Abstract
Aims
To determine structure, activities and drug selection processes used by Dutch hospital drug and therapeutics (D & T) committees.
Methods
A pretested structured survey questionnaire based on the Australian process and impact indicators, previous research, and consultation of professionals was developed. Subsequently, D & T committees that met predefined selection criteria were asked to participate.
Results
The overall response rate was 70% (38/54). D & T committees varied considerably in size and representation of clinical expertise. Although responsibilities were theoretically alike, actual responsibilities were frequently passed on to other authorities, such as pharmacy staff. Few committees used detailed guidelines or decision supportive matrices to establish transparency in drug selection. With respect to drug selection, the value scores on the information resources used, the factors involved, and the selection criteria used varied. Hospital pharmacists were likely to be most involved and to have the greatest impact. A consensus was most difficult to achieve for drugs used in cardiology, oncology, and psychiatry. Interference of industrial marketing strategies on drug selection was recognized and identified.
Conclusions
Our results indicate that Dutch hospital D & T committees differ with respect to their clinical expertise and their activities, a situation comparable with that observed in other countries. Furthermore, the lack of transparency in drug selection was considerable. These findings clarify the differences previously found between Dutch hospital drug formularies.
Keywords: drug and therapeutic committees, drug selection, information resources, the Netherlands, rational pharmacotherapy, selection criteria
Introduction
Rational pharmacotherapy, which is defined as the selection and use of drugs based on effectiveness, safety, convenience, and economics [1–4], should ideally be pursued in all healthcare settings. Hospitals in particular are under great pressure from patients demanding more rapid recovery through the use of new technology drugs, which are often extremely expensive. In addition, pharmacy departments are confronted by the diversity of the patients' preadmission pharmacotherapy, which necessitates a wide variety of (interchangeable) drugs being kept in stock. Therefore, hospital drug formularies (HDFs) have been utilized as management tools to structure drug use and visualize selection. However, research has indicated that HDFs are negatively evaluated by many hospitals [5]. Moreover, HDFs may have limited or even a negative impact on clinical outcomes and overall hospital expenditure [6–10]. Therefore, as in other countries, the process of drug selection in Dutch hospitals has received attention from the government, patient organizations, healthcare professionals, and the media [11–14].
Internationally, hospitals have had Drug and Therapeutics (D & T) committees for over 50 years to ensure rational pharmacotherapy, and several methods have been described to monitor and evaluate their performance [11, 12, 14–17]. Weekes et al. have developed a well-designed set of field-tested indicators for hospital D & T committees which consider the process, impact and outcome [18]. Although the set specifically applies to Australian hospital D & T committees, it can be used as an example for other countries.
To date, few studies have been performed that have addressed the composition, objectives, and procedures of European hospital D & T committees. Information on Dutch hospital D & T committees is not available. A previous pharmacotherapeutic evaluation of Dutch HDFs showed that rational pharmacotherapy may be interpreted differently by different hospital D & T committees [40]. Therefore, the objective of this research was to evaluate the way in which Dutch hospital D & T committees operate and thereby clarify the differences previously found between Dutch HDFs.
Methods
The hospital inclusion criteria were (1) size ≥300 beds, (2) the presence of a D & T committee, (3) with a written statute, and (4) a printed HDF. As a result, 54 hospitals, including 7 teaching hospitals were selected. Subsequently, 46 hospital pharmacists, and eight clinical pharmacologists, who were members of the D & T committee were requested to participate in the survey.
Based on the Australian process and impact indicators [18], previous research [19], and consultation of four hospital pharmacists and three clinical pharmacologists affiliated to the university department, a structured survey questionnaire was developed and pretested. The final questionnaire consisted of 55 questions, 17 and 13 closed-ended questions addressing structure and activities, respectively, and 25 questions, of which 17 were closed-ended (including 2 that requested scoring on an analogue scale of 1–10) and 8 were open, addressing drug selection. All closed-ended questions included a free text option at the end. Completed questionnaires had to be circulated amongst all committee members for approval in order to ensure that the answers reflected the views of the whole committee, rather than those of individual hospital pharmacists who completed the questionnaires.
