Abstract
Aims
The present study was designed to define the kinetic behaviour of sertraline N-demethylation in human liver microsomes and to identify the isoforms of cytochrome P450 involved in this metabolic pathway.
Methods
The kinetics of the formation of N-demethylsertraline were determined in human liver microsomes from six genotyped CYP2C19 extensive (EM) and three poor metabolisers (PM). Selective inhibitors of and specific monoclonal antibodies to various cytochrome P450 isoforms were also employed.
Results
The kinetics of N-demethylsertraline formation in all EM liver microsomes were fitted by a two-enzyme Michaelis-Menten equation, whereas the kinetics in all PM liver microsomes were best described by a single-enzyme Michaelis-Menten equation similar to the low-affinity component found in EM microsomes. Mean apparent Km values for the high-and low-affinity components were 1.9 and 88 μm and Vmax values were 33 and 554 pmol min−1 mg−1 protein, respectively, in the EM liver microsomes. Omeprazole (a CYP2C19 substrate) at high concentrations and sulphaphenazole (a selective inhibitor of CYP2C9) substantially inhibited N-demethylsertraline formation. Of five monoclonal antibodies to various cytochrome P450 forms tested, only anti-CYP2C8/9/19 had any inhibitory effect on this reaction. The inhibition of sertraline N-demethylation by anti-CYP2C8/9/19 was greater in EM livers than in PM livers at both low and high substrate concentrations. However, anti-CYP2C8/9/19 did not abolish the formation of N-demethylsertraline in the microsomes from any of the livers.
Conclusions
The polymorphic enzyme CYP2C19 catalyses the high-affinity N-demethylation of sertraline, while CYP2C9 is one of the low-affinity components of this metabolic pathway.
Keywords: CYP2C19, CYP2C9, cytochrome P450, demethylsertraline, drug metabolism, human liver microsomes, monoclonal antibody, omeprazole, pharmacogenetics, selective serotonin reuptake inhibitor, sertraline, sulphaphenazole
Introduction
Sertraline is a potent and selective serotonin reuptake inhibitor (SSRI) in the central nervous system and is used to treat depression and obsessive-compulsive behaviour [1, 2]. Previous studies have found that SSRIs differentially inhibit the activity of various cytochrome P450 isoforms, including CYP1A2 [3, 4], CYP2D6 [5], CYP2C19 [6, 7], CYP2C9 [8] and CYP3A4 [9, 10]. Compared with paroxetine, fluoxetine and fluvoxamine, sertraline exhibits the most favourable profile in relation to drug interactions because it only inhibits the activities of these enzymes mildly or moderately at usual therapeutic doses [11]. Out of the above cytochrome P450 isoforms sertraline has the lowest inhibitory constant (Ki = 0.70 μm) in vitro for CYP2D6 [5] and is most likely to be involved in clinically important interactions with substrates of this enzyme. However, the low plasma concentrations of sertraline present in patients would lessen the potential for such interactions. To date, no sertraline–induced drug interactions of clinical importance in vivo have been observed, although coadministration of sertraline gives rise to modest elevations in the plasma concentrations of desipramine and nortriptyline through inhibition of CYP2D6 [12, 13]. On the other hand, studies of the possible influences of potent inhibitors or inducers of cytochrome P450 on hepatic sertraline clearance have not been thoroughly evaluated. The identification of the enzymes responsible for the metabolism of sertraline should allow physicians to anticipate and avoid unwanted drug interactions.
Sertraline is extensively metabolized by the hepatic cytochrome P450 enzymes and less than 2.5% of the drug is excreted unchanged in human urine. Sertraline mainly undergoes N-demethylation leading to the formation of the inactive metabolite N-demethylsertraline [1, 2]. However, little is known with regard to the isoforms of cytochrome P450 responsible for the metabolism of sertraline. The disposition of this drug and its demethyl metabolite does not cosegregate with the polymorphic oxidation of debrisoquine, indicating that CYP2D6 is not a major enzyme involved in sertraline biotransformation [14]. However, the competitive inhibition of S-mephenytoin 4′-hydroxylase (CYP2C19) and phenytoin p-hydroxylase (CYP2C9) activity by sertraline in human liver microsomes raises the possibility that sertraline may be a substrate for these two cytochrome P450 isoforms [6, 8].
To test the role of CYP2C19 in sertraline N-demethylation, we have studied the kinetics of sertraline N-demethylation by human liver microsomes from 6 extensive (EM) and 3 poor metabolizers (PM) with respect to CYP2C19. Various selective chemical inhibitors and specific monoclonal antibodies were also utilized to identify the isoforms of cytochrome P450 involved in sertraline N-demethylation.
