Abstract
Aims
Azathioprine is a prodrug commonly used in combination therapy to prevent allograft rejection after renal transplantation. After conversion to 6-mercaptopurine, the drug is metabolized into 6-thioguanine nucleotides (6-TGN) and catabolized by thiopurine methyltransferase (TPMT), an enzyme under monogenic control. The aim of this study was to evaluate the inter- and intraindividual variability of red blood cell thiopurine methyltransferase and 6-TGN concentrations and their relationship to the clinical effects of azathioprine in paediatric patients.
Methods
In the present study, the interand intraindividual variations in red blood cell TPMT activity and 6-TGN concentrations and their relationship to the actions of azathioprine were evaluated during the first year after renal transplantation in 22 paediatric patients.
Results
6-TGN concentration reached steady-state values after 6 months and correlated negatively with TPMT activity (P=0.004). Initial TPMT activity (median: 20.8 nmol h−1 ml−1, range 7.8–34.6) and 6-TGN concentration at steady-state (median: 80 pmol 8×108–1 cells, range not detected to 366) were not related to the occurrence of rejection episodes during the period of the study. In contrast, TPMT activity and the percentage difference in TPMT activity from the day of transplantation determined at month 1 were higher in the patients with rejection episodes by comparison with those that did not reject during the first 3 months or the first year following transplantation (P<0.005).
Conclusions
We report a relationship between TPMT activity and occurrence of rejection in paediatric kidney transplant patients undergoing azathioprine therapy. These data suggest a link between high red blood cell TPMT activity and poor clinical outcome probably caused by rapid azathioprine catabolism.
Keywords: azothioprine, paediatric, renal transplant, thiopurine methyltransferase
Introduction
Azathioprine and 6-mercaptopurine are 6-thiopurine prodrugs requiring intracellular metabolism to produce cytotoxicity [1]. In paediatric patients, azathioprine is commonly administered in association with cyclosporin and corticosteroids to prevent allograft rejection after renal transplantation [2]. 6-Mercaptopurine is used in the treatment of acute lymphoblastic leukaemia.
In vivo, azathioprine is converted rapidly to 6-mercaptopurine, which is metabolized via three competing pathways. The anabolic route for 6-mercaptopurine is metabolism by hypoxanthine guanine phosphoribosyltransferase to active 6 thiopurine nucleotides, mainly 6-thioguanine nucleotides (6-TGN), which are incorporated into DNA [3]. 6-Mercaptopurine is also oxidized by xanthine oxidase and together with the 6-thiopurine nucleotides is S-methylated by thiopurine S-methyltransferase (TPMT; E.C. 2.1.1.67). TPMT activity is polymorphic and under monogenic control. Approximately 89% of Caucasians are homozygous for the high activity alleles, 11% are heterozygous and about 1 subject in 300 individuals inherits a deficiency in TPMT as an autosomal recessive trait [4]. Three major point mutations in the TPMT gene (localized on chromosome 6p22.3) are responsible for low thiopurine methyltransferase activity [5–8]. The G238C mutation in exon 5 and the combination of two mutations in exon 7 (G460A) and 10 (A719G) are the most common defects leading to the inactivating alleles TPMT*2 A and TPMT*3 A. These are associated with the loss of catalytic activity caused by rapid degradation of the neo-synthetized protein by an ATP dependent proteasome mediated pathway [9].
High 6-TGN concentrations have been related to bone marrow failure in patients undergoing azathioprine therapy [10–13]. Furthermore, 6-TGN concentrations were negatively correlated with TPMT activity in leukaemic patients receiving 6-mercaptopurine [14]. Therefore, patients homozygous for recessive alleles encoding low TPMT activity are at higher risk of severe myelosuppression. In addition, significant modifications of TPMT activity have been observed during 6-thiopurine therapy. TPMT activity was reported either to remain constant or to increase during the first three months after renal transplantation in patients being treated with azathioprine [15]. In leukaemic patients, TPMT was also found to increase during 6-mercaptopurine therapy with a return to predrug values when 6-mercaptopurine was stopped [16].