Thirty-eight (70%) valid questionnaires were returned within the 3 month time limit. Response bias was unlikely as responders and nonresponders were similar with respect to the hospital type, size, and geographical region. All data were anonymized and processed by MS Access 7.0 for Windows. The means, s.d., ranges, and medians were calculated by MS Excel 7.0 for Windows.
Results
Structure
Thirty of the committees (79%) were responsible for 1 hospital only, while 8 (21%) were also responsible for other healthcare institutions (range 1–9), including nursing homes, psychiatric hospitals, or other specialized small hospitals within the region. Twenty-three committees (61%) had established working groups or subcommittees (range 1–20) concerned with antibiotic policies, antithrombotic therapy, drug distribution, (par)enteral nutrition, blood products, psychotropic drugs, expensive drugs, HDF editing, or the development of pharmacotherapeutic treatment guidelines.
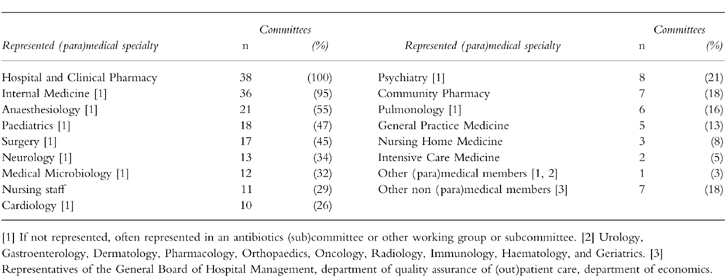
Committee members were mostly selected by the General Board of Hospital Management for a period of 1–3 years. The number of committee members varied from 3 to 14 with a median of 8. Hospital pharmacists held the position of secretary and chairman in 36 (95%) and 14 (37%) of the committees, respectively. Thirty-three committees (87%) had a fixed structure, whereas 5 (13%) had a changing structure depending on the issues addressed. Table 1 shows what (para)medical specialties were represented. Ten committees (26%) included clinical pharmacologists (range 1–3). The median of the ratio clinician:pharmacist was 4:1 (range 1:1–7:1).
Table 1.
Representation of clinical expertise in Dutch hospital D & T committees (n = 38).
Activities
Eighteen committees (47%) met bimonthly, seven (18%) monthly, and less than 10% once or twice a year, quarterly, or irregularly. Twenty-one committees (55%) restricted meetings to members. Seventeen committees (45%) had nonrestricted meetings at which clinical experts and healthcare professionals working in primary healthcare, other hospitals, or nursing homes were invited. Thirty-six committees (95%) indicated that meetings were primarily used to discuss professional literature, followed by interactive debate (92%, n = 35).
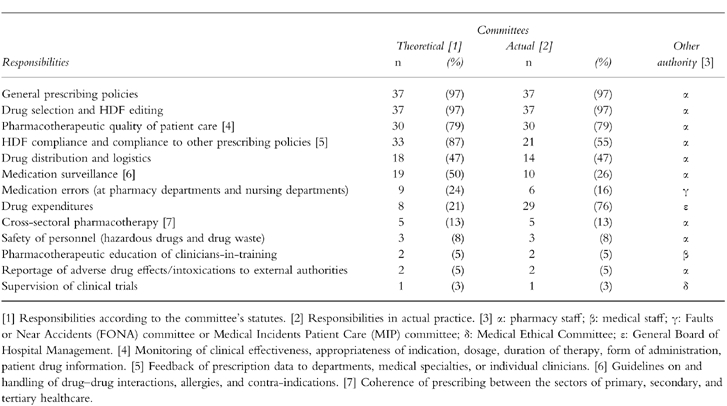
Table 2 shows both the theoretical and actual responsibilities, and whether or not they were delegated to subcommittees or working groups. Although most of the theoretical responsibilities were also actual responsibilities, in practice, some were passed on to other authorities, such as pharmacy staff.
