Abstract
Aims
To determine patterns in presentation, risk factors, management and outcome of patients with ACE inhibitor associated angioedema in one British teaching hospital.
Methods
Cases of ACE inhibitor associated angioedema in patients presenting to the City Hospital, Birmingham between 1993 and 1999 were collected and entered prospectively onto a computerised register.
Results
A total of 20 cases (mean age 60 years, range 42–82 years) of ACE inhibitor associated angioedema were reported (11 female and 9 male) with 65% (n=13) of patients being black/Afro-Caribbean. In 70% of cases (n=14), angioedema occurred within 4 weeks of starting therapy, although three patients presented following long-term treatment (24–48 months). ACE inhibitors were continued in 50% (n=10) patients, despite at least one documented episode of angioedema. Admission to hospital was necessary in 40% (n=8) patients, with three of these admitted to the intensive care unit, and one of these died as a result of severe laryngeal obstruction.
Conclusions
ACE inhibitor related angioedema is a serious and potentially fatal complication which is relatively rare in the general population, but is more common amongst black/Afro-Caribbean patients. ACE inhibitors are frequently continued following an episode of angioedema and it is important that these episodes are minimised by prompt cessation of the drug, careful patient counselling and heightened awareness in all clinicians who prescribe this common group of drugs.
Keywords: ACE inhibitors, angioedema, ethnicity
Introduction
Angiotensin converting enzyme (ACE) inhibitor associated angioedema was first reported in the early 1980s [1] and it is now well recognized as a potentially serious, but rare, side-effect. The use of ACE inhibitors, for hypertension and also in heart failure, has markedly increased over recent years and clinicians, both in hospital and in the community, should be aware of this serious complication.
Since 1993 we have maintained an angioedema register of all patients seen with this condition in our clinical practice and blood pressure clinic. This was prompted by one patient who required admission to the intensive care unit with severe respiratory distress and a patient who died of anoxic encephalopathy, related to enalapril associated angioedema. We present a case series of 20 patients from our hospital, illustrating ethnic differences in susceptibility and emphasizing the need for greater awareness and earlier diagnosis.
Methods
Cases of ACE inhibitor associated angioedema reported in our hospital between 1993 and 1999 were collected and entered prospectively onto a computerized register of such patients. The hospital covers the West of Birmingham, serving a multiracial city centre population of approximately 300 000 people. The 1991 census of West Birmingham revealed that in the age bands 30–65 years, 22% of residents were Indo-Asian and 12% were Afro-Caribbean. In the blood pressure clinic, which receives over 500 annual referrals from local general practitioners and other hospital consultants, an estimated 52% of patients are Caucasian, with 31% Afro-Caribbean and 17% Indo-Asian. Afro-Caribbean patients are estimated to account for 13% of all-cause adult admissions to our hospital.
Angioedema was defined as swelling of the lips, mouth, tongue or airway in patients receiving ACE inhibitor therapy, where no other clinical cause was identifiable and where there were no recurrent symptoms following cessation of the drug. We reviewed in detail the patient exposure to other drugs, both prescription and nonprescription, to exclude other agents as a cause of the angioedema, and cases were excluded from the series when doubt remained as to the cause of the angioedema.
Results
Illustrative case report
An Afro-Caribbean male in his sixties was admitted to a hospital with acute respiratory distress, secondary to severe angioedema with gross tongue, lip and facial swelling. He had been attending his general practitioner for longstanding hypertension, with a previous history of mild stroke and had been receiving the ACE inhibitor enalapril (2.5 mg daily) for 5 months prior to the admission. There had been no recent therapeutic changes and his other medication included bendrofluazide (2.5 mg), amlodpine (5 mg) and aspirin (150 mg). Since the introduction of enalapril he had suffered recurrent and troublesome pruritus, which had been managed with the H1-antihistamine chlorpheniramine.
On admission he was in a state of acute respiratory distress and was treated immediately with high dose oxygen, subcutaneous adrenaline (0.7 ml 1:1000) and intravenous steroids (hydrocortisone 200 mg). His condition rapidly deteriorated leading to an acute respiratory arrest, secondary to laryngeal obstruction. Conventional tracheal intubation was impossible because of gross swelling involving the mucous membranes of the mouth, pharynx and larynx and an emergency tracheostomy was performed. This proved to be a difficult and traumatic procedure and the patient remained hypoxaemic for a number of minutes.
