Abstract
Neural activity and neurotrophins induce synaptic remodeling in part by altering gene expression. A cDNA encoding a glycosylphoshatidylinositol-anchored protein was identified by screening for hippocampal genes that are induced by neural activity. This molecule, named neuritin, is expressed in postmitotic-differentiating neurons of the developing nervous system and neuronal structures associated with plasticity in the adult. Neuritin message is induced by neuronal activity and by the activity-regulated neurotrophins BDNF and NT-3. Purified recombinant neuritin promotes neurite outgrowth and arborization in primary embryonic hippocampal and cortical cultures. These data implicate neuritin as a downstream effector of activity-induced neurite outgrowth.
The developing nervous system establishes functional networks through the growth and retraction of synaptic connections from growing axons and dendrites. This plasticity extends into adulthood as a component of memory processes and in pathophysiological conditions such as epilepsy. The remodeling of synapses by repeated neuronal stimuli involves alterations in gene expression that correlate with neurotransmitter-mediated signaling, as well as with the activation of neurotrophin receptors (1–4).
The pool of identified downstream molecules that may elicit the expression of long-term synaptic changes remains small, and little is understood about the molecules’ mode of action in plasticity (5–10). Genes regulated by neuronal activity whose changes in expression can affect cellular function have been identified. They include transcription factors (9), cytoskeletal-associated proteins (7), extracellular proteases (5), and neuroregulatory molecules such as brain-derived neurotrophic factor (BDNF; refs. 11–14) and dynorphin (15). There is increasing evidence that supports an integral relationship between neurotrophin (NT) function and neuronal activity in neuronal survival and differentiation (4). An overlap of genes may therefore be expected between the pool of genes induced following NT receptor activation and those activated following neurotransmitter signaling. Identification of such coregulated downstream effector genes may provide insight into the mechanism by which NTs and neural activity separately and synergistically elicit structural and functional changes in neuronal connectivity.
To identify potential targets of neuronal stimulation, we used the glutamate analog kainic acid (KA) to generate a subtracted cDNA library from KA-activated rat dentate gyrus (DG). The library was used in a differential screen to identify genes potentially involved in plasticity (15). We describe here the identification and characterization of a glutamate and NT receptor target gene that encodes a small neuronal protein which functions extracellularly to modulate neurite outgrowth. The gene’s expression is restricted to the nervous system and is dynamically regulated throughout development and in the adult.
METHODS
Cloning of Neuritin.
Rat full-length neuritin was cloned as described (15). Human neuritin was cloned by PCR with oligonucleotides complementary to the 5′ and 3′ ends (5′-CTAGTCTAGAACCATGGGACTTAAG-3′ and 5′-GGTATAGTCGACCCGTGCTCAGAA-3′) of the rat coding sequence using cDNA generated from human cortical RNA (CLONTECH). Amplified products of the predicted size (460 bp) were subcloned and sequenced.
Recombinant Expression and Purification of Neuritin.
Mammalian recombinant neuritin was expressed in Chinese hamster ovary (CHO) d− cells using the Amgen mammalian expression vector containing a simian virus 40 promoter and dihydrofolate reductase selection cassette. The complete rat cDNA was used to express the glycosylphoshatidylinositol (GPI)-anchored version of neuritin. The human cDNA minus the putative GPI-signal peptide (terminating at N) was used to express the secreted, tagged version of neuritin containing the herpes simplex virus-hexa-histidine epitope (pGREG). Individual dihydrofolate reductase-positive colonies were expanded, and neuritin expression was assayed by Northern and Western blot analyses. Histidine-tagged neuritin was purified from serum-free conditioned media using nickel/nitrilotriacetic acid resin (Qiagen) and then eluted with 500 mM imidazole, concentrated (Ultrafree-5K MWCO, Millipore), and diafiltered into 1× PBS. The purity of neuritin was assessed by silver staining at >95%.
Animal Procedures.
Adult rats (200–210 g) were treated with KA (fresh, 10 mg/ml in saline) by i.p. injection (8 mg/kg). Six hours after injection of KA, rats were killed by decapitation for fresh tissue dissection or anesthetized with ketamine/xylazine. Intracranial injection of recombinant human BDNF was done as described (16). Briefly, BDNF (10 mg/ml in saline) or saline alone was injected (1–2 μl) stereotaxically into the lateral ventricle of postnatal day 4 rats. Brain tissue was dissected 6 hr after intraventricular injection, pooled, and immediately frozen.
Northern Blot Analysis.
A multiple-tissue Northern blot (CLONTECH) containing 10 μg of poly(A)+ RNA from human or rat tissues was probed with a 32P-labeled cRNA probe complementary to the neuritin coding sequence. Total RNA from tissues was isolated essentially as described (17). Total RNA from primary embryonic neuronal cultures (4 × 106 hippocampal or 6 × 106 cortical cells per treatment) was isolated using RNeazy lysis buffer and spin columns (Qiagen). RNA (5 or 10 μg) was size-fractionated on 0.8–1% formaldehyde agarose gels and capillary-blotted to nylon membranes (Hybond-N, Amersham) and probed with 32P-labeled cDNA or cRNA probes specific for rat neuritin. RNA loading was controlled by hybridization of blots with a glyceraldeyde 3-phosphate dehydrogenase probe.
In Situ Hybridization and Immunohistochemistry.