Table 2.
Responsibilities of Dutch hospital D & T committees (n = 38).
Drug selection
Three committees (8%) used detailed guidelines for drug selection in view of quality assurance. These guidelines defined, identified, or clarified the procedure and process of drug selection, the type of information needed, the approved information resources, and the selection criteria. Nineteen committees (50%) obliged clinicians to follow specific procedures for HDF drug applications. In view of this, eight of these (42%) had developed printed HDF drug application forms. Figure 1 shows the information requested on such forms. Thirty-eight committees (95%) identified hospital pharmacists as being in charge of collecting and evaluating information with respect to drug selection. Also identified were clinicians who requested the HDF drug application (55%, n = 21), and the pharmacotherapeutic clinical experts who were particularly involved (37%, n = 14).
Figure 1.
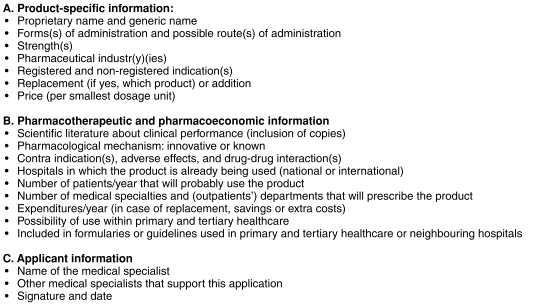
The information requested on an HDF drug application form.
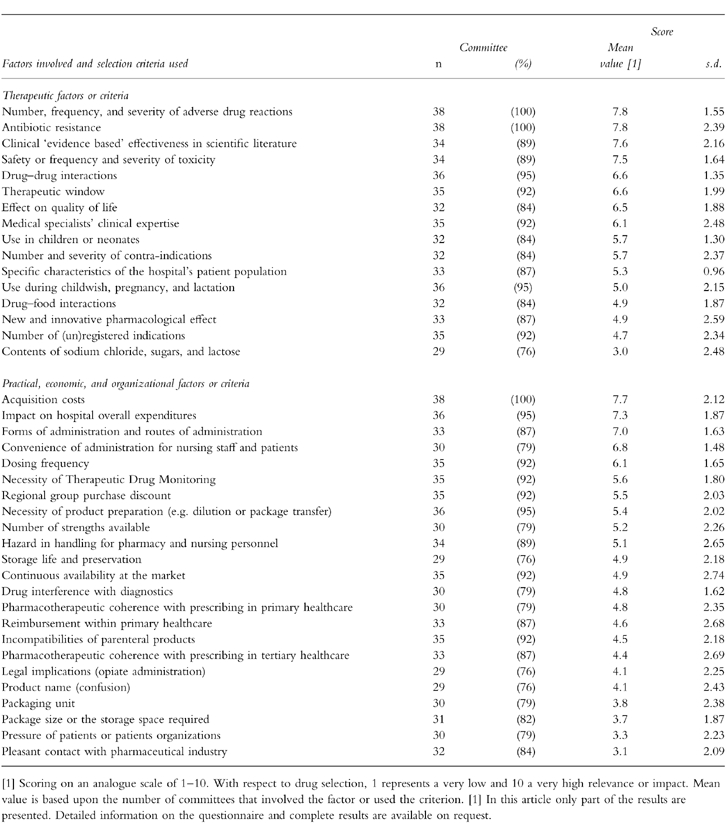
Table 3 shows the information resources used in drug selection and how these were valued. Three-quarters of all committees used the majority of resources. Published, peer-reviewed, objective, and (inter)national information was favoured over informal, subjective, and subnational information. Many committees spontaneously indicated their unfamiliarity with the methodology of meta-analysis. Table 4 shows the factors involved and criteria used in drug selection and how these were valued. Generally, therapeutic considerations were favoured over practical, economic and organizational considerations.
Table 3.
Information resources used in drug selection by Dutch hospital D & T committees (n = 38).
