Abstract
Aims
The newly revived coronary bypass graft, the radial artery (RA), is more spastic than the internal mammary artery. Thromboxane A2 is a potent vasoconstrictor for arterial grafts. This study was therefore designed to determine whether the specific thromboxane A2 (TP) receptor antagonist, GR32191B, is effective in inhibition of prostanoid or nonprostanoid receptors in the RA.
Methods
The effect of GR32191B was studied in human RA segments, taken from coronary bypass patients, in organ chambers. Two effects of GR32191B were tested: (1) the relaxation induced by GR32191B in the RA precontracted with the TP receptor agonists U46619 and PGF2α or nonprostanoid vasoconstrictors (noradrenaline [NA], angiotensin II [AII], and K+) and (2) the inhibitory effect of GR32191B on TP receptor agonists and nonprostanoid vasoconstrictors.
Results
In U46619 (10 nm, n = 7) and PGF2α (1 μm, n = 7) precontracted RA, GR32191B induced 100% relaxation (10–100 μm) but not after precontraction with nonprostanoid stimuli (5.8% for K+, 25 mm, n = 6, 24.4% for NA, 3 μm, n = 8, and 53.2% for AII, 3 nm, n = 5) (P < 0.001). Treatment with GR32191B (30 nm) significantly depressed the contraction with U46619 (from 160.1±11.0% to 116.8±13.1%, P < 0.05) or PGF2α (from 91.3±12.3% to 42.2±9.2%, P < 0.01). The contraction was further abolished by 3 μm GR32191B. However, GR32191B at 3 μm did not significantly inhibit the contraction induced by either NA, AII, or K+.
Conclusions
GR32191B is a highly potent and specific TP receptor antagonist for the human RA. It may be particularly useful in inhibiting TXA2-mediated vasoconstriction and therefore in reducing the complications related to vasospasm in this graft.
Keywords: coronary bypass, GR32191B, human artery, internal mammary artery, radial artery, thromboxane A2, thromboxane antagonists
Introduction
Various autologous arteries have been used as grafts for coronary artery bypass (CABG). Because of the superior long-term results from internal mammary artery (IMA) grafting [1], this graft is now prefered. During recent years, the radial artery (RA) has become another popular arterial graft [2]. The RA was initially applied as a graft for CABG in 1971 [3]. However, it was soon abandoned because of reported high incidence of vasospasm and low patency rates [4]. With increased knowledge of spastic characteristics of this artery and of how to overcome the spasm using pharmacological agents, this arterial graft is now re-used [2].
The RA has been demonstrated to be more spastic than the IMA [5]. We have recently classified all arterial grafts into three types [6] according to their pharmacological reactivity and embryological origins: Type I (somatic arteries), Type II (splanchnic arteries), and Type III (limb arteries). In this classification [6], the RA belongs to the Type III, a type that is more spastic than the Type I artery such as the IMA [6]. Considering the more spastic biological characteristics of this artery, together with the history of the use of the RA that was abandoned once as a graft due to its vasospasm problem, antispastic therapy for this arterial graft in CABG is particularly important. In fact, the revival of the RA was largely due to the use of calcium antagonists [2]. However, use of calcium antagonists may be contraindicated in patients with poor left ventricular function and bradycardia. Further, it is still unclear how best to prevent RA spasm.
The search for the best antispastic method for the RA is therefore an important clinical problem. Previous studies have demonstrated that thromboxane A2 (TXA2) is a potent vasoconstrictor in the human RA [5, 7]. Plasma concentrations of TXB2, the stable metabolite of TXA2, are elevated during cardiopulmonary bypass [8] and this implies that TXA2 may be a particularly important spasmogen for arterial grafts such as RA. Although many vasodilators are commonly used clinically, under some circumstances their effect on TXA2-related contraction in the grafts is limited. For example, nitroglycerin (NTG) has a limited effect if used prior to the TXA2 mimetic U46619-induced contraction [9]. We have recently proposed the use of a verapamil protocol as antispastic therapy for the RA [10]. However, it is known that calcium antagonists are very potent inhibitors of vascular contraction via voltage-operated calcium channels but less potent on contraction via receptor-operated calcium channels, which is the major mechanism for TXA2 induced contraction [9]. For the aforementioned reasons, use of specific TXA2 antagonists may have implications in coronary artery bypass surgery.