TPMT is expressed in various organs and cells, and erythrocyte activity was demonstrated to correlate with kidney and liver activities [17, 18]. In the present study, red blood cell TPMT and 6-TGN concentrations were measured repeatedly during the first year after transplantation in paediatric renal allograft recipients in order to evaluate their interand intraindividual variability and their relationship to the clinical effects of azathioprine.
Methods
Patients and protocol
Twenty-two paediatric renal transplant recipients (9 girls and 13 boys) aged 11±5 years (mean±sd) and weighing 30±13 kg (mean±s.d.) were included in this prospective study. The immunosuppressant protocol included thymoglobulins, azathioprine, corticosteroids and cyclosporin. Azathioprine was administered at an intravenous dose of 2 mg kg−1 on the day of transplantation and subsequently at a standard daily oral dose of 1 mg kg−1. Corticosteroids were administered at a standard dosage of 60 mg m−2 daily and rapidly reduced to 30 mg m−2 during the first month to achieve a 15 mg m−2 dosage 2 months later. After an initial intravenous phase, cyclosporin was administered orally to achieve a whole blood concentration of 150–250 ng ml−1. Rejection episodes were confirmed by a renal biopsy and were treated with intravenous methylprednisolone. No patients received frusemide or related compounds during the period of the study.
Erythrocyte 6-TGN concentrations were determined during the follow up visit at day 8, month 1, month 6 and month 12 after transplantation. Erythrocyte TPMT activity was determined on the day of transplantation and then at month 1, month 6 and month 12 after transplantation.
The study was approved by the Ethics Committee of Paris Bichat-Claude Bernard and written informed consent was given by the parents of all the patients.
H.p.l.c. assay for the determination of red cell 6-thioguanine nucleotides concentrations
Blood samples (5 ml) were rapidly centrifuged to isolate red cells. After washing the pellets twice with saline, red cells were counted to normalize 6-TGN concentrations to 8×108 cells and an aliquot was stored at −80° C until analysis. 6-TGN concentrations were determined by modifications of the h.p.l.c. method reported previously [19]. Briefly, red blood cells (100 μl) were diluted in water containing 10 mg dithiotreitol to a final volume of 1 ml and deproteinized with 100 μl perchloric acid (70%, v/v). After centrifugation (4500 rev min−1, 10 min, 4° C), the supernatant was removed and heated for 45 min at 100° C. After cooling, a 50 μl aliquot was injected onto the chromatographic system. Calibration curves were constructed with drug free erythrocyte lysates spiked with 6-thioguanine. The chromatographic system consisted of a C18 reversed phase column (Hypersil ODS, 3 μm, 150×4.6 mm, purchased from C.I.L., Sainte Foie la Grande, France) with u.v. detection of 6TG at 342 nm. Extraction efficiency of 6-TGN from protein precipitation, at a concentration of 60 pmol/8×108 cells (10 ng ml−1) was 73% (coefficient of variation: 6.8%, n=7). Between day precision was 7% at a concentration of 60 pmol/8×108 cells (10 ng ml−1) and 3% at a concentration of 600 pmol/8×108 cells (100 ng ml−1) (n=5). The limit of detection was 5 ng ml−1and the lower limit of quantification was 10 ng ml−1.