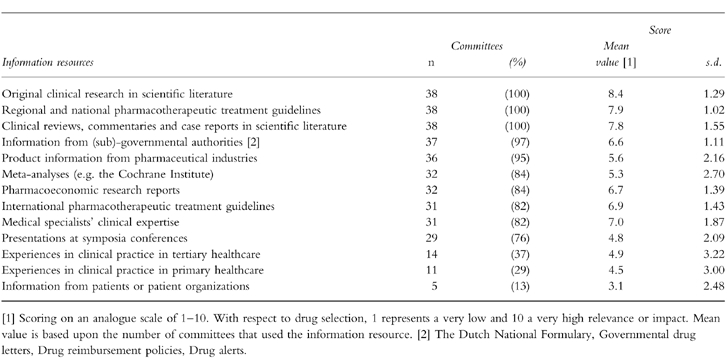
Table 4.
Factors involved and criteria used in drug selection by Dutch hospital D & T committees (n = 38).
Thirty-seven committees (97%) were familiar with decision supportive selection matrices, such as SOJA (System of Objectified Judgement Analysis) (90%, n = 34), Informatrix (37%, n = 14), the matrix of the Dutch Health Insurance Fund Council (18%, n = 7), MAUT (Multi Attributive Utility Analysis) (11%, n = 4), FDSS (Full Decision Supportive System) (8%, n = 3), or SELMED (System of Election in Medicine) (8%, n = 3) [21–25]. Four committees (11%) used self-developed matrices. Six committees (16%) actually used matrices whereas 32 (84%) considered the matrices to be complex, time-consuming, or manipulable. In addition, their usefulness was questioned because the clinical data required were not always available and consensus was easily achieved without them. Sixteen committees (42%) considered hospital pharmacists to have the highest impact on drug selection, followed by pharmacotherapeutic clinical experts (40%, n = 15) and internists (18%, n = 7). Twenty-six committees (68%) mainly replaced drugs, whereas nine committees (24%) mainly added drugs to the HDF. Some respondents spontaneously indicated that a maximum number of drugs to be included per each drug group had been defined. On average, 15 drugs were added to the HDF annually (range 10–25). It must be noted that annually about 30 new drugs (≈350 products) are introduced onto the Dutch market.
The medical specialties that were considered to be the most difficult in achieving a consensus were cardiology (47%, n = 18), internal medicine (29%, n = 11), psychiatry (26%, n = 10) and anaesthesiology (21%, n = 8). Eight committees (21%) did not consider any medical specialty more complicated than others and 6 committees (16%) did not provide information because of privacy considerations. The drug groups considered to be the most complicated were cardiovascular drugs (50%, n = 19), in particular ACE-inhibitors, β-adrenoceptor blocking agents, diuretics, angiotensin II antagonists, hypolipidaemic agents, calcium channel blocking agents, cytotoxic agents (45%, n = 17), antiemetics (37%, n = 14), and radiological contrast agents and antidepressants (both 18%, n = 7). Sixteen committees (42%) did not consider any drug group to be more complicated than others. Within cardiology, internal medicine, and psychiatry, a wide variety of equally effective products were available. Individual experience, education, and marketing strategies of pharmaceutical industries were considered to be of high impact and were important in forming the opinions of clinicians working in these medical specialties. Moreover, psychiatrists were said to be rather reluctant to select one drug over another because of the pressure exerted by patients and patient organizations. Additionally, the clinical outcomes of individual drugs differed considerably between individual psychiatric patients. Within radiology, hard scientific evidence for product performance was scarce. Oncologists had to cope with an emotional and ethical dilemma for innovative cytotoxic agents that were very expensive and the small and uncertain positive effects in terminally ill patients were often outweighed by costs. Generally, most problems within medical specialities e.g. anaesthesiology, were based upon personal conflicts and were caused by clinicians who operated individually and who had intentionally obstructive attitudes.