GR32191B has been demonstrated as a specific TXA2 (TP)-receptor antagonist [11–15]. However, the role of TXA2 antagonists in other arterial grafts such as the RA has not yet been studied.
The purpose of the present study was to determine the effect of GR32191B on prostanoid and nonprostanoid receptor-mediated contraction in the human RA in order to search for new vasodilators suitable to be used for antispastic therapy in this spastic coronary bypass graft.
Methods
One hundred and eleven human RA segments were collected from patients undergoing CABG using the RA irrespective of preoperative therapy. However, none of the patients received thromboxane antagonists. Approval to use discarded RA tissue was given by the Grantham Hospital Human Ethics Committee. Any redundant or discarded RA segments were collected and placed in a container with oxygenated, physiological solution (Krebs′) maintained at 4° C, and then transferred to the laboratory immediately. The RA was transferred into a glass dish and dissected out from its surrounding connective tissue. The vessels were cut into 3 mm long rings and suspended on wires in organ baths [5, 9]. The number of rings taken from each patient varied from 2 to 6. The Krebs' solution had the following composition (in mm): Na+ 144, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 128.7, HCO−3 25, SO2−4 1.2, H2PO−4 1.2, and glucose 11. The solution was aerated with a gas mixture of 95% O2–5% CO2 at 37° C.
Organ-bath technique
A technique that allowed the normalization of vascular rings to a physiological pressure in the organ bath was used in this study. The vascular rings were set at a tension comparable with that at the in vivo situation. The details of the technique were published before [16]. Briefly, the rings were stretched-up in progressive steps to determine the length-tension curve for each ring. A computer iterative fitting program (VESTAND 2.1, Yang-Hui He, Princeton, N.J.) was used to determine the exponential line, pressure and the internal diameter. When the transmural pressure on the rings reached 100 mmHg, determined from their own length-tension curves, the stretch-up procedure was stopped and the rings were released to 90% of its internal circumference at 100 mmHg. This degree of the passive tension was then maintained throughout the experiment.
The endothelium was intentionally preserved by cautiously dissecting and mounting the rings in our study since endothelium plays a modulatory role in the contractility of arterial grafts [5, 6]. We previously found that this technique allowed the experiments to be carried out with an intact endothelium, as determined by the functional relaxation response to substance P or calcium ionophore [5] in the RA or acetylcholine in the coronary artery [17].
Protocol
After the normalization procedure, the RA rings were equilibrated at least for 1 h. The following protocols were designed for the experiments.
Relaxation
GR32191B-induced relaxation was studied in the precontraction induced by two prostanoid receptor agonists—the TP receptor agonist U46619 (10 nm, n = 7) and the prostaglandin F (FP) receptor agonist PGF2α (1 μm, n = 7). The relaxation was also studied in the precontraction induced by three nonprostanoid receptor vasoconstrictors potassium chloride (K+, 25 mm, n = 6), noradrenaline (3 μm, n = 8), and angiotensin II (AII, 3 nm, n = 5). The concentrations of these vasoconstrictor substances were submaximal (EC50 to EC80) that were determined from the logistic-curve fitting equation [16]. These concentrations are equal to EC50-EC80 for the U46619, AII, or K+-induced contraction in the human RA from previous studies [5, 10] and for NA and PGF2α from the present study. Cumulative concentration-relaxation curves to GR32191B were then established. Only one concentration-relaxation curve was obtained from each RA ring. From 5 to 8 rings (taken from at least 3 patients), a mean concentration-relaxation curve was constructed.
Depression of contraction by pretreatment with GR32191B
After equilibration, 100 mm K+ was added into the organ bath and the contraction force was recorded. The ring was frequently washed to restore the baseline. To determine whether pretreatment with GR32191B would alter the contraction response to prostanoid receptor agonists (U46619 and PGF2α) or nonprostanoid vasoconstrictors (NA, AII, and K+), cumulative concentration-contraction curves were constructed in RA segments. The contraction was expressed as percentage of the contraction force induced by 100 mm K+.