Investigations during the admission revealed mild renal impairment with a serum creatinine of 144 μmol l−1 (normal range 44–133), 12-lead ECG demonstrated sinus tachycardia with Sokolov-Lyon voltage criteria for left ventricular hypertrophy and the chest X-ray revealed widening of the mediastinum with features suggestive of an aneurysm of the thoracic aorta. Serum C1 esterase-inhibitor levels were within the normal range. He was ventilated in the Intensive Therapy Unit, until his angioedema had settled, but unfortunately failed to regain consciousness following extubation. He died a few days following admission, as a result of a respiratory infection and extensive hypoxic brain injury. A subsequent post-mortem also revealed extensive atheromatous disease with evidence of a previous cerebral infarction, severe coronary artery disease and a large aneurysm of the descending thoracic aorta (without evidence of tracheal compression).
A total of 20 cases (mean age 60 years, range 42–82 years) of ACE inhibitor associated angioedema were reported (Table 1). There was a similar sex distribution (11 female, nine male), although there was a marked ethnic bias, with 65% (n=13) black patients. This proportion was significantly greater than expected, when compared with the proportion of Afro-Caribbean patients in the local population (65%vs 12%, P<0.001), the proportion of black patients attending the blood pressure clinic (65%vs 31%, P<0.01) and the proportion of adult black patients presenting as acute medical admissions to the hospital (65%vs 13%, P<0.01). In three black patients ACE inhibitors were being used as first-line antihypertensive agents. In 70% of cases (n=14) angioedema occurred within 4 weeks of starting therapy, although three patients presented following long-term treatment (24–48 months).
Table 1.
Characteristics of patients suffering ACE inhibitor induced angioedema.
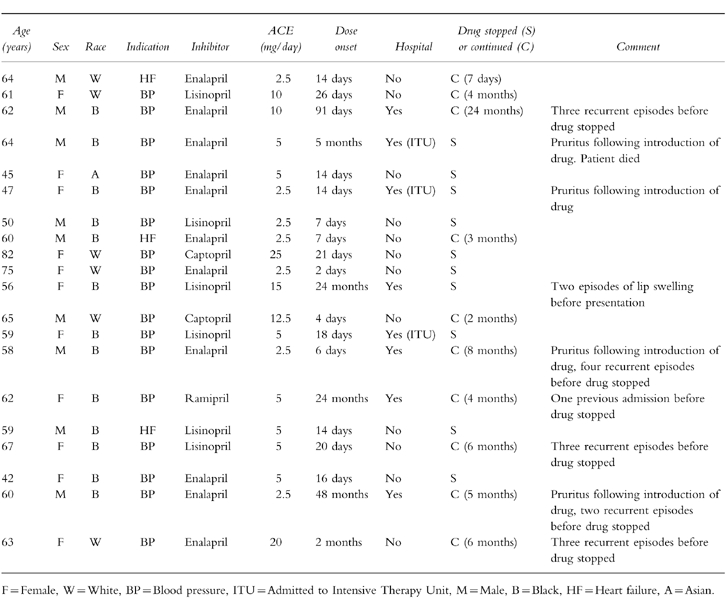
ACE inhibitors had been continued in 50% (n=10) patients, despite at least one documented episode of angioedema and six of these patients suffered recurrent episodes of angioedema until the drugs were eventually stopped, after a variable time interval (range 7 days-24 months). The dose of enalapril was increased in one patient presenting with angioedema, who had been found to be persistently hypertensive. This patient, a 58 year-old man, suffered four further episodes of angioedema, before the ACE inhibitor was eventually switched to an alternative antihypertensive agent.
Admission to hospital was necessary in 40% (n=8) patients and two of these were admitted to the intensive care unit with life threatening symptoms of laryngeal obstruction, necessitating acute intubation and ventilation. One patient died as a result of severe laryngeal obstruction, despite attempts at emergency tracheostomy. Patients who were admitted to hospital were treated with H1-receptor antagonists (6/8), steroids (6/8) and adrenaline (5/8). In all cases the angioedema had resolved within 24 h. The clinical presentation is similar to hereditary angioedema and C1 esterase-inhibitor levels were performed to exclude the diagnosis in 50% patients (n=10). The occurrence of angioedema did not appear to be related to the age of the patient, the dose of the drug, the particular type of ACE inhibitor or concurrent medical conditions.
Discussion
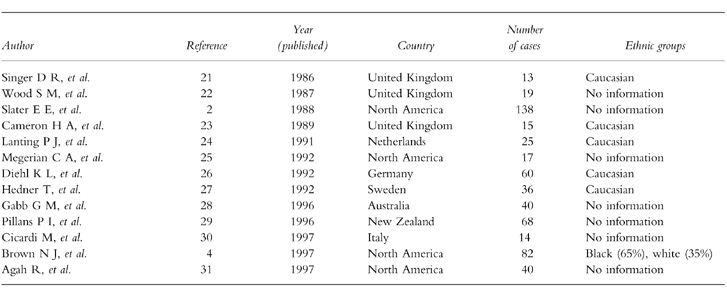
ACE inhibitors are a well documented cause of life threatening angioedema (Table 2) with a reported incidence of 0.1–0.2%, in the general population [2], although the incidence has been reported to be as high as 0.5% [3]. In our series the number of patients developing angioedema is likely to be an underestimate of the total number of patients developing this complication, as some patients may have died before reaching hospital. However, in our case series, angioedema occurred in a disproportionate number of black patients and this is consistent with studies from North America which have demonstrated a markedly increased risk in black Americans (adjusted relative risk of 4.5), compared with white subjects [4]. In addition, severe angioedema requiring admission to our intensive care unit was only observed in black patients, so it is possible that there also are differences between ethnic groups in the severity of ACE inhibitor associated angioedema [4]. It is also well established that ACE inhibitors have reduced antihypertensive efficacy when used as monotherapy in this group of patients [5].
