong was supported by the Pilkington Trusts. No commercial sponsorship was received.
References
- 1.Atherton DJ, Sheehan M, Rustin MHA, Whittle B, Guy G. Treatment of atopic eczema with traditional Chinese medicinal plants. Pediatr Dermatol. 1992;9:373–375. doi: 10.1111/j.1525-1470.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 2.Latchman Y, Whittle B, Rustin M, Atherton DJ. Brostoff J. The efficacy of traditional Chinese herbal therapy in atopic eczema. Int Arch Allergy Immunol. 1994;104:222–226. doi: 10.1159/000236669. [DOI] [PubMed] [Google Scholar]
- 3.Sadler Catharine. Chinese herbs for eczema: risks and benefits. Community Nurse. 1996;2:21–22. [PubMed] [Google Scholar]
- 4.Xu X-J, Banerjee P, Rustin MH, Poulter LW. Modulation by Chinese herbal therapy of immune mechanisms in the skin of patients with atopic eczema. Br J Dermatol. 1997;136:54–59. 10.1046/j.1365-2133.1997.d01-1142.x. [PubMed] [Google Scholar]
- 5.Liu HN, Jaw SK, Wong CK. Chinese herbs and atopic dermatitis. Lancet. 1993;342:1175–1176. doi: 10.1016/0140-6736(93)92161-l. 10.1016/0140-6736(93)92161-L. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee P. Efficacy of a new palatable formulation of Chinese herbal therapy as treatment of atopic eczema. Br J Dermatol. 1994;131(Suppl 44):26. [Google Scholar]
- 7.Latchman Y, Banerjee P, Poulter LW, Rustin MHA, Brostoff J. Association of immunological changes with clinical efficacy in atopic eczema patients treated with traditional Chinese herbal therapy (Zemaphyte®) Int Arch Allergy Immunol. 1996;109:243–249. doi: 10.1159/000237245. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan M, Rustin MHA, Atherton DJ, et al. Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis. Lancet. 1992;340:13–17. doi: 10.1016/0140-6736(92)92424-e. 10.1016/0140-6736(92)92424-E. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan MP, Atherton DJ. A controlled trial of traditional Chinese medicinal plants in widespread non-exudative atopic eczema. Br J Dermatol. 1992;126:179–184. doi: 10.1111/j.1365-2133.1992.tb07817.x. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan M, Stevens H, Ostlere L, Atherton DJ, Brostoff J, Rustin MHA. Follow-up of adult patients with atopic eczema treated with Chinese herbal therapy for 1 year. Clin Exp Dermatol. 1995;20:136–140. doi: 10.1111/j.1365-2230.1995.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan MP, Atherton DJ. One-year follow up of children treated with Chinese medicinal herbs for atopic eczema. Br J Dermatol. 1994;130:488–493. doi: 10.1111/j.1365-2133.1994.tb03383.x. [DOI] [PubMed] [Google Scholar]
- 12.Perharic L, De Smet P, Murray V. Possible association of liver damage with the use of Chinese herbal medicine for skin disease. J Vet Hum Toxicol. 1995;37:562–566. [PubMed] [Google Scholar]
- 13.Ferguson JE, Chalmers RJG, Rowlands DJ. Reversible dilated cardiomyopathy following treatment of atopic eczema with Chinese herbal medicine. Br J Dermatol. 1997;136:592–593. 10.1046/j.1365-2133.1997.d01-1241.x. [PubMed] [Google Scholar]
- 14.Koo J, Arain S. Traditional Chinese medicine for the treatment of dermatologic disorders. Arch Dermatol. 1998;134:1388–1393. doi: 10.1001/archderm.134.11.1388. 10.1001/archderm.134.11.1388. [DOI] [PubMed] [Google Scholar]