U46619, PGF2a and K+
Three rings were taken from each of six patients. One of these rings was used as a control and the other two were equilibrated for 1 h with one of the two concentrations of GR32191B. These concentrations were −7.5 (30 nm) or −5.5 log m (3 μm). A cumulative concentration-contraction curve was then constructed for U46619, PGF2α, or K+.
NA and AII
Two ring segments were taken from each of six patients. One of them was used as a control and the other was equilibrated for 1 h with a high concentration (3 μm) of GR32191B before concentration-contraction curves for these vasoconstrictor substances were established.
Data analysis
The effective concentration of the constrictor (or dilator) agent that caused 50% of maximal contraction (or relaxation) was defined as EC50. The EC50 was determined from each concentration-contraction (or relaxation) curve by a logistic, curve-fitting equation:
E = MAp/(Ap+Kp) where E is response, M is maximal contraction (or relaxation), A is concentration, K is EC50 concentration, and p is the slope parameter [16]. A computerized program was used for the curve–fitting.
From these fitted equation, the mean EC50 value±s.e.mean was calculated in each group. Unpaired t-test or analysis of variance were used to test statistical significance between different constrictors and dilators regarding the maximal response or EC50. Scheffe F-test was used as posthoc test between groups. P < 0.05 was considered as significant.
Drugs
Drugs used in this study and their sources were: (–)noradrenaline bitartrate; 5-hydroxytryptamine, and PGF2α (Sigma, St Louis, MO); U46619 (Cayman Chemical, Ann Arbor, MI); Stock solution of noradrenaline was freshly made each day. Stock solutions of U46619, PGF2α, and AII were held frozen until required. GR32191B was generously given by Glaxo Group Research Ltd. The molecular formula of GR32191B is C30H39NO4.HCl. The structure of this drug has been published [13].
Results
Resting vessel parameters [16]
The mean internal diameter of the 111 rings at an equivalent transmural pressure of 100 mmHg (D100) was 2.7±0.1 mm as determined from the normalization procedure. When the RA rings were set at a resting diameter of 0.9×D100, the equivalent transmural pressure was 66.1±1.7 mmHg, and the resting force was 2.3±0.2 g.
Relaxation by GR32191B in the RA precontracted by five vasoconstrictors
Precontraction with prostanoid receptor agonists
GR32191B caused a full relaxation in U46619 (100%) and PGF2α (100%)-precontracted RA (Figure 1).
Figure 1.

(a) Mean concentration (−log m) −response (% relaxation) curves for GR32191B in the human radial artery precontracted by U46619 (10 nm, n = 7), PGF2α (1 μm, n = 7), potassium chloride (K+, 25 mm, n = 6), noradrenaline (NA, 3 μm, n = 8), and angiotensin II (AII, 3 nm, n = 5). The rings were taken from at least three patients in each group. Vertical error bars are 1 s.e.mean of mean values. **P < 0.01; ***P < 0.001 at the maximal effect. (b) Chart records of isometric force (g) (retouched from the computer recording for publication) from one ring of the radial artery. Cumulative concentration (−log m)-relaxation curve of GR32191B is shown in the ring precontracted with PGF2α (1 μm).
Precontraction with nonprostanoid vasoconstrictors
In contrast, GR32191B only relaxed the K+-contracted rings to 5.8±2.2% (P < 0.0001, compared with the relaxation in U46619 or PGF2α-precontracted RA) (95% confidence interval[95% CI]: not available due to zero standard error in the relaxation by U46619 and PGF2α). In the NA-and AII-induced precontraction, GR32191B induced 24.4±7.7% and 53.2±16.1% relaxation (P < 0.0001 and P = 0.003, compared with U46619 or PGF2α induced relaxation (95% CI: not available due to zero standard error in the relaxation by U46619 and PGF2α) (Figure 1).
The EC50 was −6.82±0.09 log m for U46619 and −7.04±0.07 log m for PGF2α (P > 0.05). In contraction induced by nonprostanoid vasoconstrictor, in some rings, there was minimal relaxation so that the EC50 could not be calculated. In the 6 of 8 rings in the NA experiment, the EC50 was −6.56±0.15 log m. In 4 of 5 rings in the AII experiment, the EC50 was −5.59±0.61 log m (P = 0.01 compared with U46619 precontraction and P < 0.01 compared with PGF2α precontraction).