Table 2.
Case series (>10 cases) of ACE inhibitor associated angioedema.
The mechanism of ACE inhibitor-associated angioedema is not known, although a plausible mechanism involves the vasoactive peptide bradykinin [6, 7]. The increased risk in black patients may be related to racial differences in the kallikrein-kinin system and increased sensitivity to bradykinin [8]. Although the newer angiotensin receptor antagonists exert fewer effects on bradykinin, a number of cases of angioedema have been associated with angiotensin II-receptor antagonists [9, 10].
The onset of angioedema is normally within the first week, although late onset angioedema may occur in up to 25% of cases [11]. The initial presentation is well recognized following years of asymptomatic therapy [12]. Although swelling of the lips, mouth, tongue and airways were recorded in our series other manifestations of ACE-inhibitor-induced angioedema include oedema of the skin and subcutaneous fat, and involvement of the viscera [13, 14], which may cause nausea, diarrhoea and abdominal pain [15–17]. ACE inhibitors should not be prescribed to patients who have a history of heriditary or acquired angioneurotic oedema [18, 19]. The immediate discontinuation of the ACE inhibitor following an episode of angioedema is mandatory, as continued therapy dramatically increases the risk of recurrent angioedema, with serious morbidity [20]. Symptoms of pruritus preceded angioedema in 25% (n=4) of patients in our series and we would therefore recommend that ACE inhibitors are stopped in patients who subsequently develop pruritus.
We conclude that ACE inhibitor related angioedema is more common, and may be more severe, in black patients and our practice is to avoid these agents as first-line antihypertensives in this ethnic group. However, ACE inhibitors remain an important treatment in hypertensive diabetics and patients with impaired left ventricular systolic function. All patients starting ACE inhibitors should be advised to report urticarial symptoms and stop the drug immediately in the event of lip, facial of tongue swelling. Although prompt treatment with adrenaline, steroids and antihistamines may terminate a severe episode of angioedema, clinicians should be aware that mild-moderate swelling may rapidly develop into massive life-threatening swelling, requiring endotracheal intubation and/or tracheostomy. It is important that these episodes are minimized by careful patient counselling and heightened awareness in all clinicians who prescribe this common group of drugs.
References
- 1.Wilkin JK, Hammond JJ, Kirkendall WM. The captopril-induced eruption. A possible mechanism: cutaneous kinin potentiation. Arch Dermatol. 1980;116:902–905. [PubMed] [Google Scholar]
- 2.Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- 3.Kostis JB, Shelton B, Gosselin G, et al. Adverse effects of enalapril in the Studies of Left Ventricular Dysfunction (SOLVD). SOLVD Investigators. Am Heart J. 1996;131:350–355. doi: 10.1016/s0002-8703(96)90365-8. [DOI] [PubMed] [Google Scholar]
- 4.Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- 5.Drayer JIM, Weber MA. Monotherapy of essential hypertension with a converting-enzyme inhibitor. Hypertension. 1983;5(Suppl 3):108–113. doi: 10.1161/01.hyp.5.5_pt_2.iii108. [DOI] [PubMed] [Google Scholar]
- 6.Barrow SE, Dollery CT, Heavey DJ, Hickling NE, Ritter JM, Vial J. Effect of vasoactive peptides on prostacyclin synthesis in man. Br J Pharmacol. 1986;87:243–247. doi: 10.1111/j.1476-5381.1986.tb10177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferner RE, Wilson D, Paterson JR, Wilkinson R, Rawlins MD. The effects of intradermal bradykinin are potentiated by angiotensin-converting enzyme inhibitors in hypertensive patients. Br J Clin Pharmacol. 1989;27:337–342. doi: 10.1111/j.1365-2125.1989.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainer JV, Nadeau JH, Ryder D, Brown NJ. Increased sensitivity to bradykinin among African Americans. J Allergy Clin Immunol. 1996;98:283–287. doi: 10.1016/s0091-6749(96)70151-3. [DOI] [PubMed] [Google Scholar]