Embryos from timed pregnant rats [embryonic day 1 (E1) = 24 hr post coitus] were isolated and fixed overnight in fresh 4% paraformaldehyde in PBS (4% paraformaldehyde/PBS) at 4°C before dehydration and paraffin embedding. Adult rat brains were first prepared by transcardial perfusion of anesthetized animals with 4% paraformaldehyde/PBS. In situ hybridization on tissue sections (10 μm) was done as described (18) using α-35S-UTP-labeled neuritin cRNA probe. Immunohistochemical localization of neuritin was determined using sections (as above) probed with affinity-purified antisera specific for mammalian recombinant neuritin. Bound neuritin antibody was detected with biotinylated goat anti-rabbit Ig and horseradish peroxidase-labeled avidin using the Vectastain Elite ABC staining kit (Vector Laboratories).
Generation of Polyclonal Antisera.
Rabbit antisera were generated against the peptide DCQEGAKDMWDKLRK or against bacterially expressed neuritin (amino acids 30–113) purified from solubilized inclusion bodies. Antisera were affinity-purified using Sepharose beads containing the appropriate immobilized peptide (SulfoLink, Pierce, and Actigel-ALD, Sterogen, Arcadia, CA) followed by a protein A/G column (ImmunoPure, Pierce) for Ig concentration.
Primary Neuronal Culture.
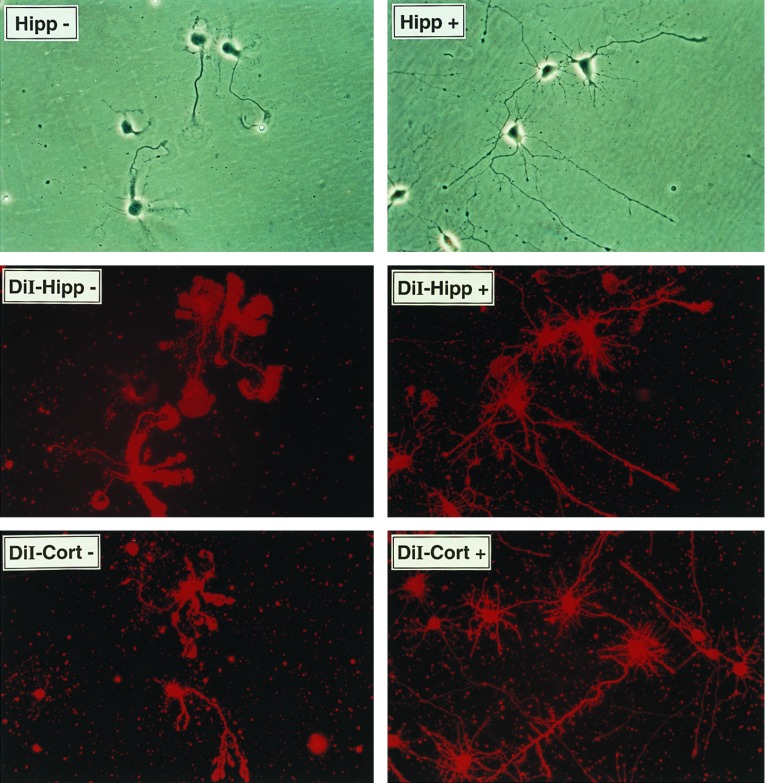
E18 rat embryo brain regions were dissected, and primary hippocampal and cortical neurons were prepared using a papain-based tissue dissociation kit (Worthington). For RNA analysis dissociated neurons were plated on six-well tissue culture plates (Falcon) precoated with poly-l-ornithine (0.1 mg/ml in 150 mM sodium borate, pH 8.4, Sigma) and laminin (1 μg/ml in PBS, GIBCO) at 2 × 105 (hippocampal) to 3 × 105 (cortical) cells per square centimeter. Cells were grown in Neurobasal media (GIBCO) supplemented with 1× B-27 supplement (GIBCO) and 50 μg/ml gentamycin sulfate (GIBCO). Primary rat neurons were grown for 7 days in vitro before treating as described. Glial content following 7 days in vitro was <5% as assessed by immunofluoresence detection of GFAP-positive cells. Neuritogenesis was assessed using cells plated on 35-mm dishes coated with poly-l-lysine (20 μg/ml in H2O) at 5 × 103 cells per square centimeter in the presence or absence of nickel-purified histidine-tagged neuritin (≈150 ng/ml). A corresponding nickel-column fraction of parental vector CHO-conditioned media was used as control. Neurite outgrowth was evaluated after 4 days in vitro by staining of the live cultures with the nonspecific lipophilic dye DiI (10 μM in dimethyl sulfoxide, visualized with a 565-nm filter) for 30 min at 37°C, followed by 3 washes in B-27-supplemented Neurobasal media.
Biochemical Analysis of the GPI Anchor.