Depression of contraction by pretreatment with GR32191B
Prostanoid receptor-agonists (U46619 and PGF2α)
One hour pretreatment of RA with GR32191B significantly depressed the maximal contraction induced by U46619 from 160.1±11.0% (control) to 116.8±13.1% (95% CI: 0.1%˜3.6%, P = 0.02, Scheffe F-test) and 7.4±2.3% (95% CI: 4.6%≈116.3%, P < 0.0001, Scheffe F-test) with the treatment of GR32191B at the concentration of 30 or 3 nm (Figure 2a).
Figure 2.

Mean concentration (−log m)-contraction (percentage of 100 mm K+-induced contraction) curves for U46619 (a) and PGF2α (b). Three rings obtained from each patient were allocated to each treatment. One ring was the control without pretreatment of GR32191B. For the other two rings GR32191B −7.5 or −5.5 log m was added into the organ bath one hour before the start of the U46619 or PGF2α curve. Symbols represent data averaged from 6 rings (from 6 patients) for each vasoconstrictor. Vertical error bars are 1 s.e.mean of mean values. *P < 0.05; **P < 0.01; ***P < 0.001 at the maximal effect. (c) Chart records of isometric force (g) (retouched from the computer recording for publication) from three rings of one segment of the radial artery from a patient. Cumulative concentration (−log m)-contraction curves of U46619 in the control ring (control, without pretreatment of GR32191B), the ring treated with GR32191B at −7.5 log m for 1 h (GR32191B −7.5), and the ring treated with GR32191B −5.5 log m (GR32191B −5.5) are shown.
In addition, The pretreatment with GR3191B significantly decreased the sensitivity of the RA to U46619. Pretreatment with GR32191B at 30 nm significantly increased the EC50 (−7.52±0.22 vs−8.28±0.21 log m, 95% CI: −0.18 ~−4.61 log m, P = 0.03). In the rings pretreated with 3 μm GR32191B, the EC50 could not be calculated because the contraction was abolished (Table 1).
Table 1.
Maximal contraction (% of 100 mm K+ contraction) and fitted EC50 values (mean±s.e.mean) for five vasoconstrictors in the human radial artery treated with GR32191B. The number of rings in all groups is 6.

Pretreatment with GR32191B at 30 nm significantly inhibited PGF2α-induced maximal contraction from 91.3±12.3% (control) to 42.2±9.2% (95% CI: 0.4%˜9%, P = 0.005, Scheffe F-test) and further abolished it at 3 μm (95% CI: 34.7%˜883.8%, P = 0.0001, Scheffe F-test) (Figure 2b). The treatment of GR32191B (30 nm) increased the EC50 9.8-fold higher although not statistically significant (−5.38±0.50 vs−6.37±0.12 log m, 95% CI: 0.01 ˜0.33 log m, P = 0.06, Table 1). However, the abolishment of the PGF2α-induced contraction was complete by treatment with GR32191B at 3 μm so that the EC50 could not be calculated.
Non–prostanoid receptor vasoconstrictors (K+, NA and AII)
In contrast to the prostanoid receptor agonist-mediated contraction, K+-mediated contraction was not affected by the pretreatment with GR32191B (Figure 3a and Table 1). Also, there was no difference regarding EC50.
Figure 3.
