Introduction
The impact of antimicrobial agents on public health over the past 50 years is unmatched by any other therapeutic class. Precise data on current antibiotic use are difficult to obtain; in the UK, in the community alone, there are over 50 million prescriptions and over 250 million defined daily doses of antibiotics each year [1].
Much current activity is focused on issues surrounding antimicrobial resistance. It is now 4 years since the American Society for Microbiology published their concerns [2]. Reports and meetings involving the World Health Organization, colleagues from the European Union and, in the UK, the House of Lords and the Department of Health have followed [1, 3]. Although resistance is reported among antiviral, antifungal and antiparasitic agents and can have a major impact on the management of infected patients, it is the antibacterials, because of the far greater quantity of prescribing and burden of disease, that attract most attention.
The threat to public health is clear; solutions less so. Once it is accepted that use correlates with selective pressure to develop resistance, attempts to reduce consumption and improve the situation must tackle failings in current clinical practice and medical education, patient expectations, surveillance, infection control, and the balance between agricultural gains from antibiotic use and some current, highly questionable, practices.
The purpose of this review is to define and examine the mechanisms of resistance, identify specific current clinical problems, possible solutions and future developments. Antibiotics currently available in the UK are listed in Table 1 and a simplified bacterial taxonomy in Table 2.
Table 1.
Antibiotics currently available in the UK (December 1998).

Table 2.
Bacterial taxonomy.

Methodology and definitions
Resistance is by definition an in vitro phenomenon. Although there is currently no standardized methodology for antibiotic susceptibility testing, guidelines do exist both in this country (British Society for Antimicrobial Chemotherapy), and in the US (National Committee for Clinical Laboratory Standards) [4–6].
Disc diffusion testing remains the most commonly employed technique for routine susceptibility testing in the UK. The surface of an agar plate is evenly inoculated with the bacterial isolate under investigation and a filter paper disc impregnated with a defined quantity of antibiotic applied to the plate. After incubation, usually overnight, a circular zone of inhibition of growth appears around the disc as a result of diffusion of antibiotic into the agar and inhibition of growth of the isolate. The zone size is compared with that obtained with control isolates and a result recorded of sensitive, intermediate or resistant. Unfortunately there are many technical variables that can influence the zone size and comparability of results.
More technically demanding is the determination of an MIC (minimum inhibitory concentration). The MIC is the lowest concentration of an antibiotic that will inhibit visible growth of the isolate under investigation after an appropriate period of incubation. A defined inoculum of bacteria is used in a series of antibiotic dilutions in broth (macrodilution and microdilution methods) or less commonly in agar. In the agar dilution method spot inoculation onto antibiotic-containing agar enables many isolates to be assessed simultaneously. Automated technology is now available. All methods provide a precise measurement which can be compared with a known MIC for a similar (but control) isolate. The terms MIC50 and MIC90 are used when reporting MICs for a group of different isolates and are the concentrations that inhibited growth of 50% and 90% of the group.
A more simple, and widely used, method of MIC determination is by the commercially available E-test (AB Biodisk). This employs diffusion into agar from a thin paper strip impregnated with a varying concentration of an antibiotic along a gradient. After similar inoculation with the bacterial isolate, application of the strip, and overnight incubation, an ellipsoid zone of inhibition of growth appears on the agar plate, intersecting with the strip. The MIC is simply read from the strip, which is labelled with a specific range of antibiotic concentrations.
The breakpoint method is a similar, but abbreviated, version of the MIC test. A breakpoint concentration of antibiotic is calculated after consideration of both microbiological and pharmacological factors, and a simple all-or-non effect (i.e. growth or no growth following spot inoculation onto an agar plate containing this specific concentration of antibiotic) defines sensitive or resistant. Unlike the MIC it does not provide information on the degree of susceptibility, namely those isolates that are highly sensitive or highly resistant. An additional higher breakpoint may therefore be used to define intermediate sensitivity. The method has many attractions, not least in the ease of interpretation, but decisions on the precise breakpoint concentrations to employ are often difficult. Breakpoints for a single antibiotic are specific for groups of bacteria (e.g. the Enterobacteriaceae vs Gram positives). Site-specificity is limited to urinary and nonurinary infection, with BSAC guidelines suggesting alternative breakpoints for antibiotics which achieve high levels in urine and lower levels in tissue.
Extrapolation from any of these in vitro methods to the complex in vivo relationship of patient-infection-antibiotic must be careful. Resistance does not necessarily equate with treatment failure, clinical outcome data are often lacking, and susceptibility testing in clinical laboratories is only a best attempt to predict the in vivo response. The frustration of both those who prescribe, and those in the laboratory, with the simple question ‘will this antibiotic work or not?’, and the relevance of a result when a patient responds apparently inappropriately is all too familiar. In recent years a number of pharmacodynamic/pharmacokinetic properties have evolved in predicting bacteriological efficacy and clinical outcome. For agents such as β-lactams, which exhibit time-dependent killing, the time during a 24 h dosing period during which the serum antibiotic concentration exceeds the MIC (T > MIC) seems to be the important determinant of bacteriological efficacy. Extrapolation to clinical outcome has been confirmed in otitis media. The quinolones and aminoglycosides differ in that they exhibit concentration-dependent killing with an AUC (area under curve):MIC ratio as the important parameter [7, 8].
Mechanisms
Resistance may be either inherent or be acquired by the processes of genetic mutation or gene transfer.
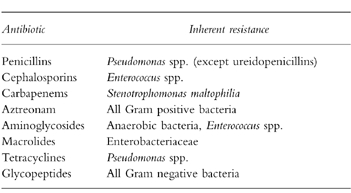
Genera or species of bacteria inherently resistant to specific antibiotics (or a class of antibiotic) are not uncommon, with examples listed in Table 3. This may occur at the level of permeability of bacterium to the particular antibiotic or at the target site. Not only is this information predictable and can therefore guide prescribing, it also explains the phenomenon of the ‘opportunistic’ bacteria where use of an antibiotic favours (‘selects’) overgrowth of inherently resistant bacteria and other microorganisms. For example, the penicillin binding protein targets in Enterococcus spp. have very low affinity for β-lactam antibiotics, particularly the cephalosporins, and their use will favour colonization and, on occasion, subsequent infection (‘super-infection’) with these bacteria. This also explains the development of oral or vaginal candidiasis (thrush) following antibiotics.
Table 3.
Examples of inherent resistance.
The mechanisms of acquired resistance fall into one of five categories, although bacteria may employ more than one mechanism:
enzymatic modification or destruction of the antibiotic
reduced antibiotic uptake into the bacterium
increased efflux of antibiotic from the bacterium
alteration or production of a new target site
over-expression of the drug target
The genetic information that encodes for these mechanisms may emerge by random spontaneous mutation or by gene transfer from other microorganisms. Mutations in particular genes that enable the bacterium to survive in the presence of antibiotic confer an enormous advantage. In the presence of certain antibiotics mutational resistance is more readily selected; examples are listed in Table 4. Use of these antibiotics should be managed carefully.
Table 4.
Antibiotic-bacteria combinations where mutational resistance is more readily selected [3].

Resistance genes may be present naturally, since many antibiotic classes are natural products and bacteria need to protect themselves, or alternatively have evolved from housekeeping genes. What is clear is that antibiotic resistant genes existed long before antibiotics were used, having been characterized in coliforms from glacial water and ice in the Arctic estimated at 2000 years old [9]. Regardless of their origin it is the processes of gene transfer coupled with rapid multiplication (as frequently as every 20 min) that disseminate this information; transformation involves transfer of naked DNA following bacterial death into a receptive bacterium; conjugation and transduction gene transfer by cell to cell contact or via bacteriophages (viruses that infect bacteria), respectively. Although in some bacteria, such as S. pneumoniae, gene exchange occurs largely by transformation, for most bacteria the most important vector of genetic exchange is the plasmid—a self-replicating circular piece of DNA, smaller and separate from the bacterial genome, which transfers into another bacterial strain or species. Smaller still is the transposon, a DNA section of the plasmid which transfers between plasmids and into the genome. Conjugative transposons and transfer by transposition to other plasmids is also common. Since the transmissibility of a particular plasmid is often restricted transposons (or ‘jumping genes’) enable resistance genes from a relatively nontransmissible plasmid to be transferred into a more transmissible plasmid. It appears that conserved DNA sequences called integrons, carried with the plasmid or transposon, may be involved in mobilization and site-specific insertion of gene sequences [10].
Enzymatic modification or destruction of the antibiotic
This is the most extensively characterized mechanism of resistance. Although the β-lactamase enzymes are most important, other examples include aminoglycoside-modifying enzymes, an acetyltransferase (chloramphenicol) and esterases (macrolides in Enterobacteriaceae).