Neuritin-expressing CHO cells (CHO15.4; 1 × 106; ≈1 × 105 cells per square centimeter) were treated with 0.4 unit/ml of phosphatidylinositol-specific phospholipase C (PI-PLC, Calbiochem) in 0.5 ml of release buffer (19). Supernatants from mock-treated and PI-PLC-treated cells were analyzed by immunoblotting with neuritin-specific antisera. Analysis of the endogenous GPI-anchored neuritin was done by extracting tissue with the detergent Triton X-114 (Calbiochem; ref. 20). Approximately 100 mg of pulverized tissue was suspended in 0.5 ml of 1× TNE buffer (20) with protease inhibitors and mixed at 4°C with 0.25 volumes of Triton X-114 pre-equilibrated with 1× TNE. The Triton X-114 soluble proteins were extracted twice by incubating the mixture at 30°C for 15 min and centrifuging at 3000 × g for 5 min at room temperature. Aliquots (20–40 μl) of the detergent fractions were precipitated with 10 volumes of MeOH (10 min at 4°C followed by spinning at 14,000 × g for 15 min at 4°C). The precipitated pellet was resuspended in SDS/PAGE sample buffer (with 2-mercaptoethanol) and analyzed by immunoblotting.
Immunoblot Analysis.
Proteins were size-separated on 16% SDS/Tris-glycine gels before electroblotting to nitrocellulose for immunodetection with neuritin-specific affinity-purified antisera and horseradish peroxidase-conjugated goat anti-rabbit antisera that is detected by peroxidase-sensitive enhanced chemiluminescence (Amersham).
RESULTS
Neuritin cDNA and Predicted Protein Sequence.
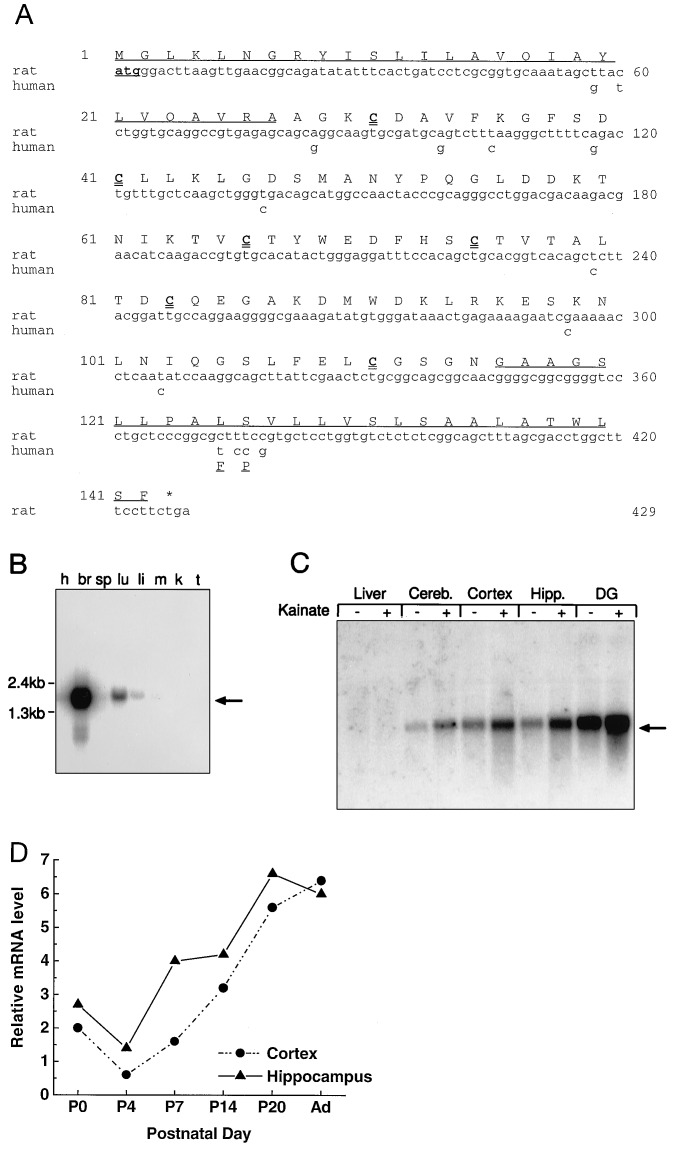
A cDNA that hybridizes to a ≈1.6-kb mRNA was identified in a differential screen of a subtracted library made from KA-activated rat hippocampal DG (15). A putative full-length clone of 1604 bp (Fig. 1A) contains an ORF predicting a 143-aa protein. This ORF contains a potential initiating methionine downstream of 5′ translation stop sites that sits within a nucleotide sequence which corresponds to the Kozak consensus for efficient translation (21). Alternative methionines within the ORF (positions 49 and 90) are not optimal for translation initiation. The 3′ noncoding region contains a polyadenylylation signal sequence (AATAAA) 12 nt from the terminal poly(A) tail.
Figure 1.
Deduced amino sequence and expression profile of neuritin cDNA. (A) The full sequence of rat neuritin is shown. The coding region of human neuritin is identical to the rat sequence in all but 14 bp. Only the nucleotides and amino acids that differ from the rat sequence are shown. The predicted signal peptides for secretion and GPI anchoring are underlined. The six cysteine residues of the mature protein are in boldface type. (B) Multiple-tissue Northern blot of 10 μg of poly(A)+ RNA from human tissues probed with a 32P-labeled cRNA probe of neuritin coding sequence. The neuritin message migrates between 1.6 and 2.0 kb. h, Heart; br, brain; sp, spleen; lu, lung; li, liver; m, muscle; k, kidney; t, testis. (C) Neuritin mRNA is present in different regions of the brain, and the expression is stimulated by KA in vivo. Northern blot of total RNA (10 μg) isolated from control or KA-treated rats (i.p. injection, 10 mg/kg for 6 hr) probed with the neuritin cRNA probe. Cereb., cerebellum; Hipp., hippocampus; DG, dentate gyrus. (D) Quantitated Northern blot analysis results of RNA harvested at different postnatal time points. Ad, adult (8 weeks).