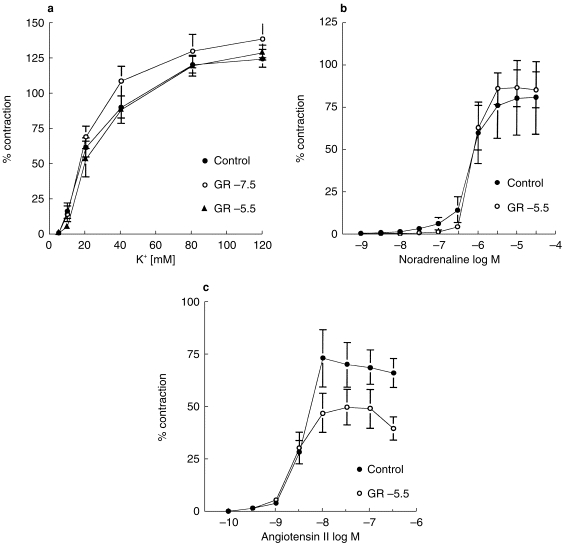
Mean concentration (−log m)-contraction (percentage of 100 mm K+-induced contraction) curves for (a) K+ (n = 6) (b) noradrenaline (NA, n = 6), and (c) angiotensin II (AII, n = 6). Three (for K+) or two (for NA or AII) rings obtained from each patient were allocated to each treatment. One ring was the control without pretreatment of GR32191B. (a) GR32191B −7.5 or −5.5 log m was added into the organ bath 1 h before the start of the K+ curve. (b) GR32191B −5.5 log m was added into the organ bath 1 h before the start of the NA curve. (c) GR32191B −5.5 log m was added into the organ bath 1 h before the start of the AII curve. Symbols represent data averaged from 6 rings (from 6 patients). Vertical error bars are 1 s.e.mean of mean values.
Similarly, pretreatment with GR32191B did not alter the NA-mediated contraction in either the maximal contraction force or the EC50 (Figure 3b and Table 1). A similar effect was also seen in AII-mediated contraction, although the maximal contraction was slightly depressed with GR32191B treatment (Figure 3c and Table 1).
Discussion
In this study, we have found that in the human RA GR32191B is a specific TXA2 (TP) receptor antagonist, which totally blocks TP receptor-mediated contraction but has little effect on the contraction mediated by nonprostanoid vasoconstrictors.
Operative results (early mortality and long-term patency) of CABG largely depend on the function of the graft. Based on the experimental studies, the RA is more spastic than Type I arteries (such as IMA and IEA) [5–7]. From the history of the revival of the RA and our clinical experience in accordance with others [2], RA contraction (or spasm) is almost inevitably encountered during surgical dissection. The spastic characteristic of the RA warrants use of vasodilators during harvesting. Development of new vasodilators has promoted the use of arterial grafts. Despite continuing studies on the mechanisms of vasospasm, the nature of vasoconstriction has not been clear. This is probably due to the complexity of vasoconstrictors and mechanisms involved in the contraction of blood vessels [18]. Identifying new vasodilators has been a continuing challenge. Such new vasodilators include potassium channel openers [19], PDE inhibitors [20], and thromboxane antagonists [15].
In fact, the use of vasodilators is a key step in the revival of the RA [2]. Acar et al. [2] suggested a protocol, in which diltiazem is used as the systemic vasodilator and papaverine is used topically during harvesting. In addition, we have developed a new protocol which includes calcium antagonist verapamil for systemic administration and verapamil and nitroglycerin for topical use [10].
The common disadvantages of calcium antagonists, on the other hand, are (1) that the high vasoconstrictor-selectivity may limit their effect under some circumstances when a vessel is contracted through receptor mechanisms (2) that the onset of the action is relatively slow, and (3) that they may have negative inotropic or chronotropic effects.
During cardiopulmonary bypass, TXA2 is increased and this may be related to the vasoconstriction after cardiopulmonary bypass [8]. TXA2 antagonists have been demonstrated to have protective effects on cardiac contraction during ischaemia and reperfusion [21]. Use of TXA2 antagonists during cardiopulmonary bypass for open heart surgery may be beneficial to the patient regarding myocardial protection. For coronary artery bypass grafting surgery, the use of such antagonists may have particular implications.
The human RA is an active artery that responds to many vasoconstrictors such as K+, TXA2 agonist, α-adrenoceptor agonists, 5-HT, and endothelin [5, 7]. In particular, TXA2 mimetic U46619 is a potent vasoconstrictor for the human RA [5]. Due to increased plasma concentrations of TXA2 during cardiopulmonary bypass, this vasoconstrictor could be implicated as a spasmogen for the human RA or other arterial grafts. Due to the limitation of the effect of NTG and calcium antagonists on TXA2 mimetic-induced contraction in arterial grafts [9, 22], an effective vasodilator, which specifically inhibits TP receptors, may have indications for coronary artery bypass grafting using arterial grafts.