With over 200 types of β-lactamase now described, although only a few are common, this field is complex. All cleave the four membered β-lactam ring common to penicillins, cephalosporins, carbapenems (imipenem and meropenem) and monobactams (aztreonam), although with varying success. Whereas the β-lactamases of Gram positive bacteria are excreted extracellularly, the site of action in Gram negatives is intracellularly within the periplasmic space. This difference in location has several consequences, and explains the key role of these enzymes in Gram negative bacteria; the enzyme is not lost by diffusion but remains within the cell, the overall degree of resistance may be enhanced by other local membrane phenomena (impermeability and efflux), and since it is the individual cell that is protected this cell will be selected, when appropriate, facilitating further evolution of β-lactamases. Target modification is more important than β-lactamase production in Gram positive bacteria.
These enzymes can be classified in a variety of ways such as by spectrum of activity, genomic (‘chromosomal’) or plasmid location, molecular weight, amino acid sequences or susceptibility to β-lactamase inhibitors. Although the original Richmond-Sykes classification in Gram negatives, now over 25-year-old, is still referred to, a more recent classification, proposed by Bush et al. is outlined in Table 5 [11].
Table 5.
β-lactamase classification [11].

Group I β-lactamases (molecular class C) such as AmpC are genomic enzymes and primarily cephalosporinases. Some bacteria produce tiny amounts of these enzymes which offer little protection, others exhibit inducible expression in the presence of antibiotic. Antibiotics in turn vary in their inducing power and lability, i.e. sensitivity to hydrolysis. Those antibiotics that are strong inducers but labile (ampicillin, first generation cephalosporins) will have very poor activity against bacteria with inducible β-lactamase. Strong inducers that are resistant to hydrolysis (carbapenems, cefoxitin) maintain their activity. Weak inducers that are labile (second and third generation cephalosporins, ureidopenicillins, monobactams) may seem preferable to those strong inducers that are labile; the drawback is the rare selection among the bacterial population of stably derepressed mutants that hyperproduce these β-lactamases. This process of selection, occurring only with antibiotics that are labile weak inducers, and at a frequency of approximately 1 in 106, is a clear advantage to the bacterium enabling overgrowth of a resistant population. Up to 40% of Enterobacter spp. isolates are derepressed for AmpC in certain European centres. More recently plasmidic AmpC β-lactamases have been reported in Klebsiella spp. and, to a lesser extent, in E. coli, most likely representing escape of AmpC genes to plasmids.
Among the plasmid-mediated enzymes TEM-1, TEM-2 and SHV-1 are the most common, with TEM-1 predominant and responsible for 90% of ampicillin resistance in E. coli. TEM-1 and TEM-2 differ by a single amino acid. SHV-1 is a genomic enzyme in the case of Klebsiella spp. but plasmid-mediated in other Enterobacteriaceae. These enzymes are produced by up to 80% of certain bacterial species among the Enterobacteriaceae, and also by H. influenzae and N. gonorrhoea. Ampicillin, first generation cephalosporins, carboxypenicillins (ticarcillin) and ureidopenicillins are all hydrolysed.
The extended spectrum β-lactamases (ESBLs) first appeared over 10 years ago, represent mutants of TEM-1/TEM-2 and SHV-1, and usually first appear in isolates of Klebsiella spp. [12]. Although their spectrum is extended to include second and third generation cephalosporins and monobactams, the carbapenems are not hydrolysed. Functionally many of these ESBLs exhibit much slower rates of hydrolysis compared with TEM-1 and ampicillin. However, there has been rapid spread in the hospital setting; ESBLs were present in 23% of Klebsiella spp. from a recent survey of European intensive care units [13]. They are often associated with cross-resistance to other antibiotics such as aminoglycosides and quinolones, and their appearance in organisms with permeability defects adds to the overall protection they impart [14].
The carbapenems, imipenem and meropenem, are considered the most potent of any antibiotic class, and are for use in serious infections or when resistance compromises all other agents. These antibiotics are stable to ESBLs and usually remain active against stably derepressed Gram negative mutants that hyperproduce genomic β-lactamases [15, 16]. The activity of these latter enzymes may however, become more significant in the presence of permeability defects (e.g. in occasional isolates of P. aeruginosa and E.cloacae). Certain bacteria, and in particular S.maltophilia, produce a genomic enzyme that hydrolyses carbapenems. More rarely this is observed in Bacteroides spp., Aeromonas spp. and certain Enterobacteriaceae. Fortunately the plasmid-mediated carbapenemase, IMP-1, is rarer still, at present confined to Japan, and reported in isolates of P.aeruginosa and Enterobacteriaceae. A new carbapenemase in a UK isolate of P.aeruginosa has recently been reported [17]. Interestingly, many of these carbapenemases are zinc-dependent enzymes (metallo–lactamases); cephalosporinase activity varies and most cannot hydrolyse the monobactam aztreonam.
Use of the specific β-lactamase inhibitors in combination with a β-lactam molecule is a logical attempt to overcome enzyme-mediated resistance. Amoxycillin-clavulanate (coamoxiclav), ticarcillin-clavulanate, piperacillin-tazobactam and ampicillin-sulbactam (not available in the UK) are such combinations. These clavulanate and penicillanic acid sulphone molecules possess the β-lactam ring, bind the β-lactamase in a competitive fashion, and acylate the enzyme which is then transiently inhibited. Alone these inhibitors have moderate to very low level, or species-specific, antibacterial activity, and differ in the range of β-lactamases they inhibit. The genomic induced or derepressed Group 1 enzymes are, in general, not inhibited by the action of clavulanate, although sulbactam and particularly tazobactam have better activity. Activity against ESBLs is more uniform [12]. In certain countries sulbactam is available as a single agent for coadministration with antibiotics as the prescriber sees fit. Being β-lactam molecules these inhibitors can themselves also induce β-lactamases. Since tazobactam is the weakest inducer, and piperacillin has a lower affinity for these enzymes, the piperacillin-tazobactam has advantages over other combinations.
Given the ability of bacteria to adapt to new antibiotic pressures the characterization of TEM-like β-lactamases that are inhibitor-resistant (IRTs) is perhaps not surprising. First described over 5 years ago in isolates ofE. coli, over 10 enzymes have now been reported [18]. Derived by amino acid substitutions most are TEM-1 mutants and produced by E. coli, although a TEM-2 mutant from P.mirabilis has recently been characterized, as have occasional TEM-1 mutants in Klebsiella spp. No IRTs derived from SHV-1 have yet been described. The effect of IRT-producingE. coli strains on β-lactam-inhibitor activity varies; most strains remain susceptible to piperacillin-tazobactam, reflecting the greater inherent activity of piperacillin, whereas other combinations are rendered inactive. It is noteworthy that unlike the ESBLs, which are largely confined to the hospital setting, IRT-producing isolates are not uncommon in community isolates of Enterobacteriaceae, although not found in H. influenzae despite widespread coamoxiclav use in respiratory infections. Finally, occasional isolates of P. aeruginosa producing inhibitor-resistant mutants of the OXA-enzymes (rather than TEM-1 or -2) have been reported.
Of the other antibiotic classes enzyme-mediated resistance is most significant with the aminoglycosides. At least 20 enzymes have been characterized from Gram negative and Gram positive bacteria that modify these compounds by acetylation, adenylylation or phosphorylation [19]. Genes encoding for this pattern of resistance can be located on the genome or more commonly, on a plasmid or transposon, and in association with other antibiotic resistances. The Gram negative bacteria produce the far greatest number of these enzymes, and whereas gentamicin is susceptible to modification by a large number of enzymes amikacin is affected by far fewer—hence cross-resistance among aminoglycosides varies. Resistance is still an unusual phenomenon in Gram negatives, but Streptococcus spp. have only a low level of sensitivity, and Enterococcus spp. are inherently resistant, to aminoglycosides. However, in combination with a penicillin the aminoglycosides act synergistically against these Gram positive bacteria and can exhibit bactericidal activity. Synergy in Enterococcus spp. is abolished in the presence of high-level gentamicin resistance mediated by a plasmid-encoded bifunctional acetylation and phosphorylation enzyme, although a streptomycin combination may remain synergistic in some isolates [20].
Reduced antibiotic uptake into the bacterium
Gram positive and Gram negative bacteria differ in the structure of the cell wall that extends between the outer polysaccharide capsule (if present) and inner cytoplasmic membrane (Table 6).
Table 6.
A comparison of cell wall structure.