The amino acid sequence derived from the putative ORF has a calculated molecular mass of 15.3 kDa and contains a hydrophobic stretch of approximately 27 aa at the N terminus that functions as a secretory signal peptide of (Fig. 1A). The C-terminal 27-aa tail is also enriched in hydrophobic residues and contains a consensus cleavage signal found in GPI-anchored proteins (19). The predicted mature, membrane-bound protein of ≈90 aa (12 kDa) has 6 cysteine residues for potential inter- or intramolecular disulfide bond formation. It does not contain any obvious phosphorylation or glycosylation sites. A comparison of current GenBank DNA and protein sequence databases (release no. 98) did not reveal a significant general sequence or motif homology with any known protein.
The human cDNA corresponding to the rat ORF was cloned from a human cortical cDNA library and is 97% identical to the rat. Of the 12 nucleotide changes in the human cDNA, 10 of them occur in the wobble position of the codons found in the mature protein, resulting in a human protein that is 100% identical with that of the rat (Fig. 1A). The two additional nucleotide changes are clustered in the putative GPI signal peptide and result in amino acid changes (rat to human) L125F and S126P but do not affect the hydrophobic nature of the signal peptide.
Neuritin mRNA Is Enriched in Brain and Is Induced by KA.
Northern blot analysis of poly(A)+ mRNA from select rat tissues detects a single 1.6-kb mRNA band expressed predominantly in rat brain. Minor hybridization is observed to mRNA from lung and liver tissue following extended exposure times (Fig. 1B). The highest basal expression within the brain is in the DG (Fig. 1C) and the lowest is in the striatum (unpublished observations). Six hours after i.p. injection of KA (8 mg/kg) neuritin mRNA is induced in the adult rat brain (Fig. 1C), with the DG showing the largest induction (3- to 5-fold) of neuritin mRNA. Examination of mRNA isolated from animals treated with KA for 0, 6, and 9 hr shows that maximal expression is at 6 hr, and the elevated levels of message in the DG are maintained even after 9 hr of treatment (data not shown).
The developmental expression of neuritin mRNA was monitored in the embryo and postnatal brain. Neuritin mRNA is detected by in situ hybridization in the developing nervous system on E15 (Fig. 2A) and by Northern blot analysis of mRNA extracted from various central nervous system structures of E15–20 animals (Fig. 3 and data not shown). Expression is first detected by Northern blot analysis in mRNA isolated from the heads of E12 animals. Both the in situ and the Northern blot analyses indicate that neuritin mRNA levels increase throughout embryonic development. Northern blot analysis of postnatal neuritin expression in cortical and hippocampal tissue (Fig. 1D) shows that the relative levels of neuritin mRNA continues to increase postnatally into adulthood.
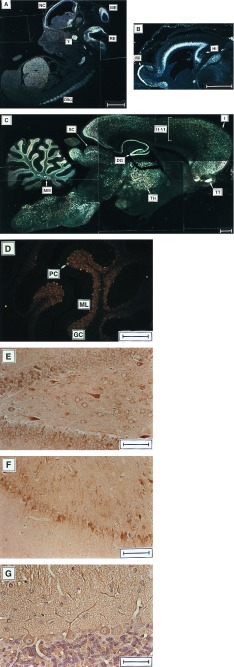
Figure 2.
Expression of embryonic neuritin mRNA and protein in situ. Neuritin mRNA is detected with a 32P-labeled neuritin cRNA probe in parasaggital sections of rat embryos. (A) Whole embryo, E15. NC, neocortex; ME, mesencephalon; RE, rhombencephalon; V, trigeminal ganglion; DRG, dorsal root ganglia. (B) E18 brain. Mi, mitral cell layer of olfactory bulb; Hi, hippocampus. (C and D) Adult rat brain. High levels of expression are observed in cortical layers II–VI, pyramidal and granule cells (DG) of the hippocampal formation, thalamus (TH), superior colliculus (SC), tenia tecta (TT), middle medial nucleus (MM), and the granule cell layer (GC) and scattered Purkinje cells of the cerebellum. ML, molecular layer. Anti-neuritin antisera on parasaggital sections of adult rat brain labels cells in DG (E) and subiculum/CA1 (F) of the hippocampus and Purkinje cells (G) of the cerebellum. Neuritin expression is detected with polyclonal antisera generated against whole recombinant (E and F) or a peptide fragment of neuritin (G). Immunopositive cells have an orange-brown appearance. [Bars = 1 mm (A–C), 500 μm (D), 100 μm (E and F), and 50 μm (G).]
