Abstract
Aims
The aim of this cohort study was to estimate the risk of clinical acute liver injury among users of oral antifungals identified in the general population of the General Practice Research Database in UK.
Methods
The cohort included 69 830 patients, 20–79 years old, free of liver and systemic disease, who had received at least one prescription for either oral fluconazole, griseofulvin, itraconazole, ketoconazole, or terbinafine between 1991 and 1996.
Results
Sixteen cases of acute liver injury were identified and validated. Ten cases occurred during nonuse of oral antifungals with a background rate of 0.6 per 100 000 person-months (95% confidence interval 0.3,1.1). Five cases occurred during current use of oral antifungals. Two were using ketoconazole, another two itraconazole, and one terbinafine. Incidence rates of acute liver injury were 134.1 per 100 000 person-months (36.8,488.0) for ketoconazole, 10.4 (2.9–38.1) for itraconazole, and 2.5 (0.4,13.9) for terbinafine. The remaining case was associated with past use of fluconazole. Ketoconazole was the antifungal associated with the highest relative risk, 228.0 (95% confidence interval 33.9,933.0), when compared with the risk among nonusers, followed by itraconazole and terbinafine with relative risks of 17.7 (2.6,72.6) and 4.2 (0.2,24.9), respectively.
Conclusions
Ketoconazole and itraconazole were the two oral antifungal associated with a marked increase of clinical acute liver injury. The risk associated with ketoconazole should be taken into account when prescribing it as initial treatment for uncomplicated fungal infections.
Keywords: acute liver injury, epidemiology, general practice research database, observational studies, oral antifungals
Introduction
Oral antifungals have been associated with different types of acute liver injury in a number of case reports and in several retrospective series. Most of the available data relate to ketoconazole. Transient asymptomatic changes in liver function tests can appear in 0.5% to 10% of patients treated with oral antifungals [1]. Clinical liver injury appears to be less frequent, although the vast majority of data come from cases reported to national pharmacovigilance systems.
Published reviews have reported a higher incidence of hepatic injury associated with ketoconazole than with other systemic antifungals [2, 3]. Some authors have estimated the incidence to lie between one per 1000 and 3000 patients, after taking into account the effect of underreporting in spontaneous monitoring systems [4, 5]. In a randomized clinical trial, patients with onychomycosis treated with ketoconazole had a threefold increased risk of developing hepatitis compared with patients treated with griseofulvin [6]. Several cases of liver injury have been reported in association with griseofulvin [7, 8], itraconazole [9, 10], and terbinafine [11–14], and most cases related with fluconazole have occurred in severely immunodepressed patients [15–20]. In a recent postmarketing surveillance study with 25 884 patients treated with terbinafine, two cases of symptomatic cholestatic hepatic injury considered potentially related to the treatment were identified [21].
In the present cohort study we estimated the risk of clinical acute liver injury among users of either oral fluconazole, griseofulvin, itraconazole, ketoconazole, and terbinafine identified in the general population of UK using the General Practice Research Database (GPRD).
Methods
Source population
Methodology used in GPRD has been detailed elsewhere [22]. GPRD contains clinical computerized information entered by general practitioners (GPs) on their patients. Recorded information includes demographics, details of each general practitioner visit, a summary of specialists’ clinical notes and hospital letters, results of laboratory tests and a free text section. Prescriptions are directly generated by the computer system with dosage instructions included.
A validation study with the GPRD has documented the recording of medical data in the general practitioners’ computers to be near to complete [23]. Several studies on drug-induced acute liver injury have been published using the GPRD resource [24]. These studies were able to quantify the risk of clinical liver injury associated with suspected hepatotoxic drugs.
Study cohort
The study cohort comprised people aged 20–79 years who had received at least one prescription for either oral fluconazole, griseofulvin, itraconazole, ketoconazole, or terbinafine between 1 January 1991 and 30 September 1996. We excluded subjects if they had a history of liver injury in the preceding 5 years. Subjects with a history of any of the following conditions were also excluded: cancer, liver disease, gallbladder disease, pancreatic disease, heart failure, alcohol abuse, HIV infection, rheumatoid arthritis, sarcoidosis, systemic lupus or inflammatory bowel disease. The final study cohort was constituted by 69 830 individuals. We followed these persons from the date of first antifungal drug prescription until the earliest occurrence of a code for liver injury (Table 1), one of the exclusion conditions mentioned above, age greater than 80 years, death or the end of the study period.