Methods
Chemicals and monoclonal antibodies
Sertraline and N-demethylsertraline were supplied by Pfizer Inc. Quinidine, troleandomycin, diethyldithiocarbamate, coumarin, NADP+, glucose-6-phosphate and glucose-6-phosphate dehydrogenase were purchased from Sigma Chemical Co. (St Louis, USA). Sulphaphenazole and ketoconazole were gifts from CibaGeigy Ltd, (Basel, Switzerland) and Janssen Research Foundation (Beerse, Belgium), respectively. Furafylline was kindly donated by Dr W. Pfleiderer (University Konstanz, Germany). Omeprazole was obtained from Astra Hässle AB (Mölndal, Sweden). Inhibitory monoclonal antibodies to human CYP1A2, 2A6, 2D6, 2C8/9/19 and 3A4/5 and antilysozyme monoclonal antibody (HyHel, IgG) as a control for the immunoinhibition experiments were generously donated by Drs T. J. Yang and H. V. Gelboin (Laboratories of Molecular Carcinogenesis and Metabolism, NIH, Bethesda, Maryland). All other supplies were of the highest grades available from commercial sources.
Preparation of human liver microsomes
Adult human liver tissue from renal transplant donors without known liver disease and patients who had undergone partial hepatectomy were collected in our liver bank. The collection and utilization of human liver tissues were approved by the Ethics Committee of Hunan Medical University. Candidate patients for liver sample collection were those who did not suffer from acute or chronic hepatitis or cirrhosis, and took no medications known to induce or inhibit cytochrome P450 activity. Portions of surgical liver ‘waste tissue’ distant from disease-affected regions and which appeared visually normal were collected. After removal, the liver sample was immediately cut into small pieces, washed with icecold isotonic saline, rapidly frozen in liquid nitrogen for 30 min, and was then stored at −80° C. Prior to use, all samples were confirmed as being normal histologically.
Donors were genotyped with respect to CYP2C19 from whole blood or liver tissue according to the method of de Morais et al. [15]. Livers from six EM and three PM subjects were used for this study. All the PMs were homozygous for the m1 mutation of CYP2C19.
Washed microsomes were prepared by differential centrifugation [16] and stored at −80° C until required. Microsomal protein concentrations were determined by the method of Lowry et al. [17].
In vitro incubation conditions
The incubation mixtures contained 100 mm phosphate buffer (pH 7.4), 0.5 mg ml−1 liver microsomes, 1.0 mm NADP+, 5 mm glucose-6-phosphate, 2 IU ml−1 glucose-6-phosphate dehydrogenase, 0.5 mm EDTA, 5 mm MgCl2 and sertraline (1–200 μm) in a final volume 0.5 ml. Preliminary experiments showed that the formation of N-demethylsertraline was linear with respect to time over 40 min and with respect to microsomal protein concentration (0.1–0.75 mg ml−1) at 37° C. Thus a 20 min incubation time and a 0.5 mg ml−1 microsomal protein concentration were selected for subsequent work. The reactions were terminated by placing the tubes immediately in ice-cold water and adding diethyl ether:hexane (80:20, 6 ml). All incubations were performed in duplicate except those involving monoclonal antibodies where because of limited quantities, single incubations were performed.
Assay and kinetics of N-demethylsertraline formation
N-Demethylsertraline was determined by gas chromatography with electron-capture detection based on the method developed by Tremaine et al. [18]. Only a one-step extraction with diethyl ether and hexane (80:20) was used, and the internal standard was diazepam rather than CP53 680. Chromatography was performed on a 5% phenylmethyl silicone capillary column (30 m× 0.25 mm, i.d., Alltech, Dalian, China). The acyl derivatives of N-demethylsertraline, diazepam and sertraline were well separated with retention times of 10.9, 12.4 and 15.2 min, respectively. The cytochrome P450 inhibitors used in the study did not produce interfering peaks. The lower limit of detection for N-demethylsertraline was 0.01 μm, and the assay had a between day variation of 6.7%.
In the kinetic experiments, 10 concentrations of sertraline (1–200 μm) were incubated with human liver microsomes from six EMs and three PMs. Several enzyme kinetic equations proposed by Schmider et al. [19] were fitted to the untransformed data (Figperfect, Version 5.0). The most appropriate model was selected on the basis of the dispersion of residuals and whether an F-test showed a significant reduction (P < 0.05) in the residual sums of squares. The following two equations best described the kinetics of sertraline N-demethylation in liver microsomes from EM and PM subjects, respectively.
| 1 |
| 2 |
where Km1, Vmax1 correspond to the high affinity, low capacity site and Km2, Vmax2correspond to the low affinity, high capacity site.