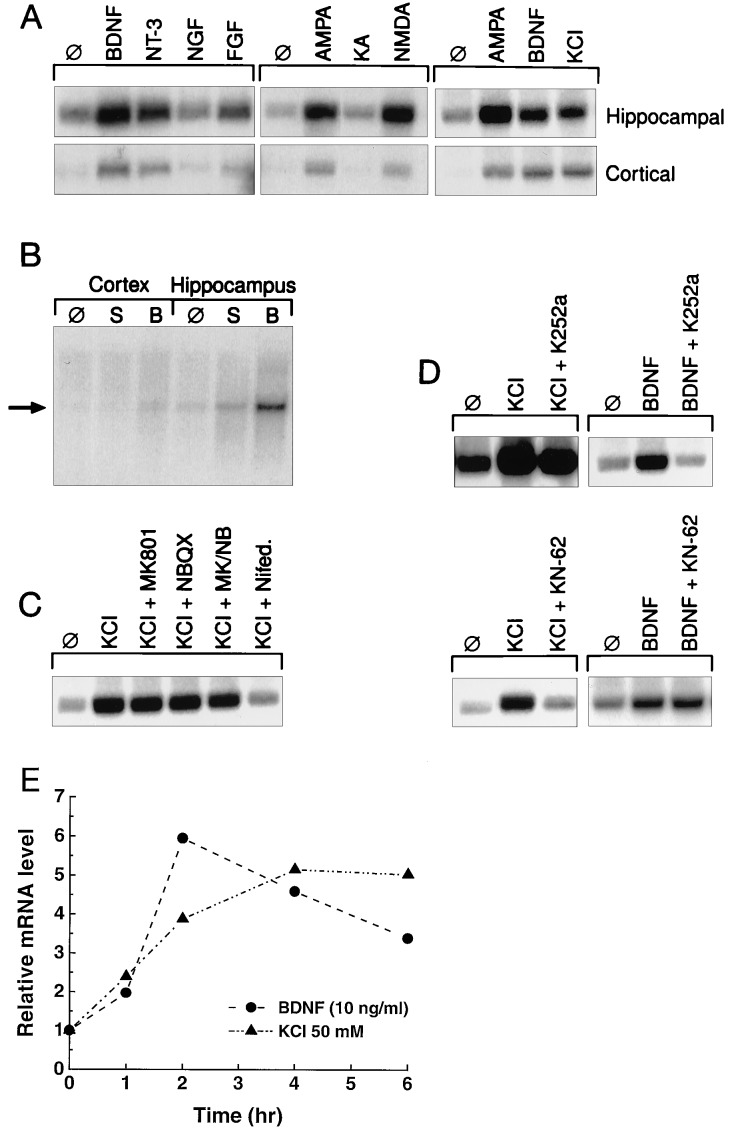
Figure 3.
Regulation of neuritin mRNA. (A) Neuritin gene expression is induced in primary hippocampal or cortical neurons by NTs, glutamate analogs, and depolarization by KCl (10 ng/ml NTs/growth factors, 30 μM glutamate analogs, and 50 mM KCl). Total RNA was harvested from primary embryonic rat neurons (E18, 7 days in vitro, treated for 6 hr) and used in Northern blot analysis (5 μg). (B) Intracranial injection of BDNF into postnatal day 4 rats induces neuritin mRNA in vivo. Total RNA from the indicated brain tissues was isolated after intraventricular injection. Ten micrograms of total RNA was analyzed by Northern blot analysis. ∅, No treatment; S, saline injection; B, BDNF. (C) Induction of neuritin mRNA by KCl occurs through activation of voltage-gated calcium channels in hippocampal neurons. (D and E) BDNF and KCl induce neuritin message in hippocampal neurons independently and with distinct kinetics. The inhibitors (NMDA, 10 μM MK-801; AMPA, 10 μM NBQX; voltage-gated calcium channels, 5 μM nifedipine; TrkB, 50 nM K252a; α-CAMKII, 10 μM KN-62) were added to cultures 30 min before induction.
Neuritin mRNA Is Expressed in Differentiating Fields of the Embryonic Nervous System and Enriched in Discrete Structures of the Adult Brain.
In situ hybridization analysis shows that neuritin mRNA is localized in the differentiating fields adjacent to the neuroepithelium of the developing neocortex, midbrain, and hindbrain in the E15 embryo (Fig. 2A; ref. 22). Neuritin is expressed in the developing trigeminal and facio-acoustic ganglia, the glossopharyngeal and vagal nerves (cranial nerves V, VII/VIII, IX, and X, respectively), and the dorsal root ganglia (23). As individual structures within the central nervous system begin to differentiate, neuritin expression becomes concentrated in the differentiating fields of the subventricular and intermediate zones of the neocortex, cingulate cortex, and hippocampus (Fig. 2C). Expression in the well defined olfactory bulb is restricted to the major output layer containing the mitral cells.
In the adult, neuritin mRNA is detected in regions well characterized with regard to their plasticity. The pyramidal neurons of the cornus ammons and the granule cells of the hippocampal DG (Fig. 2D) are highly enriched for neuritin mRNA. There is also notably strong expression in the distinct layers of the tenia tecta projecting to the olfactory bulb and in the major target of the retinal ganglion cells, the optic nerve layer of the superior colliculus (optic tectum). There is a general localization of neuritin message throughout the thalamic nuclei and the cerebral cortex; however, there does appear to be an enrichment of neuritin message within layer IV, the major afferent layer from the thalamus. An examination of neuritin in situ signals under higher magnification (Fig. 2E) reveals a specific signal associated with the cerebellar granule cells, but more striking is the heterogeneous detection of mRNA in the Purkinje cells. The punctate appearance is also observed in the hilar region of the hippocampal DG, in the middle-medial nuclei of the cerebellum, throughout layers II-VI of the cortex, and throughout the brain stem. The disjunct detection of neuritin message suggests a nonuniform regulation within individual neurons.