Table 1.
OXMIS codes in case ascertainment.
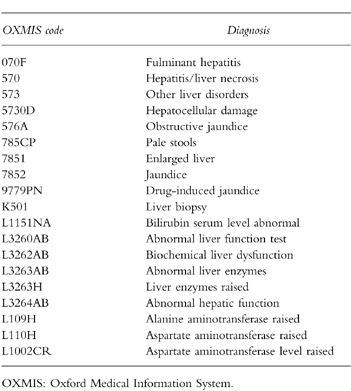
Case ascertainment
We identified 73 patients with a recorded history compatible with acute liver injury. We requested from the general practitioners all clinical records related to these events. Case validation was independently performed by three of the authors (LAGR, BHChS, and AD) without knowledge of the exposure status and agreement was reached on all cases after some discussion. A case of acute liver injury was defined as a person presenting with symptoms suggestive of liver disorder (nausea, vomiting, abdominal pain and/or jaundice) referred to a specialist or admitted to hospital, and who was free of the exclusion criteria. In addition, the following biochemical test results, based on an international consensus meeting, were a requisite as part of the case definition of liver injury [25]: an increase of more than two times the upper limit of the normal range in alanine aminotransferase (ALT), or a combined increase in aspartate aminotransferase (AST), alkaline phosphatase (APh) and total bilirubin, provided any was more than twice the upper limit of the respective normal range. The liver injury was classified as acute if the clinical and laboratory signs had lasted less than 6 months from the date of onset. The type of liver injury was designated hepatocellular when there was an increase more than twice the upper limit of the normal range in ALT alone or R≥5, where R is the ratio of serum activity of ALT over serum activity of APh. Liver injury was designated cholestatic when there was an increase of more than twice the upper limit of the normal range in APh alone or R≤2. Liver injury was designated mixed when 2<R<5.
Exposure definition
Person time at risk was aggregated in three different time windows according to use of the study antifungal drugs. Current use encompassed all the days of prescribed treatment plus an additional period of 14 days at the end of treatment. Past use was defined as the period of 90 days following the time window of current use. Finally, the time period starting after past use was defined as nonuse. We also assessed exposure to a number of suspected hepatotoxic drugs among the cases [26].
Analysis
Incidence rates of acute liver injury were calculated using as denominator both the number of patients exposed to each individual antifungal drug and the corresponding person-time at risk. Ninety-five percent confidence intervals were computed on the basis of a Poisson distribution of case counts within categories of use. The Exact program was used to obtain estimates of rate ratios [27].
Results
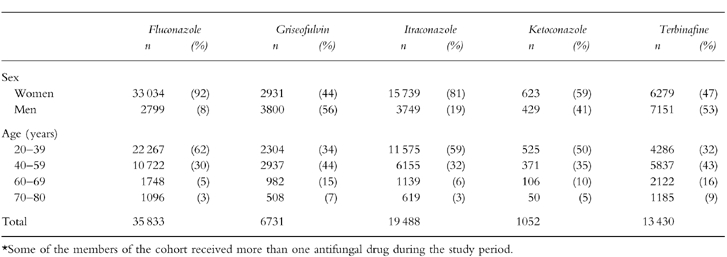
The study cohort of 69 830 subjects received a total of 149 384 prescriptions. There were marked age and sex differences among users of the five antifungal drugs with a higher proportion of women and young patients among fluconazole and itraconazole users (Table 2). These differences were a consequence of varying leading indications: candidiasis for fluconazole, itraconazole and ketoconazole, and onychomycosis for griseofulvin and terbinafine. The average treatment duration was also variable. Seventy-five percent of fluconazole users and 50% of itraconazole users received one single day treatment, respectively. Over 90% of ketoconazole users received less than 1 month of therapy. Close to 80% of terbinafine users were treated for 1–3 months. Griseofulvin users were the group with the longest duration of treatment with one third of patients taking it for a period longer than 3 months.
Table 2.
Age and sex distribution of the study cohort of users of oral antifungals*.
