Abstract
Aims
Tramadol, a centrally acting analgesic, is used as a racemate containing 50% of a (+)- and 50% of a (−)-enantiomer. This paper presents the pharmacokinetic results of postoperative patient-controlled analgesia using (+)-tramadol, (−)-tramadol or the racemate.
Methods
Ninety-eight patients recovering from major gynaecological surgery were treated in a randomised, double-blind study with (+)-tramadol, (−)-tramadol or the racemate. Following an i.v. bolus up to a maximum of 200 mg, patient-controlled analgesia with demand doses of 20 mg was made available for 24 h. Prior to each demand, the serum concentrations of the enantiomers of tramadol and its metabolite M1 were measured in 92 patients.
Results
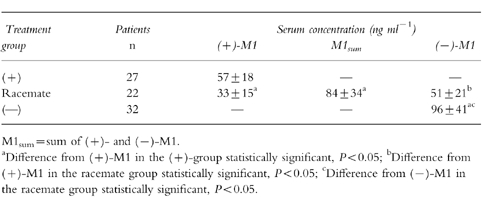
The mean concentrations of tramadol during the postsurgery phase were 470±323 ng ml−1, 590±410 ng ml−1 and 771±451 ng ml−1 in the (+)-, racemate- and (−)-group, respectively ((+) vs (−), P < 0.05); the mean concentrations of the metabolite M1 were 57±18 ng ml−1, 84±34 ng ml−1 and 96±41 ng ml−1 in the (+)-, racemate- and (−)-group, respectively ((+) vs (−) and (+) vs racemate, P < 0.05). The mean concentrations of (+)-tramadol and (+)-M1 were lower in the racemate- than in the (+)-group (P < 0.05), those of (−)-tramadol and (−)-M1 were lower in the racemate than in the (−)-group (P < 0.05). In the racemate group, the mean serum concentrations of (+)-tramadol were higher than those of (−)-tramadol (P < 0.05), whereas the mean serum concentrations of (−)-M1 were higher than those of (+)-M1 (P < 0.05).
Conclusions
The therapeutic serum concentration of tramadol and M1 showed a great variability. The lowest mean concentrations were measured in the (+)-group and the highest in (−)-group. This is in agreement with the clinical finding that (+)-tramadol is a more potent analgesic than (−)-tramadol.
Keywords: enantiomer, opioid, pharmacokinetic, postoperative pain, tramadol
Introduction
Tramadol hydrochloride (1RS,2RS)-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-cyclohexanol hydrochloride, is a clinically effective, centrally acting opioid analgesic [1, 2]. It is structurally similar to morphine in containing an aromatic ring system with three carbon atoms spaced away from a basic nitrogen centre. Tramadol displays a low affinity for opioid receptors with a marginal preference for μ-receptors [1, 2]. The analgesic effect is also mediated by the inhibition of neuronal reuptake of noradrenaline and serotonin [3].
The marketed product is a racemate mixture ((±)-T) containing 50% of a (+)-enantiomer ((+)-T) and 50% of a (−)-enantiomer ((−)-T). In vitro (+)-T exerts opioid effects and inhibits serotonin re-uptake, whereas (−)-T is a noradrenaline re-uptake inhibitor [4]. In animal models, both enantiomers revealed antinociceptive effects, with (+)-T being the more potent [4].
Tramadol undergoes biotransformation in the liver and is excreted via the kidneys. Of 11 known metabolites, only the O-demethyl-tramadol (M1) is pharmacologically active [5]. M1 has a remarkably greater affinity for the μ-receptors than tramadol, but plays no dominant role in tramadol-induced analgesia, because M1 has difficulties to penetrate the blood–brain barrier [6].
In a phase II pilot study using i.v. patient-controlled analgesia (PCA), (+)-T and (±)-T were more potent analgesics than (−)-T, whereas the incidence of adverse events was higher in (+)-T patients than in (±)-T patients [7]. It was concluded from the clinical results that the racemate is superior to either enantiomer alone, taking into account both efficacy and safety aspects. The pharmacokinetic results of this investigation are reported in the present paper.
