Abstract
Aims
To compare the pharmacokinetic profile of BeclazoneTM (beclomethasone dipropionate) in its chlorofluorocarbon (CFC)-based and CFC-free formulations.
Methods
Ten healthy adults received a single 1000 μg nominal dose (ex-valve) of beclomethasone dipropionate from a CFC inhaler (BEC-CFC) or from a CFC-free inhaler containing hydrofluoroalkane (HFA)-134a (BEC-HFA) in an open-label, randomized, two-way, crossover study. Blood samples were collected predose and over 12 h after inhalation. Comparisons were made of maximum plasma concentration of beclomethasone 17-monopropionate (17-BMP) (Cmax), and area under the plasma concentration vs time curve (AUC).
Results
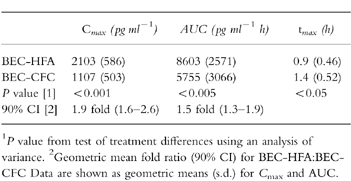
The tmax was significantly (P<0.05) earlier with BEC-HFA and plasma levels were significantly higher following administration of BEC-HFA than BEC-CFC. Geometric mean values for AUC were 1.5 fold greater (90% CI 1.3–1.9) and for Cmax were 1.9 fold greater (90% CI 1.6–2.6) following BEC-HFA than BEC-CFC.
Conclusions
Our data in healthy volunteers would not be consistent with the manufacturers’ recommendation for a microgram equivalent (1:1) nominal dose switch between these HFA and CFC formulations. Further well designed trials are required in asthmatic patients to properly define their respective dose–response relationships for antiasthmatic and systemic adverse effects.
Keywords: asthma, beclomethasone dipropionate, bioequivalence, chlorofluorocarbon, hydrofluoroalkane, pharmacokinetics
Introduction
In 1998, a new pressurized chlorofluorocarbon (CFC)-free metered-dose inhaler (BeclazoneTM) containing hydrofluoroalkane (HFA)-134a beclomethasone dipropionate (BDP) was introduced in Ireland. This inhaler was recommended to replace the existing CFC formulations on a μg for μg (one for one puff) basis in terms of the nominal dose ex-valve.
The use of pharmacokinetic profiles has been suggested as an indirect method for comparing the relative lung dose for inhaled corticosteroids [1]. For beclomethasone dipropionate there is a first-pass biotransformation to both active (beclomethasone-17-monopropionate) and inactive (beclomethasone-21-monopropionate) in both the lung and the liver (after swallowing). The early pharmacokinetic profile (as Cmax) for beclomethasone-17- monopropionate may therefore reflect absorption mainly from the lung, whereas the area under curve may reflect total systemic bioavailability from lung and gut.
We therefore performed a pharmacokinetic comparison of 250 μg strength formulations of CFC and HFA-BDP using a single nominal 1000 μg dose of inhaled BDP, which is within the recommended dose range for both products (up to 2000 μg day−1). Measurement of serum cortisol, another marker of systemic bioactivity was not included, as the study was not sufficiently powered for this analysis.
Methods
Ten adult volunteers (both sexes, age range 18–50 years), were recruited. The entry criteria required nonsmokers, within 20% of their ideal body weight based on height and body frame size, who demonstrated acceptable use of a placebo inhaler. All subjects had to be generally healthy as judged by physical examination, clinical history, 12-lead electrocardiogram, and clinical laboratory tests. Subjects were excluded if they had received any new or prescription drug within 4 weeks of recruitment, any inhaled β2-adrenoceptor agonist or steroid within 2 months, or any oral or intramuscular steroid within 6 months. All subjects gave written informed consent and an independent medical ethics committee approved the protocol.
In this randomized, open-label, two-period crossover study, subjects received a 1000 μg dose of BDP in each period, 7 days apart. After training in the correct use of an inhaler, subjects self-administered the drug under supervision, using a 10 s breath hold and 30 s spacing between puffs. The drug was administered as four puffs from a CFC-containing inhaler in one period (BEC-CFC; Beclazone 250 μg (nominal dose ex-valve) per actuation, Norton-Waterford, Ireland) and as four puffs from a hydrofluoroalkane (HFA)-134a-containing inhaler in the other period (BEC-HFA; Beclazone CFC-free 250 μg per actuation). All inhalers were from commercial lots and were primed before use.