H.p.l.c. assay for the determination of red cell thiopurine methyltransferase activity
Blood sample preparation and incubation conditions were similar to those previously described by Jacqz-Aigrain & Médard [20, 21]. Briefly, the amount of methyl 6-mercaptopurine formed during incubation of erythrocyte lysate in the presence of 6-mercaptopurine and S-adenosyl methionine (as the methyl donor) was measured by h.p.l.c. after a liquid-liquid extraction with dichloromethane-isopropyl alcohol. Results were expressed as nmol of methyl 6-mercaptopurine formed h−1 ml−1 of packed red blood cells. The 6-methylmercaptopurine calibration curve was linear over the range of 10–150 ng ml−1. The intra-assay and interassay coefficients of variation over the concentration range of 10–150 ng ml−1 were less than 10%. A quality control lysate was included in each assay run. Mean within-day activity was 13.0 nmol h−1 ml−1 (coefficient of variation: 2.6%, n=5) and mean between-day TPMT activity was 13.6 nmol h−1 ml−1 (coefficient of variation: 5.2%, n=8).
Determination of TPMT genotypes
TPMT genotypes were determined in 19 out of the 22 patients included in the study. Total genomic DNA was isolated from peripheral blood leucocytes using a conventionnal chloroform/phenol extraction procedure [22]. The known mutations in exon 5 (G238C), exon 7 (G460A) and exon 10 (A719G) were detected by PCR assays, as reported previously [23].
The nomenclature proposed by Weinshilboum and coworkers [6] was used for the different TPMT alleles. The TPMT wild type gene has been designated TPMT*1, the allele containing the G238C mutation on exon 5 TPMT*2. The mutations G460A and A719G were presumed to be combined on the same allele TPMT*3 A as hypothesised previously [6–8].
Statistical analysis
6-TGN concentrations and TPMT activities are expressed as median and range. The statistical analysis was performed using nonparametric tests. Correlations between variables were evaluated with Spearman rank test. Intraindividual differences were compared using the repeated measure anova (Friedman) and Wilcoxon tests. In order to perform a Friedman anova test in all patients, the mean TPMT value between month 6 and month 12 was calculated as the TPMT activity after month 1 was not available for two patients. The relationships between the number of rejection episodes that occurred during the period of the study and 6-TGN concentration, TPMT activity, cyclosporin concentration, creatinine concentration and creatinine clearance were assessed using multivariate anova (Kruskall-Wallis anova) and Mann-Witney tests. Two-tailed P-values less than 0.05 were accepted as statistically significant. All statistics were performed using Statview software (Abacus Concepts Inc., Berkeley, CA, USA).
Results
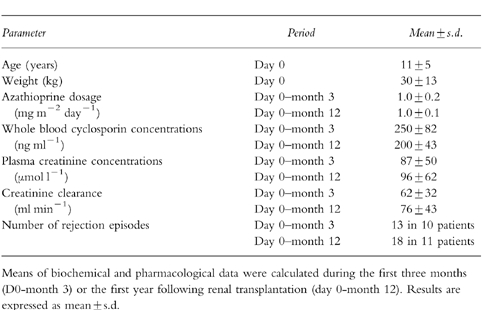
The age, weight, biological and pharmacological parameters of our patients (n=22) are summarised in Table 1. During the period of the study, no patients experienced leucopaenia or other side-effects requiring azathioprine withdrawal for more than 1 week. The azathioprine dosage was adjusted according to the peripheral white blood cell and platelet counts and ranged from 0.6 to 1.2 mg m−2 day−1 (median 1.0 mg m−2 day−1). The median intrapatient coefficient of variation for azathioprine dosage was 11.9% (range 0–38%). No significant difference was detected between dosages at 1, 6 and 12 months (Fr=0.97; P >0.50).
Table 1.
Age and weight, biochemical and pharmacological parameters determined in the 22 renal transplant patients.