Peptidoglycan is the major component in Gram positives—a sugar (glycan) polymer of N-acetyl-muramic acid and N-acetyl-glucosamine with associated stem and cross-linking peptides producing a thick three dimensional structure. External to a much smaller peptidoglycan component in Gram negatives is a membrane structure consisting of lipopolysaccharides and lipoproteins anchored to peptidoglycan, and large outer membrane proteins (Omp) called porins. The porins function as aqueous channels, providing a hydrophilic route through the membrane structure into the periplasmic space, and are present in variable numbers and sizes, e.g. P. aeruginosa has much larger, but far fewer, porins than E. coli.
Whereas the intrinsic resistance of bacteria such as P. aeruginosa to many antibiotics is related to the low number of porin molecules, genetic mutations that result in alteration of either the shape or number of existing porins will further influence permeability to antibiotics. Such phenomena are well described among Gram negative bacteria, and may result in resistance across different antibiotic classes. Those particularly affected are the β-lactams (e.g. D2 porin-deficient P. aeruginosa and imipenem-resistance), quinolones (e.g. E. coli and OmpF mutants), chloramphenicol (H. influenzae, Salmonella spp.) and trimethoprim [21, 22].
Increased efflux of antibiotic from the bacterium
Reduced drug accumulation causing increased MICs of antibiotics is best described with macrolides, tetracyclines and quinolones. Although target site modification is the more prevalent macrolide resistance mechanism, plasmid-encoded MSB resistance entails active efflux of the antibiotic via production of an ATP-binding protein and produces resistance to macrolides and type B streptogramin molecules.
Efflux may account for more macrolide resistance in S. pneumoniae than MLSB target site modification (see below). An analogous energy-dependent efflux mechanism occurs with tetracyclines mediated via membrane-located efflux proteins.
Efflux of quinolones from Gram negative bacteria is by diffusion through the inner membrane and by a saturable energy-requiring process. Radiolabelled studies have shown reduced uptake in cfxB and nfxB mutants of E. coli which recover wild type uptake if this efflux system is blocked [22]. In fact it appears that genomically mediated multidrug resistance (MDR) efflux systems, capable of extruding quinolones and other structurally unrelated antibiotics are probably distributed in all bacterial species [23]. Some seem only to produce low-level resistance and may need to function in combination with other mutations to exert a clinical effect. The best studied systems are in P. aeruginosa [24]. The relevance of efflux pump systems as opposed to genomic mutations that alter the enzyme targets of quinolones may have been underestimated [25].
Alteration or production of a new target site
Many classes of antibiotic including β-lactams, glycopeptides and quinolones can be compromised by this mechanism. The effects of β-lactams are mediated through covalent binding at the cytoplasmic membrane to several penicillin binding proteins (PBPs). This effectively blocks the transpeptidase and carboxypeptidase function of PBPs in the final stages of cell wall synthesis. Endogenous autolysins are then triggered resulting in cell lysis and death. Alterations in PBPs are known to be responsible for specific resistances, namely, methicillin-resistant S. aureus (MRSA) and penicillin-resistant S. pneumoniae(PRP). In other cases it represents a secondary mechanism of resistance, e.g. penicillin-resistant N. gonorrhoea and ampicillin-resistant H. influenzae. Interestingly, in PRP and those resistant isolates of Neisseria spp. mediated via PBP changes, examination of the PBP-2 gene sequence reveals a partly mosaic structure, probably as a result of interspecies recombination events from oral streptococci and commensal Neisseria spp. in the nose and oropharynx [26]. The consequence of these genetic events is to reduce the affinity of the specific PBP for the antibiotic. In PRP a series of changes in PBP-1 and PBP-2 have been described which are acquired in a step-wise fashion, although variable order, to produce high-level resistance. The situation with MRSA is different; an extra mecA gene rather than a mosaic gene encodes production of PBP-2a, a molecule with much reduced affinity for β-lactams [27]. Both homogenous and heterogeneous MRSA strains exist; in the latter only one bacterium in approximately 106 (all producing PBP2a) express high-level resistance. Regulatory systems of the mecA gene and a new family of fem genes have been identified. Expression of the mecA gene, essential for high-level resistance, seems to be enhanced in vitro by prior selection in β-lactams, high salt media and 30° C. At least three other mechanisms explain low-level resistance (MIC 4–8 mg l−1) occurring in the absence of mecA: overproduction of PBP-4 with lower affinity for β-lactams than PBP-1, -2, and -3, modification of PBP-2 to a lower affinity molecule, and overexpression of β-lactamase.
It is now over 10 years since glycopeptide (vancomycin and teicoplanin)-resistance was first described in clinical isolates of the normally susceptible Enterococcus spp. [28]. These antibiotics normally inhibit cell wall synthesis in Gram positive bacteria by their action on the pentapeptide of the peptidoglycan precursors. Binding of glycopeptide to the terminal d-alanyl-d-alanine residues (linked by the activity of a d-ala-d-ala ligase) prevents the transglycosylation and transpeptidation reactions required for peptidoglycan polymerization. Four resistance phenotypes have been described; VanA, VanB, VanC and VanD [29–32]. The VanA strains are reported most frequently and exhibit inducible, transferable resistance to vancomycin (MIC 64 − >1000 mg l−1) and teicoplanin (MIC 16–512 mg l−1) associated with a novel 39 kDa cytoplasmic membrane protein. The vanA gene cluster encodes seven polypeptides: VanA, VanH, VanX, VanY, VanZ, VanR and VanS, with the first three essential for resistance. VanH and VanA act in sequence to convert pyruvate to lactate, and as d-ala-d-ala ligase analogue, respectively, to form d-ala-d-lac; VanX destroys all normal d-ala-d-ala, ensuring that all peptidoglycan precursors use the modified structure. Vancomycin binds 1000 fold less tightly to this structure than to d-ala-d-ala, enabling bacteria to survive in far higher concentration of antibiotic. Although most frequent in isolates of E. faecium, this resistance pattern is also encountered in E. faecalis and other Enterococcus spp. Mutants with genomic vanA genes that exhibit constitutive expression have also been described.
In the VanB phenotype the vancomycin MICs are lower (4–1024 mg l−1), and isolates usually remain susceptible to teicoplanin (MICs 0.5–1 mg l−1). In vitro detection of this lower level vancomycin resistance may be overlooked if, for example, discs with only the standard 30 μg vancomycin content are employed. The VanC phenotype differs to other phenotypes in that it represents an intrinsic low level resistance to vancomycin with teicoplanin susceptibility, and is inducibly or constitutively expressed in most isolates of E. gallinarum, E. casseliflavus andE. flavescens.
If Enterococcus spp. had not evolved enough strategies to protect them from the action of glycopeptides the emergence of vancomycin-dependence provides a further option [33]. Such bacteria will not be isolated in the laboratory on normal media and their presence overlooked. Originally reported from the faeces of patients with renal disease, these isolates will grow on an agar plate around a vancomycin disc, and may well be derived from vancomycin-resistant strains although the mechanism is unknown.
The two target enzymes of the fluoroquinolones are DNA gyrase (topoisomerase II) and DNA topoisomerase IV, each composed of four subunits (two A and two B) encoded by gyrA and gyrB, and parC and parE, respectively. The primary target is the gyrase in Gram negative, but topoisomerase in Gram positive bacteria, with genomic mutations in the gyrA or parC responsible for most resistant isolates. These mutations may occur in conjunction with, or following, efflux changes further increasing the MICs to the fluoroquinolone class of antibiotics [34]. With the dominance of most wild type alleles over mutant gyrA genes when a cell expresses both, and the fact that these agents can both inhibit plasmid conjugation and eliminate plasmids, the lack of plasmid-mediated resistance has, until recently, been reassuring. Quinolone resistance from a transferable plasmid obtained from a clinical isolate of Klebsiella pneumoniae by an, as yet, unknown mechanism, is worrying, as are natural plasmids encoding the expression of DNA topoisomerases [35, 36].
In MLSB resistance susceptibility to three classes of antibiotic are affected—the macrolides, lincosamides (clindamycin and lincomycin) and the type B streptogramins (quinupristin)—all of which share a common or overlapping 50S ribosomal RNA binding site. The constitutive or inducible expression of this resistance, which involves dimethylation of adenine residues in 23rRNA, determines the precise spectrum of resistance, and over 30 erm (erythromycin ribosome methylation) genes have been isolated and characterized.
Target site modification is also described with the aminoglycosides, tetracyclines, rifampicin and the folate antagonists. Gram negative bacteria readily develop resistance to streptomycin by alteration of the 30S bacterial ribosome subunit. As other aminoglycosides bind to multiple rather than single ribosomal sites this mechanism of resistance by a single mutational step is not encountered. Protection of the ribosomal binding site that enables tetracyclines to prevent attachment of aminoacyl-t-RNA molecules is one of the two principle resistance mechanisms employed against these antibiotics; the tetM gene being the most widely distributed resistance determinant [37]. Similarly rifampicin-resistant isolates may possess alterations in the DNA-dependent RNA polymerase binding site; sulphonamide and trimethoprim-resistant isolates an altered dihydropteroate synthase and dihydrofolate reductase, respectively [38].