Finally, all committees recognized the marketing strategies of pharmaceutical companies. Fourteen committees (37%) obliged members to declare any conflicts of interest in terms of personal gifts, sponsorship of meetings or research grants with pharmaceutical companies so that there was absolute transparency in the drug selection process. Twenty committees (53%) indicated that conflicts of interest had to be declared for a few drug groups only while four (11%) committees indicated that the members did not have to disclose any interests. Ten committees (26%) noted that specific industries supplied some products free of charge because they easily recovered costs through community pharmacists. Seventeen committees (45%) mentioned that industrial influence was manifested by incomplete and biased clinical data presented at meetings and by deviant prescribing behaviour in outpatients' departments. Furthermore, five committees (13%) stated that as a result of visits to symposia and conferences sponsored by pharmaceutical industries, clinicians' prescribing behaviour changed and particular drugs were requested for the HDF. Additionally, if clinicians prescribed certain products they would receive personal gifts and trips or a considerable amount of cash per prescription. Two committees (5%) explicitly mentioned that in return for prescribing certain products, pharmaceutical industries financed research projects or the affiliation of extra clinicians.
Discussion
The research presented in this study has never been conducted before, and therefore provides a useful insight. However, it is important to be aware of the limitations. Although the results were anonymized, they are based upon reports rather than actual facts. Therefore, the committees may have answered in a socially desirable way, in particular on the issues that requested scoring. For example, the use of original clinical research in scientific literature may be overestimated, whereas interference by industrial marketing strategies may have been underestimated. However, most committees were very enthusiastic to participate and mentioned that they had waited for this kind of research.
Structure
Although hospital D & T committees have been established since the 1930s in America, the presence of D & T committees in Dutch hospitals, as in other European countries, dates back to the 1970s [14, 26–28]. It was not until 1984 that Dutch legislation officially obliged hospitals to install a D & T committee that included at least one pharmacist, one clinician, and one representative of the nursing staff [29]. In 1986, 87% of all general hospitals had a D & T committee of which 25% worked from a written statute [28]. The legislation has been changed recently, and hospitals today are allowed to implement authorities, methods, and policies with respect to rational pharmacotherapy based on their own views. Nonetheless, as a consequence of former legislation, 98% of all general hospitals now have a D & T committee and 89% work from a written statute. These percentages are similar to those in other countries [11, 12, 14, 18, 26].
The number of representatives (range of 3–14 with a median of 8) on Dutch committees is similar to some countries, e.g. Ireland or the USA (range 2–12, median 7 and range 8–12, median 10, respectively), but differs from others, e.g. Germany (range of 5–40, median 12) [12, 14, 30]. Furthermore, representation of clinical expertise varies considerably since there are few regulations that define structure. The wide variety of (para)medical specialties represented is due to the personal pharmacotherapeutic interests of individual clinicians, the regulations of the General Board of Hospital Management, and whether or not certain medical specialties are represented within the hospital. Generally, medical specialties confronted with a wide range of drug groups in clinical practice or those that account for a high proportion of the drug expenditure were considered the most important to be represented. Although clinical pharmacologists are outstanding experts with respect to pharmacotherapy, they seem to be underrepresented, akin to the situation in other countries [11, 12].
Activities
The model statute developed by the Dutch Association of Hospital Pharmacists suggests five major issues for which hospital D & T committees should be responsible: (1) a HDF, (2) important advice with respect to pharmacotherapeutic policies, (3) efficient drug distribution, (4) drug information and education, and (5) collection, organization, and interpretation of data with respect to drug use [32]. Internationally, the theoretical responsibilities appear to be similar and activities comprise communication, advice, policy making, monitoring, and regulation [12, 14, 30, 31]. However, in contrast to the situation observed outside the Netherlands, Dutch hospital pharmacy staff frequently bear sole responsibility. Furthermore, most Dutch committees are theoretically not responsible for drug expenditure. Nonetheless, most General Boards of Hospital Management have informally passed this responsibility onto the committees. Finally, Dutch committees do not employ educational activities, which is an explicit activity of committees abroad [11, 14, 31, 33].