The aim of the present study was to determine the therapeutic serum concentrations of the enantiomers of tramadol and its metabolite M1 during treatment of postoperative pain with tramadol racemate or either enantiomer alone.
Methods
Demographic data and design of this randomised, double-blind study have been published in detail [7]. After Institutional Ethics Committee approval, patients gave written informed consent. The study included 99 female patients after vaginal hysterectomy or gynaecological laparotomy during opioid-free anaesthesia. Patients suffering from severe pain were randomly allocated to treatment with either (+)-T, (±)-T or (−)-T, which were obtained from Grünenthal GmbH (Stolberg, Germany). Initially, an intravenous bolus injection (50 mg up to a maximum of 200 mg) was performed, until acceptable pain relief was obtained. This was followed by single on-demand boluses of 20 mg of the respective study medication through a PCA system (lockout time: 5 min).
Blood sampling
For determination of the therapeutic serum concentrations, 3 ml blood samples were taken prior to each on-demand application via the PCA system (maximal 50 ml per patient). Blood was sampled via an indwelling cannula, which was not used to apply the study medication. After complete coagulation (about 20 min) the blood samples were centrifuged for 10 min at 2800 g and the serum was stored at −20° C until analysis.
Analytical methods
The concentrations of (+)-T and (−)-T in serum were determined by a modified gas chromatographic-mass spectrometric method on a column coated with CP-Cyclodextrin–2,3,6-m-19 [8, 9]. The method involves the use of [2H2,15N]-tramadol hydrochloride as internal standard and chemical ionization with isobutane employing single-ion monitoring for quantification.
The (+)-M1 and (−)-M1 serum concentrations were determined after derivatization with diazoethane by gas chromatography on a column coated with CP-Cyclodextrin-β-2,3,6-m-19 and with a nitrogen-selective (NDP) detector [10].
The serum samples of this study were analytically determined in 15 series of (+)-T and (−)-T and 21 series of (+)-M1 and (−)-M1 determinations. Each series also contained eight calibration samples (5–250 ng 0.5 ml−1) and four control samples (5, 20, 40 and 100 ng 0.5 ml−1). If more than two concentrations of the control samples were outside±15% of the nominal concentration, the results were rejected and the series was repeated.
The lower limit of quantification was 5 ng ml−1. Calibration curves were linear and showed only small differences (0–8%) between nominal and recalculated calibration values. The mean accuracy was 5.8±5.6% for (+)-T, 2.4±1.7% for (−)-T, 4.5±1.2% for (+)-M1 and 4.6±4.0% for (−)-M1. The mean precision (%CV) was 7.4±6.6% for (+)-T, 7.1±6.0% for (−)-T, 8.7±6.8% for (+)-M1 and 11.4±6.5% for (−)-M1.
Statistics
Inter-individual means and standard deviations (s.d.) were calculated for statistical analysis. Analysis of variance including Scheffe's test for pair-wise comparison was used to compare the concentrations between the three treatment groups, the unpaired t-test to compare the concentrations of the (+)-enantiomers between the (+)- and (±)- group and of the (−)-enantiomers between the (−)- and the (±)- group and the paired t-test to compare the concentrations of the two enantiomers in the racemate group (SPSS, P < 0.05).
Results
Ninety-eight patients received study medication. One of the patients allocated to the (−)-group did not receive drug due to respiratory depression. The clinical results have been published in detail [7]. The duration of PCA using (+)-T, (±)-T and (−)-T was 20, 21 and 12 h, respectively. Patients of the (+)-, (±)- and (−)-group received 289 mg, 316 mg and 356 mg of the respective study medication. Twenty-seven patients treated with (+)-T, 25 with (±)-T and 13 with (−)-T were satisfied with pain relief (responders). Nausea and vomiting were the most frequently observed events, with highest occurrence in (+)-T patients.
