Abstract
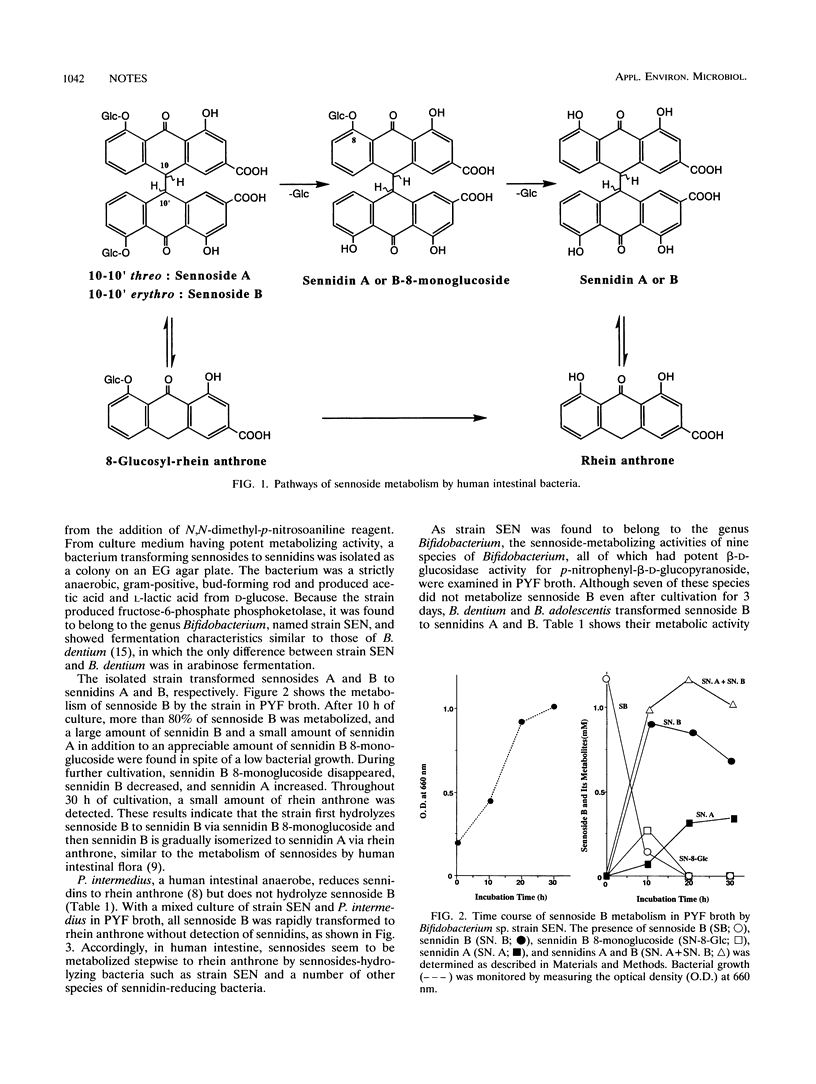
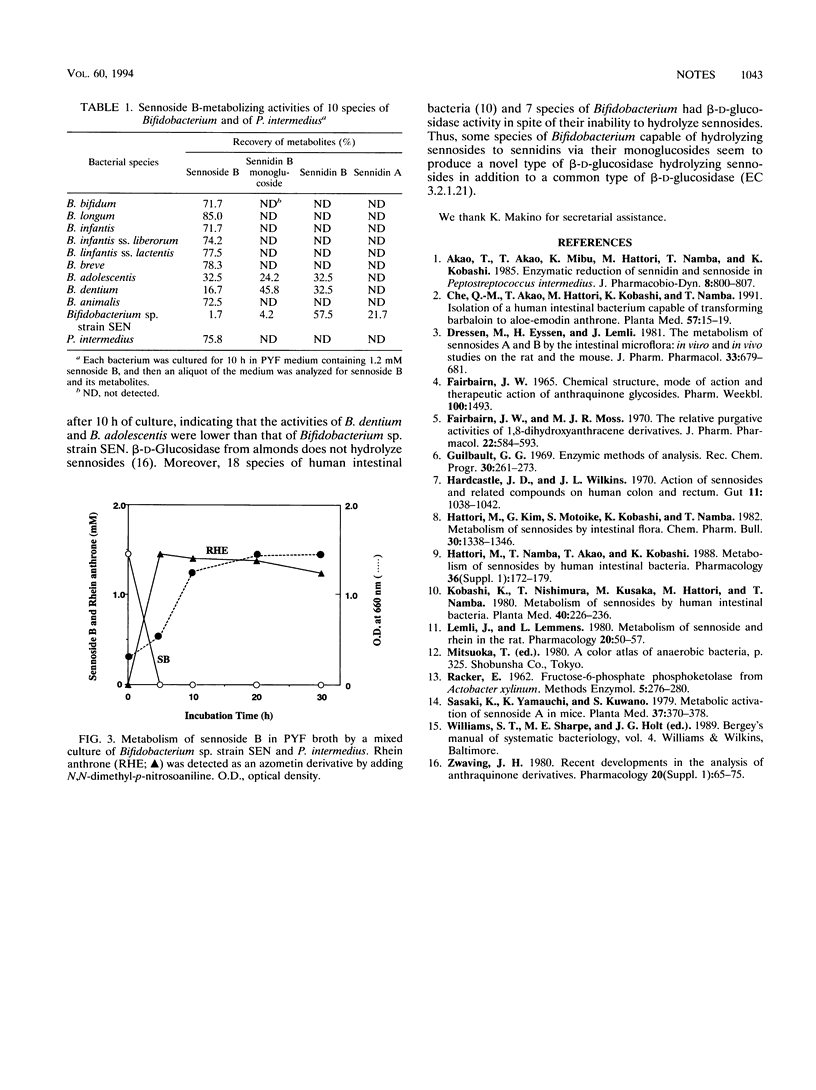
A strictly anaerobic bacterium capable of metabolizing sennosides was isolated from human feces and identified as Bifidobacterium sp., named strain SEN. The bacterium hydrolyzed sennosides A and B to sennidins A and B via sennidin A and B 8-monoglucosides, respectively. Among nine species of Bifidobacterium having beta-glucosidase activity, only Bifidobacterium dentium and B. adolescentis metabolized sennoside B to sennidin B, suggesting that the sennoside-metabolizing bacteria produce a novel type of beta-glucosidase capable of hydrolyzing sennosides to sennidins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akao T., Akao T., Mibu K., Hattori M., Namba T., Kobashi K. Enzymatic reduction of sennidin and sennoside in Peptostreptococcus intermedius. J Pharmacobiodyn. 1985 Oct;8(10):800–807. doi: 10.1248/bpb1978.8.800. [DOI] [PubMed] [Google Scholar]
- Che Q. M., Akao T., Hattori M., Kobashi K., Namba T. Isolation of a human intestinal bacterium capable of transforming barbaloin to aloe-emodin anthrone. Planta Med. 1991 Feb;57(1):15–19. doi: 10.1055/s-2006-960007. [DOI] [PubMed] [Google Scholar]
- Dreessen M., Eyssen H., Lemli J. The metabolism of sennosides A and B by the intestinal microflora: in vitro and in vivo studies on the rat and the mouse. J Pharm Pharmacol. 1981 Oct;33(10):679–681. doi: 10.1111/j.2042-7158.1981.tb13903.x. [DOI] [PubMed] [Google Scholar]
- Fairbairn J. W. Chemical structure, mode of action and therapeutical activity of anthraquinone glycosides. Pharm Weekbl. 1965 Dec 17;100(51):1493–1499. [PubMed] [Google Scholar]