Drug selection
It is not surprising that few committees use detailed guidelines with respect to drug selection since it was not until recently that the Dutch Association of Hospital Pharmacists indicated that such guidelines should form part of quality assurance [31, 33]. Additionally, although governmental policies indicate there should be transparency in drug selection, few committees use decision supportive matrices. The procedures and evaluation of HDF drug application and drug selection are similar to that of committees in other countires [11–14, 17, 30, 39]. In particular, the need for ‘evidence-based’ and pharmacoeconomic information has generally been accepted [2, 34–36]. A proactive approach of prospective continuous orientation for drugs on the market and subsequent consultation of clinicians has been shown to be effective [20]. However, Dutch committees would rather wait and act reactively in response to requests from clinicians.
Similar to the findings in other countries, hospital pharmacists are the members that are most involved and are considered to have the greatest impact on drug selection [37, 38]. They have a clear insight into the prescribing behaviour of clinicians and of drug expenditure. They also have direct access to therapeutic and organizational drug information resources, and form bridges between medical specialties [39]. The General Board of Hospital Management has little influence but may occasionally be involved in the selection of very expensive drugs [38]. Complications with respect to the achievement of consensus within specific medical specialties mostly relate to the drug groups involved. However, other personal nondrug related issues may be important as well. Evaluation of Dutch HDFs confirmed these findings as it showed remarkable differences. A wide range of drug entities were included in the drug groups considered to be the most complicated in this research [40]. Finally, industrial marketing strategies are known to have an impact on drug selection and prescribing behaviour of both pharmacists and clinicians [13, 16, 41, 42]. Although, this may be considered to be undesirable, it does not implicitly mean that the quality of the drugs selected is affected in a negative manner.
In conclusion, our results indicate that Dutch hospital D & T committees, like the committees in other countries, differ with respect to the clinical expertise represented, and the activities that ensue from their responsibilities. Importantly, there was a considerable lack of transparency in drug selection. These findings substantially clarify the differences previously found between Dutch HDFs.
Recommendations
In view of the necessity for pharmacotherapeutic quality assurance and healthcare economics, Dutch hospital D & T committees should develop detailed guidelines regarding drug selection. In this way, the demands for transparency set by both insurance companies and the government will be met. Furthermore, clinical pharmacologists should be represented on the committees. Professional representation needs to be defined more explicitly and should expand to primary and tertiary healthcare settings in order to establish cross-sectoral D & T committees of pharmacotherapeutic experts. Subsequently, these will be responsible for rational pharmacotherapy across primary, secondary, and tertiary healthcare sectors. Although this may complicate the process of drug selection and the achievement of consensus, on the whole, it is likely to be beneficial to national healthcare. Therefore, the government, insurance companies, and professional medical and pharmaceutical authorities should jointly provide healthcare professionals with detailed regulations on the development, implementation, structure, performance, and monitoring of such cross-sectoral D & T committees.
Acknowledgments
The authors would like to thank all the survey participants. Furthermore, they gratefully acknowledge the assistance of C. J. de Blaey, Scientific Institute Dutch Pharmacists and C. S. de Vries, Department of Clinical Pharmacology, University of Dundee in survey design and manuscript preparation.