One patient of the (+)-group and five of the (±)-group were excluded from the statistical evaluation of the serum concentrations, because no demand-boluses were administered or no blood was sampled prior to the boluses. Means of 6±3, 7±3 and 7±4 concentrations per individual were calculated in the patients of the (+)-, (±)- and (−)-group, respectively.
The mean concentrations of tramadol were 470 ng ml−1, 590 ng ml−1 and 771 ng ml−1 (Table 1) and those of M1 were 57 ng ml−1, 84 ng ml−1 and 96 ng ml−1 (Table 2) in the (+)-, (±)- and (−)-group, respectively. The mean concentrations of (+)-T and (+)-M1 were lower in the (±)- than in the (+)-group, those of (−)-T and (−)-M1 were lower in the (±)- than in the (−)-group. In the (±)-group, the mean serum concentrations of (+)-T were higher than those of (−)-T, whereas the mean serum concentrations of (−)-M1 were higher than those of (+)-M1.
Table 1.
Therapeutic serum concentrations (mean±s.d.) of tramadol racemate and enantiomers.
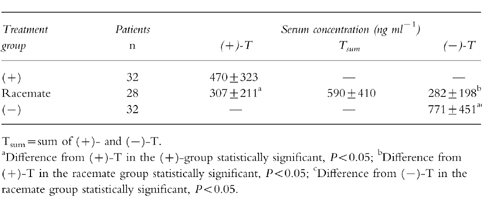
Table 2.
Therapeutic serum concentrations (mean±s.d.) of M1 racemate and enantiomers.
Discussion
The venous blood samples were taken immediately before a patient's self-controlled administration, i.e. at a point in time at which the patient was just becoming dissatisfied with analgesia. Previously, these concentrations were described as ‘minimal effective concentrations’ [11–13]. However, because no quantitative data of the part of (+)-T, (−)-T, (+)-M1 and (−)-M1 in the analgesic effect of tramadol are available, the measured concentrations cannot be defined as ‘minimal effective’. In addition, this study demonstrated a great inter and intraindividual variability in postoperative serum concentrations and could not fix narrow analgesic threshold concentrations. Therefore, the term ‘therapeutic concentrations’ was preferred.
The pharmacokinetic results can explain the clinical findings, that (+)-T is a more potent analgesic than (−)-T but is associated with a higher incidence of opioid related side-effects than (−)-T and the racemate. This is consistent with the clinical suggestion that the racemate is superior to either enantiomer alone in the treatment of severe postoperative pain.
The mean therapeutic serum concentrations of tramadol and M1 in the (+)-group were statistically significantly lower than those in the (−)-group. This finding confirms, that (+)-T is a more potent analgesic than (−)-T. The mean concentrations in the racemate group (sum of (+)-T and (−)-T; sum of (+)-M1 and (−)-M1) were between those in the (+)- and the (−)-group; of these differences, only that for M1 between the (+)- and the racemate group was statistically significant. This is consistent with the clinical suggestion, that the racemate is only sligthly less potent than (+)-T, but cannot confirm, that the racemate is markedly more potent than (−)-T.
The mean concentrations of the (+)-enantiomers of tramadol and M1, which are responsible for opioid effects, were significantly lower in the racemate group than in the (+)-group. This finding can explain that the incidence of opioid related adverse events (nausea) was lower in the racemate group than in the (+)-group.
On the assumption that the enantiomers have a similar volume of distribution, (−)-T is metabolized slightly faster to (−)-M1 than (+)-T to (+)-M1. In the racemate group, the mean serum concentrations of (+)-T were higher than those of (−)-T, whereas the mean serum concentrations of (−)-M1 were higher than those of (+)-M1.