GR32191B is a highly potent and specific TP receptor antagonist in animal vascular and nonvascular smooth muscle, as well as in human pulmonary arteries [13] and IMA [15]. Because the RA has different contractile characteristics compared with the IMA, it is necessary to study the effect of TXA2 antagonists in the RA. In the present study, we have demonstrated that GR32191B totally abolished the contraction induced by TXA2 agonist U46619 in two ways. In U46619 precontracted RA, GR32191B induced a full relaxation (Figure 1). On the other hand, when the RA was incubated with GR32191B for 1 h, U46619-induced contraction was totally prevented at a concentration of 3 μm. At the concentration of 30 nm, GR32191B reduced the maximal contraction (P = 0.03) and shifted the contraction curve rightward by 5.8 fold (P = 0.03). Those concentrations are clinically relevant. As measured by Ritter et al. [23], 12 h after GR32191B (80 mg, orally) the plasma concentration was 36.6 nm (−7.44 log m). Therefore, this study demonstrates that GR32191B is a highly potent and useful TP receptor antagonist in the human RA.
The present study demonstrates that PGF2α is another strong prostanoid vasoconstrictor for the human RA. Our study also tested the effect of GR32191B on PGF2α-induced contraction. As initially defined by Kennedy et al. [24], prostanoid receptors can be characterized by the prostaglandin that has the greatest activity, that is TP (TXA2), FP (PGF2α), EP (PGE2), etc. It has been demonstrated that the contractile effect of PGF2α is mediated by TP receptors in human airways [25, 26] and in the human IMA [15]. In contrast, PGF2α has been also suggested to contract smooth muscle through FP-receptors [22, 24, 27]. In the present study, we also tested the effect of PGF2α in the human RA in two ways. On the one hand, this prostaglandin strongly contracted the artery and produced 5.0±0.4 g force (91.3±12.3% of 100 mm K+ contraction) and the strong contraction was antagonized by the specific TP receptor antagonist GR32191B. In GR32191B-incubated RA segments, the contraction was totally (by 3 μm) abolished or significantly suppressed by 30 nm GR32191B (Figure 2b). On the other hand, the PGF2α (1 μm)-precontracted RA was totally relaxed by GR32191B and the EC50 for GR32191B in PGF2α -induced contraction was similar to that for GR32191B in U46619-induced contraction (Figure 1 and Table 1). These results suggest that, in the human RA, PGF2α may act through TP receptors. This observation is in accordance with our previous study in the human IMA [15]. It has been hypothesized that this may be due to the possible existence of subtypes of TP receptors [15, 25, 26]. However, due to lack of specific antagonists of possible subtypes of TP receptors, this is still a speculation. Further, although PGF2α strongly contracted the RA, it induced a less maximal contractile response than U46619 (91.3±12.3% for PGF2αvs 160.1±11.0% for U46619, P < 0.01). Possible explanations for this difference are two-fold. First, this may suggest that PGF2α is a partial agonist for TP receptors in the human RA. Second, this may be explained by the above-mentioned hypothesis that U46619 and PGF2α stimulate different subtypes of TP receptors. However, the possibility that PGF2α may also stimulate some relaxant prostanoid receptors such as EP2 receptors and therefore reduce the effect of contractile response through TP receptors, as suggested previously [27], may not be completely excluded and this needs to be further studied.
The high specificity of GR32191B is demonstrated in this study. We tested the effect of GR32191B on K+, α-adrenoceptor (NA), and AII mediated contraction. GR32191B did not significantly inhibit K+-induced contraction in either way (Figures 1 and 3a). Regarding NA or AII -mediated contraction, although there was a weak relaxation (Figure 1), GR32191B did not significantly inhibit the maximal contraction or change the EC50 (Figure 3b,c). Therefore, this study has demonstrated that in the human RA GR32191B is a specific TP receptor antagonist, which has significantly less effect on α-adrenoceptor or AII receptor-mediated contraction. In a study by Lumley et al. [13], GR32191B did not have significant effect upon contractions of the vessels induced by 5-HT, K+, or histamine in rat, guinea-pig, and rabbit vascular smooth muscle. Similarly, GR32191B had little effect on K+, α-adrenoceptor agonists, and AII-mediated contraction in the human IMA [15].