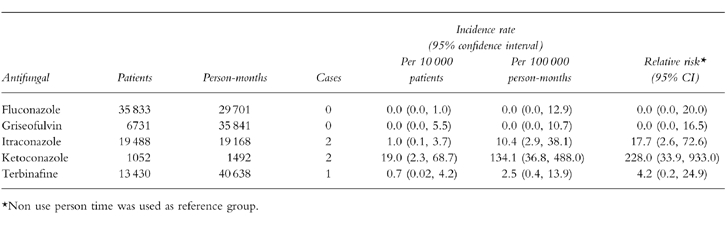
We received medical records for 66 patients (90%). In seven subjects, we did not receive information from the GPs, or the data received were insufficient to ascertain case status. Fifty patients did not meet the study case definition and the reasons for exclusion are presented in Table 3. Most of the patients presented only with minor elevations of LFTs, or had their LFTs’ derangement found through routine monitoring. Thus, 16 individuals met all case definition criteria. Eleven presented with jaundice and four were admitted to a hospital. All cases recovered from the hepatic injury and none of them resulted in a fatal outcome. Table 4 shows the clinical and laboratory features of the 16 cases classified according to their exposure status. Ten cases occurred during nonuse of oral antifungal drugs corresponding to a background rate of 0.6 per 100 000 person-months (95% CI 0.3,1.1). This risk increased considerably with age: 0.4 per 100 000 in persons younger than 60 years and 2.8 per 100 000 in older persons. No material difference was observed between males and females (0.8 vs 0.5 per 100 000 person-months). Half of the cases among nonusers were exposed to a hepatotoxic drug (Table 4). Estimates of incidence rate for current use of individual antifungal drugs are presented in Table 5. Two cases occurred during current use of ketoconazole and itraconazole, respectively, one case with terbinafine, and no cases were found with current use of fluconazole and griseofulvin. All five cases developed during the first month of starting antifungal treatment. Ketoconazole presented the greatest absolute risk with an incidence rate of 134 per 100 000 person-months followed by itraconazole (10.4 per 100 000 person-months) and terbinafine (2.5 per 100 000 person-months). Ketoconazole was the antifungal drug associated with the greatest relative risk compared to the background risk among nonusers (Table 5), followed by itraconazole and terbinafine.
Table 3.
Exclusions after review of medical records.
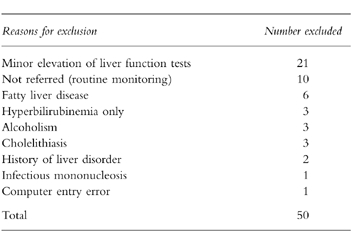
Table 4.
Clinical and laboratory findings and drug exposure of cases of acute liver injury.
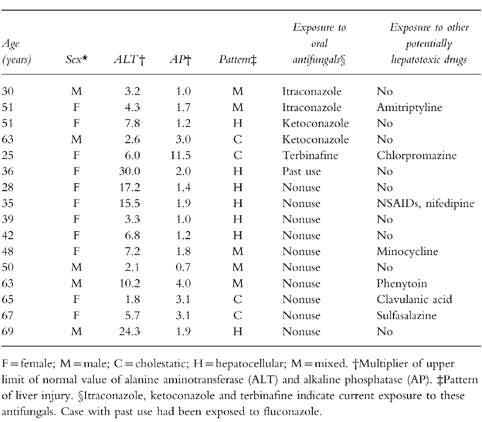
Table 5.
Crude incidence rates of acute liver injury among current users of oral antifungals.
Discussion
In this retrospective cohort study, we assessed the risk of acute liver injury to antimycotics in a large population under everyday circumstances. Because of the low number of detected cases, effect modification by gender or age could not be assessed. Therefore, only crude rates are presented. When compared to the risk in nonusers, the risk of acute liver injury was markedly increased in users of ketoconazole and itraconazole, and slightly increased in users of terbinafine. The risk of acute liver injury among ketoconazole users was the greatest with a relative risk exceeding 200 compared with nonuse. In view of the low background risk of idiopathic symptomatic hepatic injury in the general population, almost 134 extra cases occurred per 100 000 person-months of treatment with ketoconazole. Thus almost 1 in every 500 users of ketoconazole developed acute liver injury. This is higher than has been estimated in earlier studies with the exception of one clinical trial [6]. In a recent review of eight studies that estimated the risk of acute liver injury associated with several drugs using GPRD, only two drugs, chlorpromazine and isoniazid, presented a comparable level of increased risk of liver injury, (incidence greater than one per 1000 users) [24].