- 9.Sharma PK, Yium JJ. Angioedema associated with angiotensin II receptor antagonist losartan. South Med J. 1997;90:552–553. doi: 10.1097/00007611-199705000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Acker CG, Greenberg A. Angioedema induced by the angiotensin II blocker losartan. N Engl J Med. 1995;333:1572. doi: 10.1056/nejm199512073332316. [DOI] [PubMed] [Google Scholar]
- 11.Israili ZH, Dallas Hall W. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. Ann Int Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 12.Chin HL, Buchan DA. Severe angioedema after long-term use of angiotensin-converting enzyme inhibitor. Ann Int Med. 1990;112:312–313. doi: 10.7326/0003-4819-112-4-312_2. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med. 1993;32:424–426. doi: 10.2169/internalmedicine.32.424. [DOI] [PubMed] [Google Scholar]
- 14.Pavletic A. Angioedema of the intestine. N Engl J Med. 1996;335:1534. doi: 10.1056/NEJM199611143352014. [DOI] [PubMed] [Google Scholar]
- 15.Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust. 1996;165:319–321. doi: 10.5694/j.1326-5377.1996.tb124991.x. [DOI] [PubMed] [Google Scholar]
- 16.Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen.] Arch Mal Coeur Vaiss. 1994;87:1371–1374. [PubMed] [Google Scholar]
- 17.Farraye FA, Peppercorn MA. Abdominal pain, angioedema, and angiotensin-converting enzyme inhibitors. Am J Gastroenterol. 1994;89:1117–1118. [PubMed] [Google Scholar]
- 18.Agostini A, Cicardi M. Contraindications to the use of ACE inhibitors in patients with C1 esterase-inhibitor deficiency. Am J Med. 1991;90:278. [PubMed] [Google Scholar]
- 19.Orfan N, Patterson R, Dykewicz MS. Severe angioedema related to ACE inhibitors in patients with a history of idiopathic angioedema. JAMA. 1990;264:1287–1289. [PubMed] [Google Scholar]
- 20.Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor-associated angioedema. JAMA. 1997;278:232–233. doi: 10.1001/jama.278.3.232. [DOI] [PubMed] [Google Scholar]
- 21.Singer DR, MacGregor GA. Angioneurotic oedema associated with two angiotensin converting enzyme inhibitors. Br Med J. 1986;293:1243. [Google Scholar]
- 22.Wood SM, Mann RD, Rawlins MD. Short Report. Angioedema and urticaria associated with angiotensin converting enzyme inhibitors. Br Med J. 1987;294:91–92. doi: 10.1136/bmj.294.6564.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron HA, Higgins TJ. Clinical experience with lisinopril. Observations on safety and tolerability. J Hum Hypertens. 1989;3:177–186. [PubMed] [Google Scholar]
- 24.Lanting PJ, Brouwers TM, van Buuren HR, et al. [Angioedema caused by enalapril] Ned Tijdschr Geneeskd. 1991;135:335–337. [PubMed] [Google Scholar]
- 25.Megerian CA, Arnold JE, Berger M. Angioedema. 5 years’ experience, with a review of the disorders’ presentation and treatment. Laryngoscope. 1992;102:256–260. doi: 10.1288/00005537-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Diehl KL, Wernze H. [Angioneurotic edema caused by angiotensin-converting enzyme inhibitors.] Dtsch Med Wochenschr. 1992;117:727–732. doi: 10.1055/s-2008-1062369. [DOI] [PubMed] [Google Scholar]
- 27.Hedner T, Samuelsson O, Lunde H, Lindholm L, Andren L, Wiholm BE. Angio-oedema in relation to treatment with angiotensin converting enzyme inhibitors. Br Med J. 1992;304:941–946. doi: 10.1136/bmj.304.6832.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabb GM, Ryan P, Wing LM, Hutchinson KA. Epidemiological study of angioedema and ACE inhibitors. Aust NZ J Med. 1996;26:777–782. doi: 10.1111/j.1445-5994.1996.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 29.Pillans PI, Coulter DM, Black P. Angioedema and urticaria with angiotensin converting enzyme inhibitors. Eur J Clin Pharmacol. 1996;51:123–126. doi: 10.1007/s002280050171. [DOI] [PubMed] [Google Scholar]
- 30.Cicardi M, Conciato L, Agostoni A. Angioedema due to angiotensin-converting enzyme inhibition: an association frequently unrecognised. Ann Ital Med Int. 1997;12:8–10. [PubMed] [Google Scholar]
- 31.Agah R, Bandi V, Guntupalli KK. Angioedema: the role of ACE inhibitors and factors associated with poor clinical outcome. Intensive Care Med. 1997;23:793–796. doi: 10.1007/s001340050413. [DOI] [PubMed] [Google Scholar]