Blood samples were collected predose, and at 0.5, 1, 2, 4, 6, 8, 10, and 12 h postdose during each period. Plasma levels of beclomethasone 17-monopropionate (17-BMP) were measured using a liquid chromatographic/mass spectrometric assay with a calibration range of 75–2000 pg ml−1 in 1 ml plasma. The interday coefficient of variation at the limits of the assay was less than 12%.
The primary objective of this study was to compare the systemic availability of 17-BMP attained using the same nominal dose of BEC-CFC and BEC-HFA. The study had at least 80% power to detect a 50% difference in Cmax and AUC, using a sample size of 10 subjects. The primary parameters to assess differences were the maximum plasma concentration (Cmax) and the area under the plasma concentration vs time curve (AUC) of 17-BMP, calculated from time zero until the time of the last quantifiable plasma concentration using the trapezoidal rule. The primary null hypothesis was tested for each parameter using an analysis of variance with terms for sequence, period, subject and treatment effects in the model. The log10 transformation was used for 17-BMP AUC and Cmax. 90% confidence intervals were calculated for the geometric mean fold difference between the two formulations
Results
The study population consisted of seven males and three females, mean ages 36.6 (s.e.mean 3.9) and 40.7 years (s.e.mean 2.7), respectively. All subjects completed both study periods. No subject reported any adverse event. Physical examinations and clinical laboratory tests remained normal at the end of the study.
Plasma levels of 17-BMP were significantly greater following BEC-HFA than following BEC-CFC (Figure 1). Geometric mean values for AUC were 1.5 fold greater and for Cmax were 1.9 fold greater following BEC-HFA than BEC-CFC. The time to reach the peak 17-BMP levels (tmax) was significantly earlier with BEC-HFA. All pharmacokinetic comparisons between the two formulations were statistically significant (Table 1).
Figure 1.
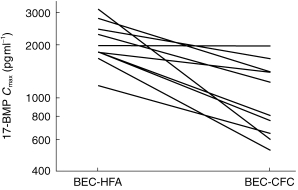
Peak plasma 17-BMP levels in 10 subjects following administration of 1000 μg BDP as BEC-CFC and BEC-HFA.
Table 1.
Beclomethasone-17-monopropionate pharmacokinetic results.
Discussion
The HFA formulation produced up to two fold greater absorption of drug than BEC-CFC for the same nominal dose (ex-valve). Pharmacokinetic parameters for early (Cmax) and total (AUC) absorption differed significantly between the two products, which could indicate clinically important differences in systemic adverse effects and perhaps in efficacy. We did not perform a charcoal block study to discriminate between the lung and gut moieties of absorption for beclomethasone-17-monopropionate and so it is not possible to assess whether the differences were due to a higher lung dose with the HFA formulation. Nonetheless the early absorption profile (as Cmax) is likely to reflect lung rather than gut bioavailability. Indeed the tmax was significantly later for the CFC formulation, perhaps reflecting a component of Cmaxdue to delayed gut absorption. A much higher degree of lung delivery has been reported with another similar HFA-134a formulation of BDP (Qvar, 3 m Healthcare, Loughborough, UK) compared with CFC-BDP [2]. In our study the ratio between HFA and CFC formulations for Cmax (1.9 fold) was relatively higher than the ratio for AUC (1.5 fold), perhaps suggesting that early lung absorption was greater for the HFA formulation. Our data would not be consistent with the manufacturer’s recommendation for a microgram equivalent (1:1) nominal dose switch between these HFA and CFC formulations.
Little published data on the Beclazone-HFA-134a formulation are available. In a study by Milanowski et al. no apparent differences in antiasthmatic efficacy were reported in asthmatic patients receiving either 400 μg day−1 or 2000 μg day−1 of HFA-134a and CFC formulations of BDP [3]. However, this study design was flawed because there was no difference in FEV1 response between low and high doses after 6 weeks of treatment, with the 400 μg dose being on the plateau of the response curve. In order to properly compare the potency of two inhaled corticosteroid formulations it is necessary to compare the effects of at least three doses on the steep part of the response curve [4]. For example, data using another similar HFA-134a formulation of BDP metered dose inhaler (Qvar, 3M Healthcare, Loughborough, UK), revealed a relative potency ratio of 2.6 for HFA vs CFC-BDP, as assessed by comparing effects on the steep part of the dose–response curve for FEV1 in patients with moderate to severe asthma [5]. In other studies, half the dose of HFA-BDP were as effective as CFC-BDP in the control of moderately severe asthma [6, 7]. It is therefore perhaps not surprising that significant pharmacokinetic differences were found in the present study for the supposedly bioequivalent formulations of BEC-CFC and BEC-HFA.