In the current study, it is highly unlikely that the results are explained by bias or confounding. Because the complete patient population attended by about 1500 general practitioners served as the source population for identifying our study cohort, selection bias is rather unlikely. Patients with a history of liver disease were excluded. This means that our estimates of absolute risk for all oral antifungal drugs are conservative as a consequence of depletion of susceptible persons. Information bias might result from the awareness of doctors and patients that ketoconazole and itraconazole might cause liver injury. If this leads to more frequent visits and laboratory assessments, the ascertainment of liver injury might differ between antimycotics. Our study, however, was restricted to symptomatic cases which makes such information bias an improbable explanation for our results. Also confounding by indication is unlikely as uncomplicated candidiasis (1 case), tinea pedis (1 case) and mycotic skin infections (3 cases) are not known as risk factors for acute liver injury.
The risk of hepatic injury was highest with ketoconazole and itraconazole. Both agents are azole derivatives which have been associated with several cases of symptomatic hepatic injury but most reports concern ketoconazole [3–5, 9, 10]. Among the cases with ketoconazole reported in the literature, the onset was mostly within 6 months after starting therapy with 200–400 mg daily [25]. Biochemically, the pattern was mostly hepatocellular but several cases of mixed cholestatic-hepatocellular or cholestatic injury have also been reported. Most patients recovered after discontinuation but several cases of fatal hepatic necrosis have been reported. Histology confirmed the hepatocellular pattern in most patients, varying from cellular unrest to confluent centrolobular or even massive necrosis. In high doses, ketoconazole induced significant hepatic injury in several animal species after 2–4 weeks administration. Although the precise mechanism is unknown, it has been speculated that hepatotoxicity of ketoconazole could result from its interference with membrane sterol synthesis or by inhibiting hydrogen peroxidase degrading enzymes, e.g. catalase and cytochrome c peroxidase [28].
In conclusion, ketoconazole and itraconazole were the two oral antifungal drugs associated with a marked increased risk of clinical acute liver injury in our study. Specially the high risk observed with ketoconazole may be an argument for reevaluating its role as initial treatment for uncomplicated fungal infections and restricting its use to deep mycoses.
Acknowledgments
We thank to Dr Felix Arellano for his early contribution in the design of the study, and to the staff at EPIC and the participating general practitioners for their excellent collaboration.
CEIFE received a research grant from Novartis. Dr Stricker has no financial relationships with Novartis nor with CEIFE.
References
- 1.Hay RJ. Risk/benefit ratio of modern antifungal therapy. Focus on hepatic reactions. J Am Acad Dermatol. 1993;29:s50–s4. doi: 10.1016/s0190-9622(08)81838-5. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KR, Schiff ER. Hepatotoxicity of antimicrobial, antifungal, and antiparasitic agents. Gatroenterol Clinics North Am. 1995;24:923–926. [PubMed] [Google Scholar]
- 3.Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86:503–513. [PubMed] [Google Scholar]
- 4.Stricker BHCh, Blok APR, Bronkhorst FB, Van Parys GE, Desmet VJ. Ketoconazole-associated hepatic injury. A clinicopathological study of 55 cases. J Hepatol. 1986;3:399–406. doi: 10.1016/s0168-8278(86)80495-0. [DOI] [PubMed] [Google Scholar]
- 5.Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J. 1987;294:419–422. doi: 10.1136/bmj.294.6569.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien RN, Yang LJ, Lin PY, Liaw YF. Hepatic injury during ketoconazole therapy in patients with onychomycosis: a controlled cohort study. Hepatology. 1997;25:103–107. doi: 10.1002/hep.510250119. [DOI] [PubMed] [Google Scholar]