- Fairbairn J. W., Moss M. J. The relative purgative activities of 1,8-dihydroxyanthracene derivatives. J Pharm Pharmacol. 1970 Aug;22(8):584–593. doi: 10.1111/j.2042-7158.1970.tb10575.x. [DOI] [PubMed] [Google Scholar]
- Guilbault G. G. Enzymic methods of analysis. Rec Chem Prog. 1969 Dec;30(4):261–273. [PubMed] [Google Scholar]
- Hardcastle J. D., Wilkins J. L. The action of sennosides and related compounds on human colon and rectum. Gut. 1970 Dec;11(12):1038–1042. doi: 10.1136/gut.11.12.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Kim G., Motoike S., Kobashi K., Namba T. Metabolism of sennosides by intestinal flora. Chem Pharm Bull (Tokyo) 1982 Apr;30(4):1338–1346. doi: 10.1248/cpb.30.1338. [DOI] [PubMed] [Google Scholar]
- Hattori M., Namba T., Akao T., Kobashi K. Metabolism of sennosides by human intestinal bacteria. Pharmacology. 1988;36 (Suppl 1):172–179. doi: 10.1159/000138437. [DOI] [PubMed] [Google Scholar]
- Kobashi K., Nishimura T., Kusaka M., Hattori M., Namba T. Metabolism of sennosides by human intestinal bacteria. Planta Med. 1980 Nov;40(3):225–236. doi: 10.1055/s-2008-1074963. [DOI] [PubMed] [Google Scholar]
- Lemli J., Lemmens L. Metabolism of sennosides and rhein in the rat. Pharmacology. 1980;20 (Suppl 1):50–57. doi: 10.1159/000137398. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yamauchi K., Kuwano S. Metabolic activation of sennoside A in mice. Planta Med. 1979 Dec;37(4):370–378. doi: 10.1055/s-0028-1097352. [DOI] [PubMed] [Google Scholar]
- Zwaving J. H. Recent developments in the analysis of anthraquinone derivatives. Pharmacology. 1980;20 (Suppl 1):65–75. doi: 10.1159/000137400. [DOI] [PubMed] [Google Scholar]