Another major effect of GR32191B is inhibition of platelet aggregation. GR32191B has been found to inhibit platelet aggregation at concentrations similar to that blocking TP receptors in vascular smooth muscle [13], and causes only a modest prolongation of bleeding time. This is unaffected by therapeutic doses of NTG. These features of GR32191B may prove advantageous when used after CABG for patients having received arterial grafts such as the RA in addition to its blocking effect on the TP receptor in vascular smooth muscle.
In conclusion, the results from our study suggest that GR32191B is a highly potent and specific TP receptor antagonist for the human RA. It may be particularly useful in inhibiting TXA2-mediated vasoconstriction and therefore reducing the complications related to vasospasm in this graft.
Acknowledgments
This study was supported by Hong Kong Research Grants Council (Grant HKU7280/97 m), Committee of Research and Conference Grant (337/048/0018, 335/048/0079) and University Grant (014/048/9602 and 344/048/0001), The University of Hong Kong. The authors gratefully acknowledges the technical assistance of the surgical medical officers at Division of Cardiothoracic Surgery, the Operating Theater nurses and technicians at Grantham Hospital, and Mr Kenneth Wong at Department of Surgery, University of Hong Kong.
References
- 1.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 2.Acar C, Jebara VA, Portoghese M, et al. Revival of the radial artery for coronary bypass grafting. Ann Thorac Surg. 1992;54:652–660. doi: 10.1016/0003-4975(92)91007-v. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier A, Guermonprez JZ, Deloche A, Frechette C, Dubost C. The aorto-to-coronary radial artery bypass graft: a technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier A, Discussion of Geha AS, Krone RJ, McCormick JR, Baue AE. Selection of coronary bypass: anatomic, physiological, and angiographic considerations of vein and mammary artery grafts. J Thorac Cardiovasc Surg. 1975;70:414–431. [PubMed] [Google Scholar]
- 5.He G-W, Yang C-Q. Radial artery has higher receptor-selective contractility but similar endothelium function compared to mammary artery. Ann Thorac Surg. 1997;63:1346–1352. doi: 10.1016/s0003-4975(97)00106-9. 10.1016/S0003-4975(97)00106-9. [DOI] [PubMed] [Google Scholar]
- 6.He G-W, Yang C-Q. Comparison among arterial grafts and coronary artery. An attempt at functional classification. J Thorac Cardiovasc Surg. 1995;109:707–715. doi: 10.1016/S0022-5223(95)70352-7. [DOI] [PubMed] [Google Scholar]
- 7.Chardigny C, Jebara VA, Acar C, et al. Vasoreactivity of the radial artery. Comparison with the internal mammary artery and gastroepipoic arteries with implications for coronary artery surgery. Circulation. 1993;88:115–127. [part II] [PubMed] [Google Scholar]
- 8.Davies GC, Sobel M, Salzman EW. Elevated plasma fibrinopeptide a and thromboxane B2 levels during cardiopulmonary bypass. Circulation. 1980;61:803–813. doi: 10.1161/01.cir.61.4.808. [DOI] [PubMed] [Google Scholar]
- 9.He G-W, Buxton B, Rosenfeldt F, Angus JA. Reactivity of human isolated internal mammary artery to constrictor and dilator agents. Implications for treatment of internal mammary artery spasm. Circulation. 1989;80(Suppl):I141–I150. [PubMed] [Google Scholar]
- 10.He G-W, Yang C-Q. Use of verapamil and nitroglycerin solution for preparation of radial artery for coronary bypass grafting. Ann Thorac Surg. 1996;61:610–614. doi: 10.1016/0003-4975(95)00920-5. 10.1016/0003-4975(95)00920-5. [DOI] [PubMed] [Google Scholar]
- 11.Jones RL, Wilson NH, Lawrence RA. EP 171: a high affinity thromboxane A2-mimetic, the actions of which are slowly reversed by receptor blockade. Br J Pharmacol. 1989;96:875–887. doi: 10.1111/j.1476-5381.1989.tb11898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas M, Lumley P. Preliminary assessment of a novel thromboxane A2 receptor-blocking drug, GR32191, in healthy subjects. Circulation. 1990;819(Suppl):I-53–I-58. [PubMed] [Google Scholar]