Intraindividual variation in red blood cell 6-TGN concentrations
Red blood cell 6-TGN increased initially and reached steady state after 6 months. However, inter-patient variation in 6-TGN was very large. Median values (range) were 25 pmol/8×108 cells at day 8 (not detected to 103; n=22), 41 pmol/8×108 cells at month 1 (not detected to 166; n=19), 80 pmol/8×108 cells at month 6 (range: not detected to 366; n=19) and 62 pmol/8×108 cells at month 12 (range: not detected to 372; n=16). 6-TGN concentrations measured after 8 days and 1 month of treatment were similar (Wilcoxon test, P >0.30) and steady state was achieved between 6 and 12 months since metabolite concentrations did not differ between these time points (Wilcoxon test, P >0.50). Thus, mean 6-TGN concentrations were calculated during the first month (from values measured at day 8 and month 1) and at steady state. The difference in 6TGN concentrations tested between month 1 and steady-state was significant (Fr=13.23, P=0.0003, n=19) and the median intrapatient coefficient of variation in 6-TGN concentrations was 80.5% (range 5.7–200.). Individual values are presented in Table 2. 6-TGN was not detectable in six patients during the first month post-transplantation and for two of them, remained undetectable after month 6.
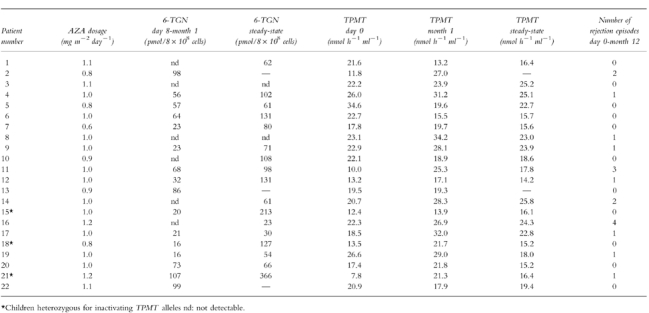
Table 2.
Azathioprine dosage, 6-TGN concentrations and TPMT activity determined during the first year post transplantation in the 22 renal transplant patients.
Intraindividual variation in red blood cell TPMT activity
TPMT activity was determined sequentially from day 0 to month 12. The median TPMT activity was 20.8 nmol h−1 ml−1 (range 7.8–34.6; n=22) on the day of transplantation, 21.7 nmol h−1 ml−1 (range: 13.2–34.3; n=22) at month 1 and 18.3 nmol h−1 ml−1 (range: 14.2–25.8; n=20) between month 6 and month 12. The intraindividual variation in TPMT activity tested between day 0, month 1 and steady-state was significant (Fr=6.10; P=0.047) and the median intrapatient coefficient of variation was 17.1% (range 0.9–55.5).
After 1 month of treatment, large differences in TPMT activity between initial values at day 0 were found (−43.5% to 174.1%, median 20.1%; n=22) together with a significant negative correlation between the two parameters (P=0.003). The patient with the lowest initial TPMT activity (7.8 nmol h−1 ml−1) experienced an increase of 174% at month 1, whereas the one with the highest activity (34.6 nmol h−1 ml−1) experienced a reduction of 40% during the first month post-transplantation. Individual data are presented in Table 2. The relationship between 6-TGN concentration and TPMT activity was not significant during the first month (r=−0.185; P=0.407) but a negative correlation was detected at steady-state (r=−0.622; P=0.004). TPMT activity did not correlate significantly with creatinine clearance or creatinine concentration at any time post-transplantation (P >0.15).
Relationship between 6-TGN, TPMT and the occurrence of rejection episodes
During the period of the study, no patient experienced leucopaenia requiring azathioprine withdrawal. However, 11 of the 22 patients experienced 18 allograft rejection episodes (median 50 days post-transplantation, range 5–331) and 13 of them (72%) occurred in 10 patients during the first three months (median 41 days, range 5–91). The relationship between the occurrence of rejection and the biochemical (creatinine concentrations and clearances) and pharmacological (6-TGN concentrations and TPMT activities) variables was tested during the first 3 months (day 0-month 3) and the first year post-transplantation (day 0-month 12).
Cyclosporin concentrations, creatinine concentrations and clearances were not different in the patients who rejected and in those who did not reject during the first three months (n=10 vs 12,Table 3) or during the first year post-transplantation (n=11 vs 11). However, creatinine concentrations were higher in patients who rejected than in the patients who did not reject during the first 3 months (P=0.06) (Table 3).