References
- 1.Hogerzeil HV. Promoting rational prescribing: an international perspective. Br J Clin Pharmacol. 1995;39:1–6. doi: 10.1111/j.1365-2125.1995.tb04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner F, Burgess NG, Dean Martin E. Approaches to rationing drugs in hospitals. An Australian perspective. Pharmacoeconomics. 1996;10:467–474. doi: 10.2165/00019053-199610050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Relman AS. The trouble with rationing. N Engl J Med. 1990;323:911–912. doi: 10.1056/NEJM199009273231310. [DOI] [PubMed] [Google Scholar]
- 4.Scott DK, Ferner RE. ‘The strategy of desire’ and rational prescribing. Br J Clin Pharmacol. 1994;37:217–219. doi: 10.1111/j.1365-2125.1994.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fijn R, De Jong-Van den Berg LTW, Brouwers JRBJ. Rational pharmacotherapy in The Netherlands: Formulary management in Dutch hospitals. Pharm World Sci. 1999;41:70–79. doi: 10.1023/a:1008654609916. [DOI] [PubMed] [Google Scholar]
- 6.Horn SD. Unintended consequences of drug formularies. Am J Health Syst Pharm. 1996;53:2204–2206. doi: 10.1093/ajhp/53.18.2204. [DOI] [PubMed] [Google Scholar]
- 7.Hepler CD. Where is the evidence for formulary effectiveness? Am J Health Syst Pharm. 1997;54:95. doi: 10.1093/ajhp/54.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Thornton JP, Brown D, Stonich TL, Hutchinson RA. Pharmacy managers should evaluate the full impact of formulary decisions. Am J Hosp Pharm. 1989;46:1131–1132. [PubMed] [Google Scholar]
- 9.Hemeryck L, Chan R, Sabra K, Feely J. Poor utilisation and limited impact of formularies on quality of prescribing by hospital doctors. Ir Med J. 1996;89:173–174. [PubMed] [Google Scholar]
- 10.Sloan FA, Gordon GS, Cocks DL. Hospital drug formularies and use of hospital services. Med Care. 1993;31:851–867. doi: 10.1097/00005650-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Weekes LM, Brooks C. Drug and Therapeutics Committees in Australia: Expected and actual performance. Br J Clin Pharmacol. 1996;42:551–557. doi: 10.1111/j.1365-2125.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 12.Thürmann PA, Harder S, Steioff A. Structure and activities of hospital drug committees in Germany. Eur J Clin Pharmacol. 1997;52:429–435. doi: 10.1007/s002280050315. 10.1007/s002280050315. [DOI] [PubMed] [Google Scholar]
- 13.Segal R, Pathak DS. Formulary decision making: identifying factors that influence P & T Committee drug evaluations. Hosp Form. 1988;23:174–178. [PubMed] [Google Scholar]
- 14.Summers KH, Szeinbach SL. Formularies: the role of pharmacy and therapeutics (P & T) committees. Clin Ther. 1993;15:433–441. [PubMed] [Google Scholar]
- 15.Bochner F, Martin ED, Burgess NG, Somogyi AA, Misan GMH. Controversies in treatment. How can hospitals ration drugs? Br Med J. 1994;308:901–908. doi: 10.1136/bmj.308.6933.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rucker TD. Quality control of hospital formularies. Pharm World Sci. 1988;10:145–150. doi: 10.1007/BF01959422. [DOI] [PubMed] [Google Scholar]
- 17.Mannebach MA, Ascione FJ, Christian R. Measuring performance of pharmacy and therapeutics committees. Proc Am Pharm Assoc Ann Meet. 1996;143:10. [Google Scholar]
- 18.Weekes LM, Brooks C, Day RO. Indicators for Drug and Therapeutics Committees. Br J Clin Pharmacol. 1998;45:393–398. doi: 10.1046/j.1365-2125.1998.t01-1-00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fijn R, De Vries CS, Engels SAG, Brouwers JRBJ, De Blaey CJ, De Jong-Van den Berg LTW. The quality of Dutch hospital drug formularies. An evaluation of technical features and organisational information. Pharm World Sci. 1999 doi: 10.1023/a:1008680021854. in press. [DOI] [PubMed] [Google Scholar]