Neuritin Protein Is Localized to Somata and Neuritic Projections.
Immunohistochemical analysis of adult rat brain sections with neuritin-specific antisera shows that the pattern of immunostaining generally parallels that observed for neuritin mRNA. In addition to the immunoreactive dentate granular neurons, the hilar region of the DG contains scattered, strongly immunoreactive cells that correlate with the pattern observed by in situ hybridization of neuritin mRNA (Fig. 2 C and E). The irregular staining of Purkinje neurons also correlates with the punctate message localization (Fig. 2 D and G) as well as within the subicular complex of the hippocampus, where there are a high number of the intensely stained cells (Fig. 2F).
The subcellular localization of the neuritin protein shows that it is concentrated on neuronal cell bodies and unevenly dispersed along neuritic projections in the nonmyelinated regions of the brain. The granular appearance of the immunoreactive regions is particularly evident along the projections of immunoreactive neurons in the subicular complex of the hippocampus (Fig. 2F). The concentration of neuritin along neurites is further documented in the staining of the dendritic arbors of positive Purkinje cells (Fig. 2G).
Neuritin mRNA Is Induced by Neurotrophins and Neural Activity.
The KA-induction paradigm used to clone the neuritin cDNA together with its distinct expression profile indicates that neuritin expression is dynamically regulated by neuronal activity. To confirm the specificity of induction by neuronal activity, we treated hippocampal and cortical cultures with the glutamate analogs N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA); this treatment resulted in a 5-fold increase in neuritin mRNA (Fig. 3A). A similar magnitude of induction was observed with depolarizing concentrations of KCl (Fig. 3 A and E). The up-regulation of neuritin by KCl occurs by the activation of nifedipine-sensitive voltage-gated calcium channels and not by enhanced glutamate release and subsequent activation of glutamate receptor subtypes (Fig. 3C).
BDNF message also exhibits a sustained induction via activated voltage-gated calcium channels after depolarization with KCl (24). This prompted us to test the ability of BDNF and other NTs to induce neuritin mRNA. Recombinant human BDNF or NT-3 added to hippocampal or cortical cultures induced neuritin mRNA levels up to 5-fold after 2–4 hr (Fig. 3 A and E). Likewise, intraventricular injection of BDNF into neonatal rat pups induced neuritin message in vivo in the hippocampus and cortex (Fig. 3B). This direct induction of neuritin message by BDNF can be blocked by inhibiting TrkB with K252a (Fig. 3D). However, the induction of neuritin by KCl is not blocked by K252a. This result indicates that the initial induction of neuritin by KCl is not due to activity-dependent release of BDNF (4). Furthermore, the induction of neuritin by KCl, but not by BDNF, is blocked by the α-CAMKII inhibitor, KN-62 (Fig. 3D). These data suggest that neuritin mRNA is activated by at least two independent mechanisms. BDNF induces neuritin mRNA to maximal levels within 2–4 hr, at which time they begin to decline (Fig. 3E). Induction by KCl, however, shows maximal induction of neuritin mRNA by 6 hr (Fig. 3E) and is maintained for as long as 10 hr (data not shown).
Neuritin Is a GPI-Anchored Protein.
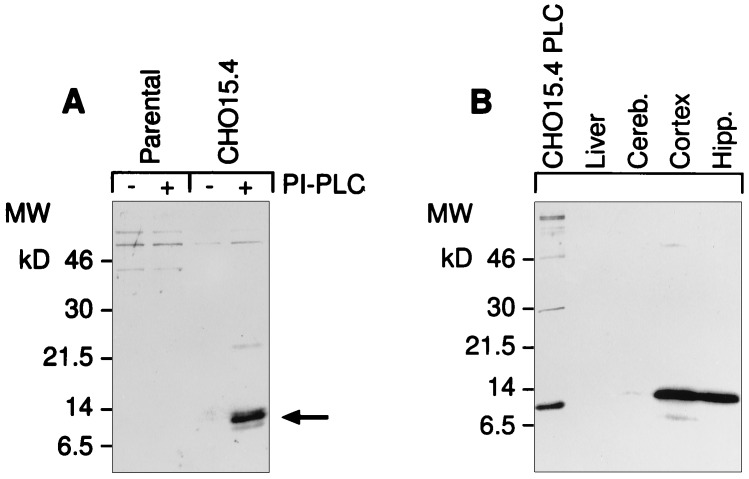
Biochemical analysis of mammalian recombinant neuritin demonstrates that the protein enters the secretory pathway and is attached to the cell surface by a GPI-lipid group. This was shown by treating stable cell lines expressing neuritin with PI-PLC and immunoblotting the cleaved products (Fig. 4A). Immunoblots of Triton X-114 extracts, in which hydrophobic membrane-associated proteins are selectively enriched (20), specifically detect neuritin in neuronal tissues at levels corresponding to their respective mRNA levels (Fig. 4B).
Figure 4.