Table 3.
Comparison of biochemical and pharmacological parameters in patients who did (n=10) and did not (n=12) experience rejection episodes during the first 3 months post renal transplantation (number of patients).
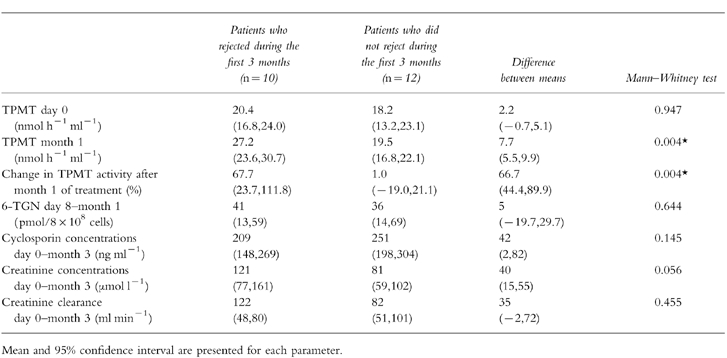
There was no difference in 6-TGN concentrations between the patients who did and did not experienced rejection during the first 3 months post-transplantation. At 6-TGN steady state, five rejection episodes occurred in four patients, but no link was found between the occurrence of rejection and 6-TGN concentrations (P=0.341).
Initial TPMT activity measured at day 0 was not different in the patients who rejected (n=10) and those who did not reject (n=12) during the first three months post-transplantation. TPMT activity at month 1 and the percentage change in TPMT activity from day 0 determined at month 1 were higher in the patients who rejected during the first three months (n=10,Table 3) and the first year post-transplantation (n=11) (P<0.05). In addition, the 11 patients who did not reject during the first year had TPMT activity below 25.0 nmol h−1 ml−1 while 16 out of 18 (88%) rejection episodes occurred in nine patients having TPMT activity over 25.0 nmol h−1 ml−1 (range 25.3–34.2) at month 1. The three patients in whom TPMT activity increased by over 100% at month 1, experienced 6 rejection episodes, 5 of which occurred in the 2 patients having a TPMT activity over 25.0 nmol h−1 ml−1.
TPMT genotype in relation to enzyme activity
TPMT genotypes were determined in 19 of the 22 patients. Sixteen (84%) were found to be homozygous for wild type alleles and had an initial TPMT activity ranging from 10.0 to 34.6 nmol h−1 ml−1 (median 21.9 nmol h−1 ml−1). Three patients were heterozygous for inactivating alleles (two patients genotyped TPMT*1/*3 A with an initial TPMT activity of 7.8 and 13.5 nmol h−1 ml−1, and one patient genotyped TPMT*1/*2 with an initial TPMT activity of 12.4 nmol h−1 ml−1). The patient genotyped TPMT*1/*2 did not show an increase in TPMT activity during the period of the study and did not reject, while TPMT activity in the two patients genotyped TPMT*1/*3 A increased by 60.9 and 174.1% at month 1 (Figure 1).
Figure 1.
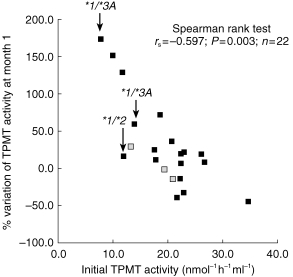
Correlation between initial TPMT activity, measured on the day of transplantation (day 0) and the percentage change after the first month of azathioprine treatment. TPMT genotype is quoted for the three heterozygous patients ( : genotype not available).
: genotype not available).