Over-expression of the drug target
This mechanism of resistance has received little attention over the years, although has been the subject of a recent review [39]. Only described in clinical isolates of Mycobacterium spp. gene duplication or promoter mutations are likely to be responsible. Hyperproduction of TEM β-lactamases leading to clavulanate resistance has been reported, and could be considered over-expresssion of drug target [40].
Current therapeutic challenges
If a single antibiotic is considered, the impact of in vitro resistance to either the individual, or in clinical practice, is very variable. This will depend on the infective indications of the antibiotic (spectrum and severity of disease, e.g. life-threatening or of major public health concern), the specific bacteria involved in these infections, and the number and suitability of alternative antibiotics (oral or intravenous, safety profiles). The transmissibility of the bacterium concerned and, particularly in hospitals, infection control practices are also critical. Transmission will lead to additional antibiotic prescribing and further aggravate the situation.
Although long considered the domain of Gram negative bacteria and particularly in the hospital setting, antibiotic resistance is an increasing problem in Gram positive isolates associated with both community-and hospital-acquired infections. Discussed below are some of the bacteria and resistances most relevant to prescribing and public health.
Staphylococcus aureus
This pus-producing bacterium causes superficial and deep-seated skin, wound, and tissue infections; and is second only to E. coli as a cause of bacteraemia. Carried on healthy skin by around one third of the population, it is readily transmissible.
Initially susceptible to penicillin (1944) and the isoxazolyl penicillins such as methicillin and flucloxacillin (1960s), very few isolates are now susceptible to penicillin, and MRSA has for many years been the focus of concern. Of those blood and cerebrospinal fluid isolates of S.aureus reported from laboratories in England and Wales to the Public Health Laboratory Service Communicable Disease Surveillance Centre (PHLS CDSC) the proportion of MRSA has risen exponentially from 1.5% in 1989–91 to 21.1% in 1996; figures for 1998 are expected to be around 25% [41, 42]. Outside the UK figures vary from <1% in Scandinavia and Netherlands to over 70% in Japan and Korea. Simultaneous increases in resistance to other classes of antibiotics such as macrolides, aminoglycosides and quinolones have occurred.
The resistance of MRSA is heterogeneous and encompasses many strains with differing antibiograms; in the UK the epidemic strains EMRSA-15 and EMRSA-16 currently predominate. Resistant to all β-lactams, only susceptibility to the glycopeptides teicoplanin and vancomycin can be guaranteed, and in the clinical setting these have been adopted as the agents of choice for treating MRSA infection. Numerous other antibiotics such as fusidic acid, rifampicin, ciprofloxacin, trimethoprim, gentamicin, chloramphenicol, the tetracyclines, clindamycin, nitrofurantoin and fosfomycin may be susceptible in vitro, although published clinical efficacy data are either anecdotal case reports, small series or noncomparative [27, 43]. Combination therapy with a glycopeptide for deep-seated infections, such as endocarditis and bone infection, is not uncommon in clinical practice, although no advantage over glycopeptide monotherapy has been demonstrated in randomised controlled trials. The combination of specific β-lactamase inhibitors with a β-lactam molecule, e.g. amoxycillin-clavulanate and ampicillin-sulbactam, has shown some promise in MRSA infection (at least in animal studies) probably as a result of a much greater affinity of ampicillin than methicillin for PBP-2a [44].
In 1997 the first clinical isolates of S. aureus with reduced vancomycin susceptibility appeared, first in Japan shortly followed by the USA and France [45–47]. Although a problem with Enterococcus spp. for over a decade, and not unexpected given documented clinical isolates of S. aureus with intermediate resistance to teicoplanin, this event has raised yet further concerns. At the moment resistance is only low-level (vancomycin MIC 8 mg l−1), and the term vancomycin-intermediate S.aureus (VISA) has been adopted. Patients have responded to alternative antibiotics such as ampicillin-sulbactam plus the aminoglycoside arbekacin, and the investigational agent quinupristin-dalfopristin. The exact mechanism in VISA is unclear; when compared with vancomycin-susceptible MRSA strains the Japanese isolate produced 3–5 times more PBP-2 and PBP-2a, was negative for VanA, VanB and VanC, but had a cell wall twice as thick as control strains suggesting cell wall synthesis rather than composition may be responsible. Across Japan a heterogeneous form of vancomycin resistance has been identified in S.aureus; strains with vancomycin MICs of 2–4 mg l−1 containing subpopulations with MICs of 5–9 mg l−1, occurring at a frequency of 1 in 106, selected in vitro by increasing concentrations of vancomycin [48]. The relatively low concentrations achievable in deep tissues or abscesses may serve to select out these populations in vivo.
Streptococcus pneumoniae
This bacterium is particularly associated with three infections; community-acquired pneumonia (the most common cause), meningitis and otitis media. It is the third most commonly reported organism in cases of bacteraemia. Penicillin has, for over 50 years, been the drug of choice with MICs consistently <0.1 mg l−1. In those allergic to penicillin, macrolides are most often substituted. Low level penicillin resistance in Streptococcus pneumoniae (PRP), defined as MIC 0.1–1 mg l−1, first appeared in Australia in 1967, with high level resistance (MIC > 1 mg l−1) some 10 years later in South Africa [49, 50].
In the UK the proportion of strains resistant to penicillin and erythromycin rose between 1989 and 1995 from 0.3% to 2.9% and 3.3% to 10.9%, respectively [51]. Corresponding figures in 1997 were 7.5% and 11.8%. Elsewhere in Europe penicillin-resistance varies from < 1% to over 50% in Spain and Hungary. Cross resistance to more traditional agents such as chloramphenicol and tetracyclines is not uncommon, and to alternatives such as rifampicin, trimethoprim and clindamycin.
Although a disturbing trend this in vitro phenomenon often has no impact on clinical outcome since antibiotic levels at the infected site (most commonly respiratory tract) well exceed these MICs. High dose oral amoxycillin is often used to treat respiratory infections caused by S.pneumoniae isolates with high level resistance, although in meningitis, where clinical failure is much more likely, alternative agents are required. Empirical treatment of meningitis in many centres is now cefotaxime and ceftriaxone, but as these agents also bind to the PBPs that mediate this resistance MICs are also often higher, and cefotaxime resistant strains have now been reported. Vancomycin remains effective but penetration is poor at this site.
Enterococcus spp
Enterococcus spp. often form part of the normal gut flora. Previously dismissed as harmless commensals these bacteria can cause community-acquired infections such as endocarditis and urinary tract infection, and over the past two decades have become increasingly important in hospital-acquired infections in both susceptible immunocompromised (e.g. transplant, renal dialysis, intensive care), and immunocompetent, patient populations. These range from superficial wound and parenteral line infection to deep intra-abdominal infection, bacteraemia and endocarditis.
The relative resistance of these bacteria to many antibiotics is characteristic. The agents of choice are either ampicillin, a ureidopenicillin (e.g. piperacillin), or glycopeptide. Alone these β-lactams are usually bacteriostatic, and for serious infections are used in a synergistic combination with an aminoglycoside to achieve bactericidal activity. Cephalosporins have no activity and relatively few isolates in the UK are now sensitive to macrolides, tetracyclines, trimethoprim and chloramphenicol.
The appearance in the 1980s of high-level aminoglycoside resistance, which abolishes the β-lactam bactericidal synergy, and then glycopeptide resistance (GRE) has presented a major therapeutic problem. Fortunately, compared with S. aureus and S. pneumoniae, these bacteria are less pathogenic; many patients are colonized and do not require therapy. When antibiotics are considered necessary bacteriostatic agents such as ampicillin, novobiocin, tetracycline and quinolone (alone or in combination) may suffice for uncomplicated urinary tract infections. For more serious infections where bactericidal activity is desirable, such as in bacteraemia and endocarditis, the options are more limited. Various combinations have been employed with varying success, including the use of a glycopeptide despite in vitro resistance [18, 26]. The lack of correlation between in vitro susceptibility pattern and clinical outcome is often apparent.
Originally a phenomenon of the tertiary centres in the UK, the range of hospitals reporting multidrug resistant Enterococcus spp. had expanded to 71 hospitals by the end of 1995 [52]. Community-acquired isolates, environmental spread and outbreaks have all been reported. More precise surveillance data are not available. Outside the UK many countries are experiencing similar problems; in the USA the percentage of isolates causing hospital-acquired infection that were resistant to vancomycin increased from 0.4% to over 10% from 1989 to 1995 [53].