Neuritin is a GPI-anchored protein that promotes neuritogenesis in primary hippocampal and cortical cells. (A) Recombinant human neuritin is sensitive to PI-PLC. Stably transfected CHO cells carrying the neuritin expression vector or the empty parental vector were treated with PI-PLC release buffer alone (−) or in the presence (+) of PI-PLC (0.4 unit/ml). Supernatants were analyzed by probing Western blots with affinity-purified antisera to a synthetic peptide derived from the C-terminal part of mature neuritin. (B) Endogenous neuritin is solubilized in Triton X-114. Tissues were harvested from control or KA-treated rats (i.p., 10 mg/kg, 8 hr) and proteins were extracted by phase separation with Triton X-114. Proteins were analyzed as in A. The first lane is PI-PLC extract from CHO cells expressing recombinant human neuritin (CHO15 cells). The difference in migration is due to the altered mobility of proteins containing an intact GPI anchor (Triton extracts). (C) Histidine-tagged neuritin produced in CHO cells and purified from conditioned media by nickel chromatography promotes neuritogenesis in embryonic hippocampal and cortical cells. Primary neurons from E18 rats were plated at 5 × 103 cells per square centimeter on poly-l-lysine-coated culture dishes in the presence of 150 ng/ml neuritin (+), or a corresponding control nickel-column fraction of parental vector CHO-conditioned media (−). The factor was added at the time of plating and neurite outgrowth was monitored daily. On day 4 of culture, cells were nonspecifically labeled with the lipophilic dye DiI (10 μM in dimethyl sulfoxide) and photographed using phase contrast (Top) or fluorescent (Middle and Bottom) microscopy.
Recombinant Neuritin Promotes Neuritogenesis.
To examine the function of neuritin, a histidine-tagged version of the molecule lacking the GPI signal peptide was produced in CHO fibroblasts and purified from conditioned media by nickel chromatography to >95% homogeneity. Hippocampal and cortical neurons from E18 rats were plated on poly-lysine-coated dishes at low density in the presence of 150 ng/ml recombinant neuritin. An equivalent nickel affinity fraction derived from conditioned media from CHO cells transfected with the expression vector without insert served as control. After 4 days in culture, the neurons plated in the presence of neuritin showed extensive neuritogenesis over the control cultures (Fig. 4C). General staining with the lipophilic fluorescent dye DiI revealed a striking difference in the organization of the soma and neurite lamellapodia. Untreated cells had flat cell bodies and broad, unfocused, lamellapodia along the length or toward the end of many neurites, whereas neuritin-treated cells had well differentiated cell bodies with thin, well defined extensions. Similar neuritogenic activity was observed with purified bacterial neuritin (unpublished results). These results demonstrate that application of exogenous neuritin to low density embryonic cultures exhibits a differentiation effect on neurite arborization that is characteristic of neurons grown at a high density.
DISCUSSION
Efforts to define the mechanisms of synaptic plasticity have focused on identifying components of the molecular processes induced by neuronal activity. The method by which neuritin was identified is indicative of a gene regulated by neuronal activity. Here we demonstrate that neuritin expression is regulated by glutamatergic neurotransmitter signaling and depolarization in vitro and is induced in vivo by KA. This modulation by neuronal stimulation is corroborated by a recent observation which showed that neuritin mRNA is up-regulated physiologically by light-induced neuronal activity in the visual cortex (ref. 25; neuritin = cpg15). We further demonstrate that neuritin expression is dynamically regulated in vivo and in vitro by administration of exogenous BDNF. Significantly, BDNF is up-regulated using a light-induction model (26) that is similar to the one used to induce neuritin. BDNF is also induced by KA as an immediate early gene in vitro and in vivo (12, 27). Collectively, the data show an induction of neuritin by exogenous BDNF and a similar regulation of BDNF and neuritin by light and KA, which places neuritin downstream of BDNF and suggests that it may mediate some of BDNF’s known effects.
Although neuritin can be directly induced by BDNF via TrkB, the induction following standard experimental glutamatergic stimulation or neuronal depolarization is not dependent on TrkB activation and apparently parallels or is similar to the pathway by which BDNF itself is induced. The significance of potentially distinct regulatory pathways is not clear; however, they may be important for molecules involved in establishing the differentiated state of a neuron in development and adjusting or maintaining that state in the adult.
Throughout development, neurons migrate from the neuroepithelium through the subventricular zone, where they differentiate to form individual structures. Neuritin mRNA is found in these differentiating layers of the developing cortex, hippocampus, and olfactory bulb. Cells in these fields characteristically begin to express phenotypic markers specific for neuronal and glial cell types as the number of nestin-tagged progenitors, along with the mitotic index, decreases (28). Therefore, the onset of neuritin expression may characterize the state of differentiation of cells progressing across the layers. As the embryo continues to develop and individual neuronal tissues differentiate, the expression of neuritin becomes more restricted within individual structures. For example, by E18, neuritin mRNA is highly restricted to the mitral cell layer of the developing olfactory bulb. This pattern of neuritin expression continues as development progresses into adulthood, during which neuritin mRNA is enriched in structures characterized for their activity-modulated plasticity (hippocampus, olfactory bulb, and Purkinje cells). The overall increase in the central nervous system expression of neuritin levels postnatally may be correlated with the enhanced activity-dependent morphological changes associated with the maturing central nervous system (29, 30) and with a similar increase in the postnatal expression of BDNF mRNA (31). In situ analysis reveals that throughout the adult brain, neuritin mRNA has a heterogeneous, punctate localization, particularly in the cells of the hilar region of the DG and the Purkinje cells of the cerebellum. Immunostaining of the neuritin protein shows a similar heterogeneous pattern of expression. This expression profile is similar to other genes characterized by their activity-dependent regulation (7, 9) and is interpreted to reflect the activity history of these neurons (7). For neuritin, the distinctive patterns of expression in the embryonic and adult rat may depict its dynamic regulation by both NTs and synaptic activity.