- 13.Lumley P, White BP, Humphrey PPA. GR32191: a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br J Pharmacol. 1989;97:783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maconochie J, Kensington J, Lumley P. Evaluation of the thromboxane A2 receptor blocking activity of GR 32191 in man. Br J Clin Pharmacol. 1988;26 [Google Scholar]
- 15.He G-W, Yang C-Q. Effects of thromboxane A2 antagonist GR 32191B on prostanoid and nonprostanoid receptors in the human internal mammary artery. J Cardiovasc Pharmacol. 1988;26:13–19. doi: 10.1097/00005344-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 16.He G-W, Angus JA, Rosenfeldt FL. Reactivity of the canine isolated internal mammary artery, saphenous vein, and coronary artery to constrictor and dilator substances: Relevance to coronary bypass graft surgery. J Cardiovasc Pharmacol. 1988;12:12–22. doi: 10.1097/00005344-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 17.He G-W, Yang C-Q, Graier WF, Yang J-A. Hyperkalemia alters EDHF-mediated hyperpolarization and relaxation in coronary arteries. Am J Physiol. 1996;271:H760–H767. doi: 10.1152/ajpheart.1996.271.2.H760. [DOI] [PubMed] [Google Scholar]
- 18.He G-W, Yang C-Q, Starr A. An overview of the nature of vasoconstriction in arterial grafts for coronary surgery. Ann Thorac Surg. 1995;59:676–683.23. doi: 10.1016/0003-4975(94)01011-0. 10.1016/0003-4975(94)00918-W. [DOI] [PubMed] [Google Scholar]
- 19.He G-W, Yang C-Q. Inhibition of vasoconstriction by the potassium channel opener aprikalim in human conduit arteries. Br J Clin Pharmacol. 1997;44:353–359. doi: 10.1046/j.1365-2125.1997.00640.x. 10.1046/j.1365-2125.1997.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He G-W, Yang C-Q. Inhibition of vasoconstriction by phosphodiesterase III inhibitor milrinone in human conduit arteries used as coronary bypass grafts. J Cardiovasc Pharmacol. 1996;28:208–214. doi: 10.1097/00005344-199608000-00005. 10.1097/00005344-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kulatilake N, Gonzalez-Lavin L, Grover GJ. Thromboxane A2 receptor blockade improves contractile function following cardiopulmonary bypass in dogs and pigs. J Surg Res. 1991;51:336–340. doi: 10.1016/0022-4804(91)90117-5. 10.1016/0022-4804(91)90117-5. [DOI] [PubMed] [Google Scholar]
- 22.He G-W, Yang C-Q, Gately H, et al. Potential greater than additive vasorelaxant actions of milrinone and nitroglycerin on human conduit arteries. Br J Clin Pharmacol. 1996;41:101–107. doi: 10.1111/j.1365-2125.1996.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 23.Ritter JM, Benjamin N, Doktor HS, et al. Effects of selective thromboxane receptor antagonist (GR32191B): and of glyceryl trinitrate on bleeding time in man. Br J Clin Pharmacol. 1990;29:431–436. doi: 10.1111/j.1365-2125.1990.tb03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy I, Coleman RA, Humphrey PPA, Levy GP, Lumley P. Studies on the characterization of prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- 25.Armour CL, Johnson PRA, Aifredson ML, Black JL. Characterization of contractile prostanoid receptors on human airway smooth muscle. Eur J Pharmacol. 1989;165:215–222. doi: 10.1016/0014-2999(89)90715-2. 10.1016/0014-2999(89)90715-2. [DOI] [PubMed] [Google Scholar]
- 26.Featherstone RL, Robinson C, Holgate ST, Church MK. Observations on thromboxane receptor-mediated contraction of guinea-pig and human airways by other prostaglandins. Br J Pharmacol. 1988;94 doi: 10.1007/BF00176337. [DOI] [PubMed] [Google Scholar]
- 27.Senior J, Sangha R, Baxter GS, Marshall K, Clayton JK. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br J Pharmacol. 1992;107:215–221. doi: 10.1111/j.1476-5381.1992.tb14489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