Discussion
Our patients received a standard dose of 1 mg kg−1 of azathioprine orally and TPMT activity and 6-TGN concentrations in red blood cells were monitored during the first year post-transplantation. The 6-TGN concentrations increased during the first 6 months of treatment and then remained stable. Median 6-TGN concentrations were below 100 pmol/8×108 RBC during the period of the study and no patient experienced leucopaenia requiring withdrawal of azathioprine. Similar low concentrations of RBC 6-TGN were also previously reported in adult renal allograft recipients receiving low doses of azathioprine [11]. As 6-TGNs are pharmacologically and therapeutically active metabolites, the administration of higher doses of azathioprine, at least in the first months of treatment should be considered in this patient group. In addition, wide interindividual variation in 6-TGN concentrations was found at steady state. At least part of this variability could be related to differences in TPMT activity, as demonstrated by the negative correlation between TPMT activity and 6-TGN concentrations at steady state.
In adult renal transplant recipients receiving azathioprine, TPMT activity was reported either to remain stable or to increase during the first 3 months post-transplantation [15]. In our study, individual TPMT activity varied widely and a significant correlation was detected between the initial pretransplant TPMT activity and its subsequent change after 1 month (P<0.01). This suggests that the variation in TPMT activity under azathioprine could be predicted from the initial value, measured at day 0. The increase in TPMT activity at the initiation of azathioprine therapy could be due to enzyme induction by its substrate, 6-mercaptopurine [24], but this would not take place in peripheral red blood cells in the absence of protein synthesis. Other mechanisms, beside induction of enzyme activity, may also be involved in the changes in TPMT activity that we observed. We demonstrated previously that steroids, cyclosporin and trimethoprime had no effects on TPMT activity in vitro [20], while sulphalazine [25] and frusemide [26] could inhibit TPMT in vitro. In the present study, no patient received frusemide or related compounds. However, many other drugs currently administrated with thiopurines, could modify TPMT activity and should be tested. The mechanism leading to the apparent increase of TPMT activity remains unknown, but it would lead to a significant increase of the catabolism of 6-mercaptopurine.
In renal transplant recipients, the relationship between TPMT activity and clinical outcome remains controversial [15, 24, 27]. In our study, 13 rejection episodes confirmed by a renal biopsy, occurred in 10 of 22 patients during the first 3 months and 18 occurred in 11 of 22 patients during the first year post-tranplantation. The initial TPMT activity or the concentrations of the active 6-TGN metabolites had no predictive value for rejection. In contrast, both TPMT activity at month 1 and the percentage of change from baseline determined at month 1 were significantly higher in the patients who rejected than in the patients who had no rejection during the first three months or the first year post-transplantation. In addition, 16 of the 18 rejection episodes occurred in nine patients with TPMT activity over 25.0 nmol h−1 ml−1. Therefore, our results are in agreement with previous studies reporting that initial TPMT activity did not predict the occurrence of rejection [27] and that high TPMT activity in adult kidney transplant receiving azathioprine were related to a worse clinical outcome [24]. Indeed, high TPMT activity would increase the catabolism of azathioprine and reduce the amount of active 6-TGN. In addition, TPMT activity in red blood cells is correlated with the TPMT activity in hepatocytes [17] and if similar changes in TPMT activity occur in the liver during azathioprine treatment, the catabolism of 6-mercaptopurine would increase, subsequently decreasing the amount of drug available for cellular immunosuppression. It is also possible that rejection per se associated with deterioration of renal function, modifies TPMT activity in treated patients In uraemic patients under haemodialysis, a significant increase of TPMT activity was reported and the presence and role of inhibitors were discussed [24, 28]. Moreover, the concentration of S-adenosyl homocysteine, an endogenous inhibitor of S-adenosyl-methionine-dependent reactions, such as thiopurine methyltransferase, was reported to increase in renal failure [29]. Although this would cause a decrease in TPMT activity, it is also possible that rejection episodes associated with deterioration of renal function could lead to an increase in TPMT activity.