A morphological effect attributed to neuronal plasticity is the alteration in the architecture of neuritic connections. Neuritin is a novel GPI-anchored protein expressed on the surface of developing and mature neurons. In studies with primary hippocampal and cortical neurons, we demonstrate that soluble recombinant neuritin promotes neurite outgrowth and especially branching of neuritic processes. In correlation with these observations, previous studies demonstrate that glutamatergic stimulation, depolarization (both reviewed in ref. 32), and direct application of NTs (33, 34) induce neurite growth. The regulation of neuritin by BDNF and neuronal activity in vivo and in vitro suggests that neuritin may represent an effector of NT and neuronal activity function. Gene targeting and other in vivo approaches should help determine whether neuritin is indeed a downstream component of neurite outgrowth promoting stimuli in the integrated system.
Acknowledgments
We thank R. Rosenfeld for his help and advice with protein purification, J. Carnahan for the intraventricular injections of BDNF, D. Duryea for the paraffin sections, T. Meng for help with the bacterial expression, F. Manu for the human cortical cDNA, and R. Basu and L. Selander for sequencing. We also thank J. Corey for critical reading of the manuscript and helpful discussions.
ABBREVIATIONS
- NT
neurotrophin
- KA
kainic acid
- DG
dentate gyrus
- CHO
Chinese hamster ovary
- GPI
glycosylphoshatidylinositol
- PI-PLC
phosphatidylinositol-specific phospholipase C
- E
embryonic day
- NMDA
N-methyl-d-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionicacid
Footnotes
References
- 1.Bailey C H, Kandel E R. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 3.McNaughton B L. Annu Rev Physiol. 1993;55:375–396. doi: 10.1146/annurev.ph.55.030193.002111. [DOI] [PubMed] [Google Scholar]
- 4.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 5.Qian Z, Gilbert M E, Colicos M A, Kandel E R, Kuhl D. Nature (London) 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 6.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyford G L, Yamagata K, Kaufmann W E, Barnes C A, Sanders L K, Copeland N G, Gilbert D J, Jenkins N A, Lanaham A A, Worley P F. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 8.Tsui C C, Copeland N G, Gilbert D J, Jenkins N A, Barnes C A, Worley P F. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worley P F, Christy B A, Nakabeppu Y, Bhat R V, Cole A J, Baraban J M. Proc Natl Acad Sci USA. 1991;88:5106–5110. doi: 10.1073/pnas.88.12.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagata K, Sanders L K, Kaufmann W E, Yee W, Barnes C A, Nathans D, Worley P F. J Biol Chem. 1994;269:16333–16339. [PubMed] [Google Scholar]
- 11.Dugich-Djordjevic M M, Tocco G, Willoughby D A, Najm I, Pasinetti G, Thompson R F, Baudry M, Lapchek P A, Hefti F. Neuron. 1992;8:1127–1138. doi: 10.1016/0896-6273(92)90133-x. [DOI] [PubMed] [Google Scholar]
- 12.Isackson P J, Huntsman M M, Murray K D, Gall C M. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 13.Lu B, Yokoyama M, Dreyfus C F, Black I B. Proc Natl Acad Sci USA. 1991;88:6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 15.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Nature (London) 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 16.Nawa H, Pelleymounter M A, Carnahan J. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Simonet W S, Bucay N, Lauer S J, Wirak D O, Stevens M E, Weisgraber K H, Pitas R E, Taylor J M. J Biol Chem. 1990;265:10809–10812. [PubMed] [Google Scholar]
- 19.Kodukula K, Gerber L D, Amthauer R, Brink L, Udenfriend S. J Cell Biol. 1993;120:657–664. doi: 10.1083/jcb.120.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borchelt D R, Rogers M, Stahl N, Telling G, Prusiner S. Glycobiology. 1993;3:319–329. doi: 10.1093/glycob/3.4.319. [DOI] [PubMed] [Google Scholar]
- 21.Kozak M. Proc Natl Acad Sci USA. 1995;92:2662–2666. doi: 10.1073/pnas.92.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman J, Bayer S A. Atlas of Prenatal Rat Brain Development. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 23.Schambra U B, Lauder J M, Silver J. Atlas of the Prenatal Mouse Brain. San Diego: Academic; 1992. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 25.Nedivi E, Fieldust S, Theill L E, Hevroni D. Proc Natl Acad Sci USA. 1996;93:2048–2053. doi: 10.1073/pnas.93.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castren E, Zafra F, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gates M A, Thomas L B, Howard E M, Laywell E D, Sajin B, Faissner A, Gotz B, Silver J, Steindler D A. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer N C. J Neurol. 1991;22:659–673. doi: 10.1002/neu.480220702. [DOI] [PubMed] [Google Scholar]
- 30.Goodman C S, Shatz C J. Neuron. 1993;10:77–98. [Google Scholar]
- 31.Maisonpierre P C, Belluscio L, Friedman B, Alderson R F, Wiegand S J, Furth M E, Lindsay R M, Yancopoulos G D. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Ari Y, Represa A. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Cory S, Fraser S E. Nature (London) 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 34.Schnell L, Schneider R, Kolbeck R, Barde Y, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]