- 20.Crane VS, Gonzalez ER, Hull BL. How to develop a proactive formulary system. Hosp Form. 1994;29:700–710. [PubMed] [Google Scholar]
- 21.Janknegt R, Steenhoek A. The System of Objectified Judgement Analysis (SOJA) Drugs. 1997;53:550–562. doi: 10.2165/00003495-199753040-00002. [DOI] [PubMed] [Google Scholar]
- 22.Brenninkmeijer RF, Vermeij DJB, Hes R, Van der Kleijn E. InforMatrix [in Dutch] Pharmaceutisch Weekblad. 1994;129:1185–1190. [Google Scholar]
- 23.Senthilkumaran K, Shatz SM, Kalies RF. Computer-based support system for formulary decisions. Am J Health Syst Pharm. 1987;44:1362–1366. [PubMed] [Google Scholar]
- 24.Schumacher GE. Multiattribute evaluation in formulary decison making as applied to calcium-channel blockers. Am J Hosp Pharm. 1991;48:301–308. [PubMed] [Google Scholar]
- 25.Martinez Bengoechea MJ, Messori A, Berto V, Becagli P, Font M, Martini N. Hospital formulary and drug selection. Eur Hosp Pharm. 1997;3:89–90. [Google Scholar]
- 26.Anonymous. Die Arzneimittelkomission im Krankenhaus—ein Beitrag zur rationalen und kostenbewussten Therapie [in German] Der Arzneimittelbrief. 1998;21:33–35. [Google Scholar]
- 27.Fitzpatrick RW. Is there a place for drug and therapeutics committees in the new NHS? Eur Hosp Pharm. 1997;3:143–147. [Google Scholar]
- 28.Van Wijmen FCB. Geneesmiddelencommissies in ziekenhuizen. Pharmaceutisch Weekblad. 1986;121:3–11. [Google Scholar]
- 29.Ministerie van Welzijn Volksgezondheid en Cultuur. Besluit eisen voor erkenning van ziekenhuizen [in Dutch] Staatscourant. 1984;234:17. [Google Scholar]
- 30.Ferrando MC, Henman MC. A survey of drug and therapeutics committees operating in Ireland. J Clin Hosp Pharm. 1986;11:131–140. doi: 10.1111/j.1365-2710.1986.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 31.Ashp. ASHP statement on the pharmacy and therapeutics committee. Am J Hosp Pharm. 1992;49:2008–2009. [PubMed] [Google Scholar]
- 32.Nederlandse Vereniging van Ziekenhuisapothekers. Modelreglement geneesmiddelencommissies [in Dutch] Taken en functies van de ziekenhuisfarmacie. 1989:1–20. [Google Scholar]
- 33.ASHP. ASHP statement on the pharmacy and therapeutics committee. Am J Hosp Pharm. 1986;43:2841–2842. [PubMed] [Google Scholar]
- 34.Bakst A. Pharmacoeconomics and the formulary decision-making process. Hosp Form. 1995;30:42–50. [PubMed] [Google Scholar]
- 35.Beck JR. How to evaluate drugs. Cost-effectiveness analysis. JAMA. 1990;264:83–84. [PubMed] [Google Scholar]
- 36.Skaer TL. Applying pharmaoeconomics and quality-of-life measures to the formulary management process. Hosp Form. 1993;28:577–584. [PubMed] [Google Scholar]
- 37.Barner JC, Thomas J. Participation and influence of individuals and committees that decide on drug availability in HMOs. Am J Health Syst Pharm. 1998;55:56–61. doi: 10.1093/ajhp/55.1.56. [DOI] [PubMed] [Google Scholar]
- 38.Van Soest MM, Steenhoek A. Geneesmiddelenformularia in ziekenhuizen [in Dutch] Pharmaceutisch Weekblad. 1998;133:1054–1063. [Google Scholar]
- 39.Campagna KD, Newlin MH. Key factors influencing pharmacists' drug therapy decisions. Am J Health Syst Pharm. 1997;54:1307–1313. doi: 10.1093/ajhp/54.11.1307. [DOI] [PubMed] [Google Scholar]
- 40.Fijn R, Engels SAG, De Jong-Van den Berg LTW, Brouwers JRBJ. Pharmacotherapeutic evaluation of Dutch hospital drug formularies. Int J Pharm Pract. 1999 in press. [Google Scholar]
- 41.Chren M, Landefeld CS. Physician's behavior and their interactions with drug companies. JAMA. 1994;271:684–689. [PubMed] [Google Scholar]
- 42.Anonymous. Potential conflict of interest of a pharmacy member and therapeutics committee member. Am J Hosp Pharm. 1989;46:2047–2050. [PubMed] [Google Scholar]