The mean tramadol concentration in the racemate group (590 ng ml−1) was markedly higher than that in a previous investigation (298 ng ml−1) of tramadol racemate using an identical study design [13]. This can be explained by the higher tramadol consumption in the present study (316 mg vs 258 mg) and the fact, that the present patients received no opioids whatsoever during anaesthesia, whereas the other patients received at least 5 μg kg−1 fentanyl intraoperatively. A higher concentration of tramadol racemate (median: 916 ng ml−1) was measured by Hackl et al. [12] combining the PCA with a continuous infusion (12 mg h−1) independent of the demands. Previous data regarding serum concentrations of the (+)-T, (−)-T, (+)-M1, or (−)-M1 are not available, since the present study describes the first administration of the enantiomers to humans.
References
- 1.Radbruch L, Grond S, Lehmann KA. A risk-benefit assessment of tramadol in the management of pain. Drug Safety. 1996;15:8–29. doi: 10.2165/00002018-199615010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Raffa RB, Nayak RK, Liao S, Minn FL. The mechanism(s) of action and pharmacokinetics of tramadol hydrochloride. Rev Contemp Pharmacother. 1995;6:485–497. [Google Scholar]
- 3.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanisms of action of tramadol, an ‘atypical’opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- 4.Raffa RB, Friderichs E, Reimann W, et al. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267:331–340. [PubMed] [Google Scholar]
- 5.Lintz W, Erlacin S, Frankus E, Uragg H. Metabolismus von Tramadol bei Mensch und Tier. Arzneim Forsch/Drug Res. 1981;31:1932–1943. [PubMed] [Google Scholar]
- 6.Lai J, Shou-wu M, Porreca F, Raffa RB. Tramadol, M1 metabolite and enantiomer affinities for cloned human opioid receptors expressed in transfected HN9.10 neuroblastoma cells. Eur J Pharmacol. 1996;316:369–372. doi: 10.1016/s0014-2999(96)00770-4. 10.1016/S0014-2999(96)00770-4. [DOI] [PubMed] [Google Scholar]
- 7.Grond S, Meuser T, Zech D, Hennig U, Lehmann KA. Analgesic efficacy and safety of tramadol enantiomers in comparison with the racemate: a randomised, double-blind study with gynaecological patients using intravenous patient-controlled analgesia. Pain. 1995;62:313–320. doi: 10.1016/0304-3959(94)00274-I. 10.1016/0304-3959(94)00274-I. [DOI] [PubMed] [Google Scholar]
- 8.Becker R, Lintz W. Report FO-PK 323/I. D-52078 Aachen: Grünenthal GmbH; 1993. GC/NPD method for the stereoselective determination of the enantiomers of tramadol and its metabolite M 1 in human serum. [Google Scholar]
- 9.Lintz W, Uragg H. Quantitative determination of tramadol in human serum by gas chromatography-mass spectrometry. J Chromatogr. 1985;341:65–79. doi: 10.1016/s0378-4347(00)84010-4. [DOI] [PubMed] [Google Scholar]
- 10.Ossig J, Terlinden R, Lintz W. Report FO-PK 389. D-52078 Aachen: Grünenthal GmbH; 1993. GC/NPD method for the stereoselective determination of the enantiomers of tramadol and its metabolite M 1 in human serum after derivatisation. [Google Scholar]
- 11.Gourlay GK, Willis RJ, Wilson RJ. Postoperative pain control with methadone: influence of supplementary methadone doses and blood concentration-response relationships. Anesthesiol. 1984;61:19–26. [PubMed] [Google Scholar]
- 12.Hackl W, Fitzal S, Lackner F, Weindlmayr-Goettel M. Fentanyl or tramadol in patient-controlled analgesia for treatment of postoperative pain. Anaesthesist. 1986;35:665–671. [PubMed] [Google Scholar]
- 13.Lehmann KA, Kratzenberg U, Schroeder-Bark B, Horrichs-Haermeyer G. Postoperative patient-controlled analgesia with tramadol: analgesic efficacy and minimum effective concentrations. Clin J Pain. 1990;6:212–220. doi: 10.1097/00002508-199009000-00008. [DOI] [PubMed] [Google Scholar]