Weinshilboum reported initially a trimodal distribution of TPMT activity in the American population [4] while TPMT activity in the European population was not trimodally distributed [8]. In our study, the overlap in TPMT activity between patients genotyped as heterozygous (n=3) or homozygous (n=16) for high TPMT alleles was observed at the initiation and during treatment with azathioprine. Further studies are required to investigate such overlap as well as the mechanism implicated in the variation of TPMT activity under treatment. Genetic differences in the TPMT gene promoter region could be implicated [8].
In conclusion, we studied the relationship between erythrocyte TPMT activity and the occurrence of rejection episodes in paediatric kidney transplant patients undergoing azathioprine therapy. Our data suggest that a high TPMT activity is associated with an increased risk of rejection, possibly related to a high rate of catabolism of azathioprine.
Acknowledgments
This work was supported by ′ L’Institut National de la Santé et de la Recherche Médicale (INSERM) and by ‘L’Agence Française du Médicament’ (1996). We thank Caroline Delnevo for secretarial assistance.
References
- 1.Elion G. The purine path to chemotherapy. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 2.Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43:329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- 3.Van Scoïk K, Johnson A. The pharmacology and metabolism of the thiopurine drugs 6-mercaptopurine and azathioprine. Drug Metab Rev. 1985;16:157–174. doi: 10.3109/03602538508991433. [DOI] [PubMed] [Google Scholar]
- 4.Weinshilboum R, Sladek S. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocytes thiopurine methytransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 5.Krynetski E, Schuetz J, Galpin A, Pui C, Relling M, Evans W. A single point mutation leading to loss catalytic activity in human thiopurine methyltransferase. Proc Natl Acad Sci USA. 1995;14:949–953. doi: 10.1073/pnas.92.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szumlanski C, Otterness D, Chentao H, et al. Thiopurine methyltransferase pharmacogenetics: human gene cloning and characterization of a common polymorphism. DNA Cell Biol. 1996;15:17–30. doi: 10.1089/dna.1996.15.17. [DOI] [PubMed] [Google Scholar]
- 7.Tai H, Krynetski E, Yates C, et al. Thiopurine S-methyltransferase deficiency: two nucleotides transition define the most prevalent mutant allele associated with loss of catalytic activity in caucasian. Am J Hum Genet. 1996;58:694–702. [PMC free article] [PubMed] [Google Scholar]
- 8.Spire-Vayron de la Moureyre C, Debuysere H, Mastain B, Vinner E, Marez D, Broly F. Genotype and phenotype analysis of the polymorphic thiopurine S-methyltransferase gene (TPMT) in a European population. Br J Pharmacol. 1998;125:879–887. doi: 10.1038/sj.bjp.0702152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai H, Krynetski E, Schuetz E, Yanishevski Y, Evans W. Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant allele in humans (TPMT*3A, TPMT*2): Mechanism for the genetic polymorphism of TPMT activity. Proc Natl Acad Sci USA. 1997;94:6444–6449. doi: 10.1073/pnas.94.12.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstey A, Lennard L, Mayou S, Kirby J. Pancytopenia related to azathioprine—an enzyme deficiency caused by a common genetic polymorphism: review. J R Soc Med. 1992;85:752–756. doi: 10.1177/014107689208501213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergan S, Rugstad H, Bentdal O, Stokke O. Monitoring azathioprine treatment by determination of 6-thioguanine nucleotide concentrations in erythrocytes. Transplantation. 1994;58:803–808. [PubMed] [Google Scholar]