- 7.Chiprut RO, Viteri A, Jamroz C, Dyck WP. Intrahepatic cholestasis after griseofulvin administration. Gastroenterology. 1976;70:1141–1143. [PubMed] [Google Scholar]
- 8.Breinstrup AH, Sogaard-Anderson J. Cholestasis intrahepatica efter griseofulvinbehandling. Ugeskr Laeger. 1966;128:145–147. [PubMed] [Google Scholar]
- 9.Gallardo-Quesada S, Luelmo-Aguilar J, Guanyabens-Calvet C. Hepatotoxicity associated with itraconazole. Int J Dermatol. 1995;34:589. doi: 10.1111/j.1365-4362.1995.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 10.Lavrijsen APM, Balmus KJ, Nugteren Huying WM, Roldaan AC, van’T Wout JW, Stricker BHCh. Hepatic injury associated with itraconazole. Lancet. 1992;340:251–252. doi: 10.1016/0140-6736(92)90527-a. [DOI] [PubMed] [Google Scholar]
- 11.Lowe G, Green C, Jennings P. Hepatitis associated with terbinafine treatment. Br Med J. 1993;306:248. doi: 10.1136/bmj.306.6872.248-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldewijn OY, Ottervanger JP, Mostart CM, Janssens AR, Calame J, Jonkers GJ. Hepatitis attributed to the use of terbinafine. Ned Tijdschr Geneeskd. 1996;140:669–672. [PubMed] [Google Scholar]
- 13.van’T Wout JW, Herrmann WA, de Vries RA, Stricker BHCh. Terbinafine-associated hepatic injury. J Hepatol. 1994;21:115–117. doi: 10.1016/s0168-8278(94)80146-0. [DOI] [PubMed] [Google Scholar]
- 14.Lazaros GA, Papatheodoridis GV, Delladetsima JK, Tassopoulos NC. Terbinafine-induced cholestatic liver disease. J Hepatol. 1996;24:753–756. doi: 10.1016/s0168-8278(96)80273-x. [DOI] [PubMed] [Google Scholar]
- 15.Gearhart MO. Worsening of liver function with fluconazole and review of azole antifungal hepatotoxicity. Ann Pharmacother. 1994;28:1177–1181. doi: 10.1177/106002809402801009. [DOI] [PubMed] [Google Scholar]
- 16.Franklin JM, Ellas E, Hirsch C. Fluconazole induced jaundice. Lancet. 1990;336:565. doi: 10.1016/0140-6736(90)92120-7. [DOI] [PubMed] [Google Scholar]
- 17.Munoz P, Moreno S, Berenguer J, Bernaldo de Quiros JL, Bouza E. Fluconazole related hepatotoxicity in patients witch acquired immunodeficiency syndrome. Arch Intern Med. 1991;151:1020–1021. doi: 10.1001/archinte.1991.00400050150032. [DOI] [PubMed] [Google Scholar]
- 18.Trujillo MA, Galgiani JN, Sampllner RF. Evaluation of hepatic injury arising during fluconazole therapy. Arch Intern Med. 1994;154:102–104. [PubMed] [Google Scholar]
- 19.Wells C, Lever AM. Dose-dependent fluconazole hepatotoxicity proved on biopsy and rechallenge. J Infect. 1992;24:111–112. doi: 10.1016/0163-4453(92)91346-d. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson MA, Hanks DK, Ferrell LD. Fatal acute hepatic necrosis due to fluconazole. Am J Med. 1994;96:188–190. doi: 10.1016/0002-9343(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 21.Hall M, Monka C, Krupp P, O’Sullivan D. Safety of oral terbinafine: results of a postmarketing surveillance study in 25 884 patients. Arch Dermatol. 1997;133:1213–1219. doi: 10.1001/archderm.133.10.1213. [DOI] [PubMed] [Google Scholar]
- 22.García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–425. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García Rodríguez LA, Ruigómez A, Jick H. A review of epidemiologic research on drug-induced acute liver injury using the General Practice Research Database in the United Kingdom. Pharmacotherapy. 1997;17:721–728. [PubMed] [Google Scholar]
- 25.Report of an international consensus meeting. criteria of drug-induced liver disorders. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 26.Stricker BH. Drug-induced hepatic injury. Amsterdam, The Netherlands: Elsevier Science Publishers; 1992. [Google Scholar]
- 27.Martin D, Austin H. An efficient program for computing conditional maximum likelihood and exact confidence limits for a common odds ratio. Epidemiology. 1991;2:359–362. doi: 10.1097/00001648-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Park BK, Kitteringham NR, Pirmohamed M, et al. Relevance of induction of human drug-metabolizing enzymes: pharmacological and toxicological implications. Br J Clin Pharmacol. 1996;41:477–491. doi: 10.1046/j.1365-2125.1996.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