Although pharmacokinetics is a sensitive metric, it is only a systemic assessment of bioavailability of two formulations of an inhaled corticosteroid and consequently may not directly relate to antiasthmatic efficacy. However, in a dose–response evaluation of budesonide given via metered dose inhaler alone or in conjunction with spacer, a two-fold increase in respirable fraction was associated with a two-fold increase in antiasthmatic and systemic potency [8]. Systemic levels of the active metabolite (beclomethasone-17-monopropionate) will determine the propensity for producing systemic adverse effects, such as adrenal suppression. One might therefore predict that the BEC-HFA formulation would produce greater detectable systemic bioactivity than BEC-CFC formulation if these were both given in microgram equivalent nominal doses, as recommended by the manufacturer. Properly designed randomized placebo controlled studies are indicated to evaluate the dose–response relationships for adrenal suppression using appropriately sensitive endpoints in patients with asthma [4].
In summary, the results of this study in healthy volunteers show appreciable differences in the systemic absorption of HFA and CFC formulations of beclomethasone dipropionate (as Beclazone), and suggest that similar clinically significant differences could exist in systemic adverse effects. Further pharmacokinetic studies using charcoal block are required to determine the relative lung and gut components of systemic bioavailability with these products, as well as evaluating the dose–response for adrenal suppression to assess their relative systemic bioactivity profiles. Caution should be exercised in switching from one product to the other on a microgram equivalent nominal basis until well-designed clinical trials, such as a dose–response comparisons, have been completed to enable the dosing relationships between these products to be properly defined.
Acknowledgments
This study was supported by 3M Healthcare Ltd.
References
- 1.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach CL. Improved delivery of inhaled steroids to the large and small awirways. Respir Med. 1998;92(Suppl A):3–8. doi: 10.1016/s0954-6111(98)90211-6. [DOI] [PubMed] [Google Scholar]
- 3.Milanowski J, Qualtrough J, Perrin VL. Inhaled beclomethasone (BDP) with a non-CFC propellant (HFA 134a) is equivalent to BDP-CFC for the treatment of asthma. Respir Med. 1999;93:245–251. doi: 10.1016/s0954-6111(99)90020-3. [DOI] [PubMed] [Google Scholar]
- 4.Lipworth BJ, Wilson AM. Dose response to inhaled corticosteroids: benefits and risks. Sem Respir Crit Care Med. 1998;19:625–646. [Google Scholar]
- 5.Busse W, Colice G, Hannon S. CFC-BDP require 2. 6 times the dose to achieve equivalent improvement in FEV1 as HFA-BDP. Am J Respir Crit Care Med. 1998;157:405. [Google Scholar]
- 6.Davies RJ, Stampone P, O’Connor BJ. Hydrofluoroalkane-34a dipropionate extra fine aerosol beclomethasone provides equivalent asthma control to chlorofluorocarbon beclomethasone dipropionate at approximately half the dose. Respir Med. 1998;92(Suppl A):23–31. doi: 10.1016/s0954-6111(98)90214-1. [DOI] [PubMed] [Google Scholar]
- 7.Gross G, Thompson PJ, Chervinsky P, Vanden Burgt J. Hydrofluoroalkane-134a beclomethasone dipropionate, 400μg is as effective as chlorofluorocarbon beclomethasone dipropionate 800μg for the treatment of moderate asthma. Chest. 1999;15:343–335. doi: 10.1378/chest.115.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Toogood JH, Baskerville J, Jennings B, Neville M, Lefcoe NM, Johannsson SA. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. Am Rev Respir Dis. 1984;129:723–729. doi: 10.1164/arrd.1984.129.5.723. [DOI] [PubMed] [Google Scholar]