- 12.Lennard L, Van Loon J, Weinshilboum R. Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 13.Schutz E, Gummert J, Armstrong V, Oellerich M. Should 6-thioguanine nucleotides be monitored in heart transplant recipients given azathioprine. Ther Drug Monit. 1996;18:228–233. doi: 10.1097/00007691-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Lennard L, Van Loon J, Lilleyman M, Weinshilboum R. Thiopurine pharmacogenetics in leukemia: Correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987;41:18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- 15.Mircheva J, Legendre C, Soria Royer C, Thervet E, Beaune P, Kreis H. Monitoring of azathioprine-induced immunosuppression with thiopurine methyltransferase activity in kidney transplant recipients. Transplantation. 1995;60:639–642. doi: 10.1097/00007890-199510150-00003. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod H, Relling M, Liu Q, Pui C, Evans W. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995;85:1897–1902. [PubMed] [Google Scholar]
- 17.Szumlanski C, Honcel R, Scott M, Weinshilboum R. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isoenzymes. Pharmacogenetics. 1992;2:148–159. [PubMed] [Google Scholar]
- 18.Woodson L, Dunnetees J, Weinshilboum R. Pharmacogenetics of human thiopurine methyltransferase: Kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982;222:174–181. [PubMed] [Google Scholar]
- 19.Boulieu R, Lenoir A. High performance liquid chromatographic determination of thiopurine metabolites of azathioprine in biological fluids. J Chromatogr B Biomed Appl. 1995;615:352–356. doi: 10.1016/0378-4347(93)80353-6. [DOI] [PubMed] [Google Scholar]
- 20.Jacqz-Aigrain E, Bessa E, Médard Y, Mircheva Y, Vilmer E. Thiopurine methyltransferase activity in a french population: HPLC assay conditions and effect of drugs and inhibitors. Br J Clin Pharmacol. 1994;38:1–8. doi: 10.1111/j.1365-2125.1994.tb04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Médard Y, Nafa S, Jacqz-Aigrain E. Thiopurine methyltransferase: new high performance liquid chromatographic assay conditions. J Chromatogr B Biomed Appl. 1997;700:275–277. doi: 10.1016/s0378-4347(97)00287-9. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis T, Sambroock J, Fritsh E. Commonly used techniques in molecular cloning. In: Ford N, Nolan C, Ferguson M, Ockler M, editors. Molecular Cloning, a laboratory manual. Cold Spring Harbor Laboratory Press; 1982. pp. E1–E8. [Google Scholar]
- 23.Yates C, Krynetski E, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: Genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Chocair P, Duley J, Simmonds H, Cameron J. The importance of thiopurine methyltransferase activity for the use of azathioprine in transplant recipients. Transplantation. 1992;53:1051–1056. doi: 10.1097/00007890-199205000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Szumlanski C, Weinshilboum R. Sulphazalasine inhibition of thiopurine methyltransferase: a possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995;39:456–459. doi: 10.1111/j.1365-2125.1995.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lysaa R, Giverhaug T, Wold H, Aarbakke J. Inhibition of human thiopurine methyltransferase by furosemide, bendroflumethiazide and trichlormethiazide. Eur J Clin Pharmacol. 1996;49:393–396. doi: 10.1007/s002280050038. [DOI] [PubMed] [Google Scholar]
- 27.Bergan S, Rugstad H, Klemetsdal B, et al. Possibilities for therapeutic drug monitoring of azathioprine: 6-thioguanine nucleotide concentrations and thiopurine methyltransferase activity in red blood cells. Ther Drug Monit. 1997;19:318–326. doi: 10.1097/00007691-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Pazmino P, Sladek S, Weinshilboum R. Thiol S-methylation in uremia: erythrocytes enzymes activities and plasma inhibitors. Clin Pharmacol Ther. 1983;28:356–367. doi: 10.1038/clpt.1980.174. [DOI] [PubMed] [Google Scholar]
- 29.Perna A, Ingrosso D, Zappia V, Galletti P, Capasso G, De Santo N. Enzymatic esterification of erythrocyte membrane protein is impaired in chronic renal failure: evidence for high levels of the natural inhibitor S-adenosyl homocysteine. J Clin Invest. 1993;91:2497–2503. doi: 10.1172/JCI116485. [DOI] [PMC free article] [PubMed] [Google Scholar]