Gram negative bacilli and the enteric pathogens
Of the Enterobacteriaceae and nonfermenters (Table 2) E. coli is the most common cause of urinary tract infection in the community. However, it is in the hospital setting, and particularly in immunocompromised patients, where this group of bacteria have most impact as causes of, for example, bacteraemia, pneumonia, skin and soft tissue infection, and catheter-related sepsis.
Resistance rates vary by species; Klebsiella spp., Enterobacter spp., and Pseudomonas spp. are invariably more resistant than E. coli and Proteus spp. Isolates of Stenotrophomonas maltophilia and Acinetobacter spp. tend to be the most resistant. As with other hospital-acquired infections the resistance data will vary between centre and unit; with patient population and prescribing practices both very relevant. However, in general the UK has far less of a problem than many of the countries in S.Europe, the Americas and Asia, but fares less well than Scandinavia and the Netherlands.
Although there are no robust national antimicrobial susceptibility data, certain trends are apparent among those blood and cerebrospinal fluid isolates reported from England and Wales to PHLS CDSC between 1989 and 1997. There are significant increases in resistance, across all species, to ciprofloxacin, cephalosporins and trimethoprim; gentamicin resistance remains more constant (Table 7).
Table 7.
Trends in antimicrobial susceptibility data 1989–1997 (Gram negative blood and cerebrospinal fluid isolates) [3].

Most enteric pathogens of bacterial origin acquired in this country are either Salmonella spp. or Campylobacter spp. The vast majority of cases are associated with consumption of contaminated food products. Although usually self-limiting antibiotic treatment is occasionally indicated in severe cases, at either age extreme, in the immunocompromised and for infected food handlers. The agent most widely used is ciprofloxacin or, for Campylobacter spp., a macrolide.
There are currently four major serotypes of Salmonella spp. causing human disease in the UK. Ciprofloxacin resistance remains rare (<1%) in the most common Salmonella enteritidis, but exceeds 10% in S. typhimurium and S. virchow, and 60% in S. hadar. Multi-resistance to ampicillin, chloramphenicol, sulphonamides, trimethoprim and tetracyclines is a particular problem in S.typhimurium. It seems very likely that these resistance patterns are influenced by past and present veterinarian practice.
Campylobacter jejuni is usually acquired from infected poultry. Erythromycin resistance remains rare (<1%), but ciprofloxacin resistance has risen to around 10%. In the Netherlands, between 1982 and 1989, parallel increases in ciprofloxacin resistance Campylobacter spp. occurred in both man and chickens from 0% to 11% and 0% to 14%, respectively, and followed extensive use of quinolones by the poultry industry [54]. This chain of events most probably explains the UK data.
Mycobacterium tuberculosis
The greatest impact of tuberculosis (TB) is in the developing world. With 8 million new cases and 3 million deaths each year it remains the commonest infectious cause of death worldwide [55]. In the UK annual notifications ceased to decline in the late 1980s, and in 1997 reached a figure of approximately 6000 [56]. Many factors could be involved in this increase, including coinfection with the human immunodeficiency virus (HIV), other immunocompromised states, increasing poverty, homelessness and immigration.
With frequent clinical rather than microbiological diagnosis, and a period of weeks to months before the in vitro susceptibility pattern is available, treatment is often empirical. Standard therapy is currently for 6 months with isoniazid, rifampicin, and for the initial 2 months pyrazinamide and sometimes ethambutol.
In the UK resistance to single drugs has occurred for many years, first appearing soon after introduction of antituberculosis therapy. Of those isolates examined in regional or national centres the most common resistances are to streptomycin or isoniazid (approximately 5% in each case), with resistance to the other three first-line agents much less frequent (<1%) [57]. In recent years multidrug resistant isolates (MDR-TB), defined as resistance to isoniazid and rifampicin with or without resistance to other drugs, have emerged. Although MDR-TB is rare, the likelihood of treatment failure or relapse is increased, with mortality approaching 100% in early reports from HIV-infected patients. Rapid detection, a high degree of suspicion, in vitro susceptibility to at least two of the second line agents, and directly observed therapy improves outcome considerably. Outbreaks within healthcare settings have been associated with much adverse publicity, mortality and serious deficiencies in infection control practices.
Genetic mutations associated with rifampicin, isoniazid, streptomycin and ciprofloxacin resistance have been identified in some strains, with a sequential acquisition of individual mutations in different genes, each conferring resistance to a single drug, most probably responsible. Whereas rifampicin resistance involves a single mutation in the gene encoding RNA polymerase which enables rapid molecular diagnosis, isoniazid resistance involves multiple genes.
Current strategies in MDR-TB use combinations of traditional antimycobacterial drugs (such as aminosalicylic acid, cycloserine, ethionamide, clofazimine) and four other groups of antimicrobial agents: aminoglycosides (other than streptomycin), macrolides, quinolones and rifamycins. Amoxycillin-clavulanate may also be used. Treatment is guided by the in vitro susceptibility pattern.
Solutions and future developments
There are three approaches to the problem of bacterial resistance: reduce antibiotic consumption and preserve existing agents, develop new antibiotics, or develop therapeutic strategies for infection that do not involve antibiotics. Where bacterial transmissibility is high the importance of simple infection control measures (e.g. adequate handwashing), rather than antibiotic control, cannot be over-emphasized, although clearly more appropriate to the hospital than community setting.
Reducing antibiotic consumption requires first education, then co-operation, of both health care professionals and the public. The logistics are complex; the enormity of this task can not be over-emphasized and is beyond the scope of this review. Although it would seem highly implausible that such an approach could be detrimental to the overall picture of resistance, there are little data on the impact following tightening of prescribing policies. In the two most cited studies in the community, of macrolide resistance in S. pyogenes in Finland, and penicillin-resistance in S. pneumoniae in Iceland, the original problem may have been complicated by clonal spread of a single strain rather than dissemination of resistance within the species [58, 59]. In the hospital setting interpretation is further complicated by the impact of infection control measures. It must be appreciated that if prescribing policies are amended to restrict one agent, but substitute another, there may be alarming side-effects. For example, reduction of cephalosporin use in one hospital in 1996 by 80% resulted in an 87% drop in ceftazidime-resistant Klebsiella spp. in the intensive care unit, and a 44% drop throughout the hospital. However by using imipenem as first-line agent resistance of P. aeruginosa to imipenem increased from 8.9% to 16.7% during this period [60].
The development of a new antibiotic may take 10 years, will cost several hundred million pounds, and once marketed it's commercial success is inevitably compromised with time as target bacteria develop resistance. There are several new agents under development of which two are completely new classes of antibiotic: the oxazolidonones and everninomycins. Others include quinupristin-dalfopristin, glycylcyclines, and new agents of the quinolone, glyocopeptide and carbapenem classes. Most have predominantly Gram positive activity and including multiresistant isolates.
Oxazolidonones are synthetic compounds that have a unique mechanism of action in inhibiting initiation of bacterial protein synthesis, are not cross-resistant with existing antibiotics, and possess exceptional oral bioavailability. Linezolid (U-100766), a 4-morpholinyl-substituted compound, and eperezolid are already in phase III human clinical trials. Linezolid is available in both an oral and intravenous formulation and has demonstrable in vitro activity against Staphylococcus spp. (including MRSA), Streptococcus spp. (including PRP), Enterococcus spp. (including GRE), Corynebacterium spp. and a variety of anaerobic bacteria. Enterobacteriaceae and other Gram negative bacilli are not susceptible [61]. All oxazolidonones exhibit bacteriostatic rather than bactericidal activity which may affect clinical usefulness. The other novel class of antibiotic, the everninomycins, first discovered in the 1980s are now being developed as alternatives to the glycopeptides with potential activity against MRSA, PRP and GRE.
Of the other agents there has been considerable interest and optimism in recent few years in quinupristin-dalfopristin. This is an injectable agent comprising a 30:70 mixture of the structurally unrelated molecules quinupristin (RP57669, a streptogramin B) and dalfopristin (RP54476, a streptogramin A) which are both derivatives of the naturally occurring agent pristinamycin (from Streptomyces pristinaespiralis). Individually both agents are bacteriostatic, but together exert a synergistic bactericidal effect in inhibiting protein synthesis [62]. In vitro activity has been demonstrated against a number of Gram positive bacteria including MRSA, PRP and GRE (E. faecium not E. faecalis) and some anaerobes and intracellular pathogens (Mycoplasma spp., and Legionella spp.). Gram negative activity is more variable and restricted to the respiratory pathogens (H.influenzae, M.catarrhalis) [63]. Although cross resistance with other major antibiotic classes does not occur, MLSB resistance, which may be inducible or constitutive, includes resistance to streptogramin B molecules, and may reduce activity [64]. Less common mechanisms of resistance to streptogramin B molecules include inactivating enzymes (vgb hydrolase) and an efflux mechanism (MSB resistance). Resistance to the streptogramin A component differs in that it seems to result in phenotypic resistance to the streptogramin A and B mixtures; several genes have been identified that mediate active efflux (vga), inactivating enzymes (e.g. vat acetylases), and lincosamide and streptogramin A resistance (lsa) in Staphylococcus spp. and E. faecium. Although there are several reports following compassionate-use programmes the clinical impact of this antibiotic is uncertain. RPR106972, an oral agent comprising a combination of two further pristinamycin derivatives, is under development [65].
New glycopeptides such as LY333328, LY191145 and LY307599 may also prove useful against resistant Gram positive bacteria. Some appear to be more rapidly bactericidal than vancomycin against MRSA in vitro, and have similar MICs for both vancomycin-susceptible and -resistant strains of Enterococcus spp. possessing the vanA and vanB phenotypes [66–68]. Development of the lipopeptide antibiotics, daptomycin and ramoplanin, which lack cross-resistance with the glycopeptides, has not progressed. Glycylcyclines, semisynthetic derivatives of tetracyclines also have in vitro activity against resistant Enterococcus spp. and MRSA [69, 70].
There are numerous new carbapenems, including orally active agents, under development of which sanfetrinem, a member of a novel class of β-lactams called trinems is closest to clinical use. Others include L695 256 SM17466, BO2727, CS834 [71–75]. Additional in vitro activity against penicillin and erythromycin-resistant S.pneumoniae, and MRSA, is encouraging. Gram negative activity is no better than existing agents.
Ketolides are new semisynthetic 14-membered ring macrolides characterized by a 3-keto group on the erythronolide A ring instead of l-cladinose. Early studies on two compounds (HMR3647 and HMR3004 [RU64004]) have shown an antibacterial spectrum similar to existing macrolides, but with additional activity against penicillin and MLSB resistant S. pneumoniae, H. influenzae and some strains of MRSA and Enterococcus spp. [76, 77].
Finally, there is much activity in the area of quinolones, with two new agents launched in the UK in 1998—grepafloxacin and levofloxacin. A third agent, trovafloxacin, will soon be available. In general, with the exception of clinafloxacin, the in vitro Gram negative activity of new quinolones is no better than existing agents. However the Gram positive and anaerobic activity is increased, although clinical efficacy data in infections caused by PRP, MRSA and GRE are at present limited. Bacteria may exhibit cross resistance to some but not all quinolones; cross-resistance to other classes of antibiotic is, as yet, uncommon. Three additional agents, moxifloxacin, gatifloxacin and gemifloxacin, are in the late stages of development.
Numerous strategies are being investigated for the management of bacterial infections by means other than conventional antibiotics. Microbial genome sequencing, combinatorial chemistry and improved methods of screening for antimicrobial activity are providing powerful new approaches in the development of these agents [78, 79]. Genomic data for several human pathogens are now available with other projects in progress; potential new targets could include genes or gene products involved in cell division, protein synthesis, metabolite transport and virulence. One technique being used to study pathogenesis and identify virulence factors is signature tagged mutagenesis; large numbers of genes are mutated and then studied simultaneously using a unique bar code identification system [80]. Drugs developed against these highly specific targets are likely to be more narrow spectrum than conventional antibiotics. Work is in progress investigating alternative approaches using plasmids containing engineered DNA to disable genes that enable pathogens to destroy antibiotics and genes responsible for virulence. Exploiting antimicrobial peptides produced as part of our natural defences against infection is another approach. Such polypeptides include protegrins, magainins, defensins, squalamines and histanin V [81]. Synthetic protegrin analogues and antimicrobial peptides derived from animals are already under development.
A greater understanding of the pathogenesis of sepsis, involving bacterial cell wall components, activation of humoral pathways and a complex network of cytokines, has also provided impetus for new therapeutic strategies. The mortality from serious bacterial infections that produce a septic syndrome or septic shock is high despite appropriate antibiotic therapy. Substances targeting this process are called biological response modifiers (BRMs) or immunomodulators; although used synonymously BRMs stimulate some or all aspects of the immune system whereas immunomodulators are BRMs that up or down regulate a specific cell type or cytokine without necessarily causing any biological or clinical changes beyond that. Although unlikely to replace antibiotics, and for use only as adjunctive therapy, many compounds are undergoing clinical trials [82]. One compound, a human IgM monoclonal that binds the lipid A component of Gram negative lipopolysaccharide, was marketed in the early 1990s but subsequently withdrawn. Recombinant colony stimulating factors and gamma interferon are used only in immunocompromised patients. The highly complex interplay of cytokines makes targeting extremely difficult; the niche exists but is clearly restricted to only the most serious of infections. Interestingly some antibiotics also function as BRMs; polymyxin B binds lipid A, and certain cephalosporins (e.g. cefodizime) down regulate specific cytokine production in vitro [83].
In many cases protective antibody obviates the need for antimicrobial agents, and vaccine-induced immunity has reduced the burden of many infectious diseases. Although invasive H. influenzae type B has been almost eliminated in the UK following the recent introduction of immunization, there remains many bacterial infections for which currently available vaccines are either deficient (e.g. S. pneumoniae, N. meningitidis, M. tuberculosis) or nonexistent (e.g. Staphylococcus spp., N. gonorrhoea). Although passive antibody therapy has been largely abandoned since the advent of antibiotics and with the associated problems of systemic administration of animal sera, the ability of sophisticated molecular techniques to identify target epitopes associated with protective antibody and then produce human recombinant antibodies to these epitopes may bring antibody therapy once more to the forefront [84].
Quorum sensing in bacteria is a recently described phenomenon and may also provide a new approach. Essentially this involves the accumulation of low molecular weight pheromones (N-acyl homoserine lactones) which enable cells to sense when the minimum population unit or ‘quorum’ of bacteria has been achieved. specific gene expression is then activated. This may play a major role in regulating virulence determinants in Gram negative bacteria, and although not yet demonstrated in vivo, use of a molecular antagonist to either interfere with the synthesis or transmission of this signal is an attractive strategy [85].
Many compounds, termed nonantibiotics, employed in the management of noninfectious pathology have been shown to modify cell permeability and exhibit broad spectrum antimicrobial activity in vitro. Non-antibiotics have been the subject of a recent comprehensive review and include compounds as diverse as phenothiazines, anaesthetic agents, antihistamines, antihypertensives, and diuretics [86]. In some cases these compounds can also enhance the activity of certain antibiotics against specific bacteria, exert a specific type of synergy in reversing in vitro resistance, and exhibit activity against resistant isolates of M. tuberculosis. Whether these in vitro phenomena can be translated into a clinically significant anti-infective strategy remains to be proven.
Whereas there have been considerable advances in the photodynamic therapy (PDT) of cancer, the science of photodynamic antimicrobial chemotherapy (PACT) is less well developed [87]. Both use photosensitizers and visible or ultraviolet light to generate a phototoxic response via oxidative damage. Although probably only amenable to the treatment of localized infection such as those in wounds (and therefore not affecting endogenous flora elsewhere) differential phototoxicity of photosensitizers in bacterial and human cells, and specific antibody-linked photosensitizers, are being investigated.
Conclusions
Although bacterial infection remains a public health problem world-wide, the impact of antibiotics over the past 50 years has been quite remarkable. Equally impressive is the selection by bacteria of antibiotic resistance mechanisms. The re-emergence in recent years of Gram positive bacteria with additional resistance patterns (MRSA, VISA, GRE, PRP), and multiresistant Gram negative ‘superbugs’, has been extensively reported, and our concerns are justified. However, at present, with the lack of robust national susceptibility data the true incidence of these patterns is unclear. Furthermore the clinical impact of antibiotic resistance is often poorly defined; it is studied from an in vitro perspective and extrapolation is fraught with problems.
It is over 10 years since the fluoroquinolones and carbapenems were introduced. Two new classes of antibiotic are in development, as are many variants of existing agents. Powerful molecular techniques may yield new targets, and new strategies may in due course lessen our dependence on traditional antibiotics.
Antibiotic resistance is a complex, continually evolving problem which is often difficult to put into perspective. Highly simplistic approaches to reduce consumption and develop significant new agents are formidable tasks.
References
- 1.House of Lords Select Committee on Science, Technology. 7th Report. 1998. London Stationery Office.
- 2.American Society for Microbiology. Task Force on Antimicrobial Resistance Report. Washington DC: ASM; 1994. [Google Scholar]
- 3.Department of Health Standing Medical Advisory Committee: Sub-Group on Antimicrobial Resistance. The Path of Least Resistance. Department of Health Publications; 1998. [Google Scholar]
- 4.BSAC. Standardised Disc Sensitivity Testing Method. The Newsletter of the British Society for Antimicrobial Chemotherapy. Summer 1998 [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. Villanova, PA: Approved Standard NCCLS Publication M2A5; 1993. NCCLS. [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Dilution Procedures for Susceptibility Testing of Aerobic Bacteria. Villanova, PA: Approved Standard NCCLS Publication M7–A3; 1993. NCCLS. [Google Scholar]
- 7.Craig WA. The future—can we learn from the past? Diagn Microbiol Infect Dis. 1997;27:49–53. doi: 10.1016/s0732-8893(97)00022-9. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 9.Dancer SJ, Shears P, Platt DJ. Isolation and characterisation of coliforms from glacial ice and water in Canada's High Arctic. J Appl Bacteriol. 1997;82:597–609. doi: 10.1111/j.1365-2672.1997.tb03590.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones ME, Peters E, Weersink AM, Fluit A, Verhoef J. Widespread occurrence of integrons causing multiple antibiotic resistance in bacteria. Lancet. 1997;349:1742–1743. doi: 10.1016/S0140-6736(05)62954-6. 10.1016/S0140-6736(05)62954-6. [DOI] [PubMed] [Google Scholar]
- 11.Bush K, Jacoby GA, Medioros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amyes SGB, Miles RS. Extended spectrum β-lactamases: the role of inhibitors in therapy. J Antimicrob Chemother. 1998;42:415–417. doi: 10.1093/jac/42.4.415. 10.1093/jac/42.4.415. [DOI] [PubMed] [Google Scholar]
- 13.Livermore DM, Yuan M. Antibiotic resistance and production of extended spectrum β-lactamases amongst Klebsiella spp. from intensieve care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 14.Nordmann Guibert M. Extended spectrum β-lactamases in. Pseudomonas Aeruginosa. J Antimicrob Chemother. 1998;42:128–131. doi: 10.1093/jac/42.2.128. 10.1093/jac/42.2.128. [DOI] [PubMed] [Google Scholar]
- 15.Livermore DM. Carbapenamases. J Antimicrob Chemother. 1992;29:609–616. doi: 10.1093/jac/29.6.609. [DOI] [PubMed] [Google Scholar]
- 16.Thomson KS, Sanders CC, Chmel H. Imipenem resistance in Enterobacter. Eur J Clin Microbiol Infect Dis. 1993;12:610–613. doi: 10.1007/BF01973639. [DOI] [PubMed] [Google Scholar]
- 17.Woodford N, Palepou M-FI, Babini GS, Bates J, Livermore DM. Carbapenamase-producing Pseudomonas aeruginosa in the UK. Lancet. 1998;352:546–547. doi: 10.1016/s0140-6736(05)79255-2. 10.1016/S0140-6736(05)79255-2. [DOI] [PubMed] [Google Scholar]
- 18.Nicolas-Chanoine MH. Inhibitor resistant β-lactamases. J Antimicrob Chemother. 1997;40:1–3. doi: 10.1093/jac/40.1.1. 10.1093/jac/40.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Shaw KJ, Rather PN, Hare RS, Miller GH. Molecular genetics of aminoglycoside genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landman D, Quale JM. Management of infections due to resistant enterococci: a review of therapeutic options. J Antimicrob Chemother. 1997;40:161–170. doi: 10.1093/jac/40.2.161. 10.1093/jac/40.2.161. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama H, Nakae T. Mechanism of efficient elimination of protein D2 in outer membrane of imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:2385–2390. doi: 10.1128/aac.37.11.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper DC, Wolfson JS, Souza KS, Ng EY, McHugh GL, Swartz MN. Mechansims of quinolone resistance in Escherichia coli: characterisation of nfxB and cfxB, two mutant resistant loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1999;33:283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen IT, Brown MH, Skurray RA. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XZ, Livermore DM, Nikaido H. Role of efflux pump (s) in intrinsic resistance to Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez JL, Alonso A, Gomez-Gomez JM, Baquero F. Quinolone resistance by mutations in chromosomal gyrase genes. Just the tip of the iceberg? J Antimicrob Chemother. 1998;42:683–688. doi: 10.1093/jac/42.6.683. 10.1093/jac/42.6.683. [DOI] [PubMed] [Google Scholar]
- 26.Dowson CG, Coffrey TJ, Spratt BG. Origin and molecular epidemiology of penicillin binding protein mediated resistance to β-lactam antibiotics. Trends Microbiol. 1994;2:361–365. doi: 10.1016/0966-842x(94)90612-2. 10.1016/0966-842X(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 27.Michel M, Gutmann L. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet. 1997;349:1901–2006. doi: 10.1016/s0140-6736(96)11192-2. 10.1016/S0140-6736(96)11192-2. [DOI] [PubMed] [Google Scholar]
- 28.Uttley AHC, Collins CH, Naidoo J, George RC. Resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. 10.1016/S0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 29.Woodford N. Glycopeptide-resistance enterococci: a decade of experience. J Med Microbiol. 1998;47:1–14. doi: 10.1099/00222615-47-10-849. [DOI] [PubMed] [Google Scholar]
- 30.Rowe PM. Preparing for the battle against vancomycin resistance. Lancet. 1996;347:252. doi: 10.1016/s0140-6736(96)90420-1. 10.1016/S0140-6736(96)90420-1. [DOI] [PubMed] [Google Scholar]
- 31.Wade JJ, Uttley AHC. Resistant enterococci—mechanisms, laboratory detection and control in hospitals. J Clin Pathol. 1996;49:700–703. doi: 10.1136/jcp.49.9.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodford N, Johnson AP, Morrison D, Speller DCE. Current perspectives on glycopeptide resistance. Clin Microbiol Rev. 1995;8:585–615. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodford N, Johnson AP, Morrison D, et al. Vancomycin-dependent enterococci in the United Kingdom. J Antimicrob Chemother. 1994;33:1066. doi: 10.1093/jac/33.5.1066. [DOI] [PubMed] [Google Scholar]
- 34.Piddock LJV. Fluoroquinolone resistance. Br Med J. 1998;317:1029–1030. doi: 10.1136/bmj.317.7165.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Martinez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–799. doi: 10.1016/S0140-6736(97)07322-4. 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 36.Fouet A, Siraud JC, Mock M. Bacillus anthracis pXo1 virulence plasmid encodes a type 1 DNA topoisomerase. Molec Microbiol. 1994;11:471–479. doi: 10.1111/j.1365-2958.1994.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 37.Roberts MC. Tetracycline resistant determinants: mechanisms of action, regulation of expression, genetic mobility and distribution. FEMS Microbiol Reviews. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. 10.1016/0168-6445(96)00021-6. [DOI] [PubMed] [Google Scholar]
- 38.Huovinen P, Sundstrom L, Swedberg G, Skold O. Trimethoprim and sulphonamide resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chopra I. Over-expression of target genes as a mechanism of antibiotic resistance in bacteria. J Antimicrob Chemotherap. 1998;41:584–588. doi: 10.1093/jac/41.6.584. [DOI] [PubMed] [Google Scholar]
- 40.Martinez JL, Vicente MF, Dlegado-Iribarren A, Perez-diaz JC, Baquero F. Small plasmids are involved in amoxycillin-clavulanate resistance in Escherichia coli. Antimicrob Agents Chemother. 1989;33:595. doi: 10.1128/aac.33.4.595-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDSC. Bacteraemia, England and Wales; laboratory reports, weeks 42–46/98. Commun Dis Rep CDR Weekly. 1998;8:414. [Google Scholar]
- 42.Johnson AP, James D. Continuing increase in invasive methicillin-resistant infection. Lancet. 1997;350:1710. doi: 10.1016/s0140-6736(05)64319-x. 10.1016/S0140-6736(05)64319-X. [DOI] [PubMed] [Google Scholar]
- 43.Rahman M. Alternatives to vancomycin in treating methicillin-resistant Staphylococcus aureus infections. J Antimicrob Chemother. 1998;41:325–328. doi: 10.1093/jac/41.3.325. 10.1093/jac/41.3.325. [DOI] [PubMed] [Google Scholar]
- 44.Franciolli M, Bille J, Glauser MP, Moreillon P. β-lactam resistance mechanisms of methicillin-resistant Staphylococcus aureus. J Infect Dis. 1991;163:514–523. doi: 10.1093/infdis/163.3.514. [DOI] [PubMed] [Google Scholar]
- 45.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 46.Ploy MC, Grelaud C, de Martin C, Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. doi: 10.1016/s0140-6736(05)79166-2. 10.1016/S0140-6736(05)79166-2. [DOI] [PubMed] [Google Scholar]
- 47.Staphylococcus aureus with reduced susceptibility to vancomycin—United States 1997. MMWR Mor Mortal Wkly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 48.Hiramatsu K, Artiaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 49.Hansman D, Glasgow H, Sturt J, Devitt L, Douglas R. Increased resistance to penicillin of pneumococci isolated from man. N Engl J Med. 1971;284:175–177. doi: 10.1056/NEJM197101282840403. [DOI] [PubMed] [Google Scholar]
- 50.Appelbaum PC, Scragg JN, Bowen AJ, Bhamjee A, Hallett AF, Cooper RC. Streptococcus pneumoniae resistant to penicillin and choramphenicol. Lancet. 1977;ii:995–997. doi: 10.1016/s0140-6736(77)92892-6. 10.1016/S0140-6736(77)92892-6. [DOI] [PubMed] [Google Scholar]
- 51.Laurichesse H, Grimaud O, Waight P, Johnson AP, George RC, Miller E. Pneumococcal bacteraemia and meningitis in England and Wales. 1993–95. Communicable Dis Public Health. 1998;1:22–27. [PubMed] [Google Scholar]
- 52.CDSC. Vancomycin resistant enterococci in hospitals in the United Kingdom. Commun Dis Rep CDR Weekly. 1996;6:267. [PubMed] [Google Scholar]
- 53.Archibald L, Phillips L, Monnet D, McGowan E, Tenover F, Gaynes R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–215. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 54.Endtz HP, Ruijs GJ, van Klingeren B, Jansen WH, van der Reyden T, Mouton RP. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother. 1991;27:199–208. doi: 10.1093/jac/27.2.199. [DOI] [PubMed] [Google Scholar]
- 55.Murray CJL. Issues in operational, social and economic research in tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection and Control. Washington DC: American Society for Microbiology; 1994. pp. 583–622. [Google Scholar]
- 56.CDSC. Infectious diseases in England and Wales: January to March 1998. Commun Dis Rep CDR Supplement. 1998. p. S4. [PubMed]
- 57.Drobniewski FA, Yates MD. Multiple drug resistant tuberculosis. J Clin Pathol. 1997;50:89–92. doi: 10.1136/jcp.50.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seppala H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 59.Stephenson J. Icelandic researchers are showing the way to bring down the rates of antibiotic resistant bacteria. JAMA. 1996;275:175. 10.1001/jama.275.3.175. [PubMed] [Google Scholar]
- 60.Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–1237. doi: 10.1001/jama.280.14.1233. 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 61.Zurenko GE, Yagi BH, Schaadt RD, et al. In vitro activities of U-100592 and U-100766, novel oxazolidonone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brumfitt W, Hamilton-Miller JMT, Shah S. In vitro activity of RP59500, a new semi-synthetic streptogramin antibiotic against Gram positive bacteria. J Antimicrob Chemother. 1992;30(Suppl A):29–38. doi: 10.1093/jac/30.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 63.Andrews JM, Wise R. The in vitro activity of a new semi-synthetic streptogramin, RP59500, against staphylococci and respiratory pathogens. J Antimicrob Chemother. 1994;33:849–853. doi: 10.1093/jac/33.4.849. [DOI] [PubMed] [Google Scholar]
- 64.Entenza JM, Drugeon H, Glauser MP, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP59500. Antimicrob Agents Chemother. 1995;39:1419–1424. doi: 10.1128/aac.39.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King A, May J, Phillips I. Comparative activity of quinupristin/dalfopristin and RPR106972 and the effect of medium on in vitro test results. J Antimicrob Chemother. 1998;42:711–719. doi: 10.1093/jac/42.6.711. 10.1093/jac/42.6.711. [DOI] [PubMed] [Google Scholar]
- 66.Mercier RC, Houlihan HH, Ryback MJ. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:1307–1312. doi: 10.1128/aac.41.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicas TI, Mullen DL, Flokowitsch JE, et al. Activities of the semisynthetic glycopeptide LY191145 against vancomycin-resistant enterococci and other gram positive bacteria. Antimicrob Agents Chemother. 1995;39:2585–2587. doi: 10.1128/aac.39.11.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mezzatesta ML, Bonfiglio G, De Angelis L, Stefani S, Russo G. Study on the in vitro activity of LY333328 against Gram positive cocci. J Antimicrob Chemother. 1998;42:266–268. doi: 10.1093/jac/42.2.266. 10.1093/jac/42.2.266. [DOI] [PubMed] [Google Scholar]
- 69.Fraise AP, Brenwald N, Andrews JM, Wise R. In vitro activity of two glycylcyclines against enterococci resistant to other agents. J Antimicrob Chemother. 1995;35:877–881. doi: 10.1093/jac/35.6.877. [DOI] [PubMed] [Google Scholar]
- 70.Weiss WJ, Jacobus NV, Petersen PJ, Testa RT. Susceptibility of enterococci, methicillin-resistant Staphylococcus aureus and Streptococcus pneumoniae to the glycylcyclines. J Antimicrob Chemother. 1995;36:225–230. doi: 10.1093/jac/36.1.225. [DOI] [PubMed] [Google Scholar]
- 71.Malanoski G, Collins L, Eliopoulos CT, Moellering RC, Eliopoulos GM. Comparative in vitro activities of L695256, a novel carbapenem against gram positive bacteria. Antimicrob Agents Chemother. 1995;39:990–995. doi: 10.1128/aac.39.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sumita Y, Nouda H, Kamazawa K, Fukasawa M. Antimicrobial activities of SM17466, a novel carbapenem antibiotic with potent activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:910–916. doi: 10.1128/aac.39.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kato Y, Otsuki M, Nishino T. Antibacterial properties of BO2727, a new carbapenem antibiotic. J Antimicrob Chemother. 1997;40:195–203. doi: 10.1093/jac/40.2.195. 10.1093/jac/40.2.195. [DOI] [PubMed] [Google Scholar]
- 74.Sakagawa E, Otsuki M, Ou T, Nishino T. In vitro and in vivo antibacterial activities of CS834, a new oral carbapenem. J Antimicrob Chemother. 1998;42:427–437. doi: 10.1093/jac/42.4.427. 10.1093/jac/42.4.427. [DOI] [PubMed] [Google Scholar]
- 75.Johnson AP, Warner M, Speller DCE. In vitro activity of sanfetrinem against isolates of Streptococcus pneumoniae and Staphylococcus aureus. J Antimicrob Chemother. 1998;42:643–646. doi: 10.1093/jac/42.5.643. 10.1093/jac/42.5.643. [DOI] [PubMed] [Google Scholar]
- 76.Ednie LM, Spangler SK, Jacobs MR, Appelbaum PC. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumococci to RU64004, a new ketolide, compared to 16 other agents. Antimicrob Agents Chemother. 1997;41:1033–1036. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boswell FJ, Andrews JM, Ashby JP, Fogarty C, Brenwald NP, Wise R. The in vitro activity of HMR3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 78.Jenks PJ. Microbial genome sequencing—beyond the double helix. Br Med J. 1998;317:1568–1571. doi: 10.1136/bmj.317.7172.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chopra I, Hodgson J, Metcalf B, Poste G. New approaches to the control of infections caused by antibiotic-resistant bacteria. An industry perspective. JAMA. 1996;275:401–403. [PubMed] [Google Scholar]
- 80.Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 81.Mitchell P. Rearming in the fight against bacteria. Lancet. 1998;352:462. doi: 10.1016/S0140-6736(05)79202-3. 10.1016/S0140-6736(05)79202-3. [DOI] [PubMed] [Google Scholar]
- 82.Vincent JL. Search for effective immunomodulating strategies against sepsis. Lancet. 1998;351:922–923. doi: 10.1016/S0140-6736(05)60595-8. [DOI] [PubMed] [Google Scholar]
- 83.Ritts RE. Antibiotics as biological response modifiers. J Antimicrob Chemother. 1990;26(Suppl C):31–36. doi: 10.1093/jac/26.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 84.Burnie JP Matthews RC. The renaissance of antibody therapy. J Antimicrob Chemother. 1998;41:319–322. doi: 10.1093/jac/41.3.319. [DOI] [PubMed] [Google Scholar]
- 85.Finch RG, Pritchard DI, Bycroft BW, Williams P, Stewart GSAB. Quorum sensing: a novel target for anti-infective therapy. J Antimicrob Chemother. 1998;42:569–571. doi: 10.1093/jac/42.5.569. 10.1093/jac/42.5.569. [DOI] [PubMed] [Google Scholar]
- 86.Kristiansen JE, Amaral L. The potential management of resistant infections with non-antibiotics. J Antimicrob Chemother. 1997;40:319–327. doi: 10.1093/jac/40.3.319. 10.1093/jac/40.3.319. [DOI] [PubMed] [Google Scholar]
- 87.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]