Inhibition studies
Selective inhibitors [20–23] of and monoclonal antibodies to cytochrome P450 isoforms were screened for their effects on human liver microsomal sertraline N-demethylation at substrate concentrations of 50 μm and/or 5 μm concentrations, at which the low-and high-affinity components of sertraline N-demethylase, respectively, were dominant.
Initial chemical inhibition studies were performed with four liver microsomal preparations (three EMs and one PM) at a substrate concentration of 50 μm. The selective inhibitors used were furafylline (20 μm, CYP1A2 inhibitor), coumarin (200 μm, CYP2A6 substrate), quinidine (10 μm, CYP2D6 inhibitor), diethyldithiocarbamate (20 μm, CYP2E1 inhibitor), troleandomycin (50 μm, CYP3A4 inhibitor), ketoconazole (1.0 μm, CYP3A4 inhibitor), sulphaphenazole (20 μm, CYP2C9 inhibitor), omeprazole (100 μm, CYP2C19 substrate), and sulphaphenazole (20 μm) plus omeprazole (100 μm) [20–23]. Except for diethyldithiocarbamate, which was dissolved in water, solutions of the inhibitors were prepared in methanol. Since methanol can have an inhibitory effect on cytochrome P450 activity, solutions were evaporated to dryness prior to incubation. The mechanism-based inhibitors, furafylline, troleandomycin and diethyldithiocarbamate, were preincubated with microsomes and the NADPH generating system at 37° C for 15 min.
To assess the inhibitory potency of omeprazole and sulphaphenazole, a range of concentrations of omeprazole (0–250 μm) and sulphaphenazole (0–50 μm) was incubated with sertraline in three EM microsomal preparations at a low (5 μm) and high (50 μm) substrate concentration, respectively. We also examined the difference in the inhibitory potency of omeprazole (25 μm) at the low and high substrate concentrations.
The inhibitory effect of monoclonal antibodies specific to CYP1A2, CYP2A6, CYP2D6 and CYP3A4/5 was examined in three liver microsomal preparations (two EM, one PM) at a substrate concentration of 50 μm sertraline. However, immunoinhibition of anti-CYP2C8/9/19 was performed in three EM and three PM liver microsomal preparations at both low (5 μm) and high (50 μm) substrate concentrations of sertraline because the kinetic analysis and chemical inhibition studies showed an important role of CYP2C in sertraline N-demethylation. The antibodies were preincubated with microsomes at 37° C for 5 min. The ratio by weight of antibody protein to microsomal protein ranged from 1:20 to 1:5.
A one-way t-test for unpaired and paired data was used to determine the significance of differences in the inhibitory effects of chemical inhibitors and monoclonal antibodies, with P < 0.05 as the minimal level of significance.
Results
Kinetics for N-demethylsertraline formation
After iteratively fitting the different mathematical models proposed by Schmider et al. [19] to the untransformed kinetic data, we found that the kinetics of N-demethylsertraline formation in all EMs of CYP2C19 followed a two-enzyme Michaelis-Menten equation (Equation 1, Figure 1), whereas the kinetics in all PMs were best described by a single-enzyme Michaelis-Menten equation (Equation 2, Figure 2). The kinetic parameters for sertraline N-demethylation in EMs and PMs are shown in Table 1. In the liver microsomes from PMs, the high-affinity component of enzyme activity was absent. Compared with EMs, the formation of N-demethylsertraline in PM liver microsomes was significantly slower, especially at low substrate concentrations (data not shown). The mean intrinsic clearance (Vmax1/Km1) of the high-affinity component was 2.7 times that (Vmax2/Km2) of the low-affinity component in EM liver microsomes.
Figure 1.
Typical substrate vs velocity and Eadie-Hofstee plots for the formation of N-demethylsertraline in human liver microsomes from an EM with respect to CYP2C19 (HL-A). The values are the means of duplicate incubations.
Figure 2.
Typical substrate vs velocity and Eadie-Hofstee plots for the formation of N-demethylsertraline in human liver microsomes from PM with respect to CYP2C19 (HL-H). The values are the means of duplicate incubations.
Table 1.
Kinetic parameters for sertraline N-demethylation in human liver microsomes from six extensive metabolizers and three poor metabolizers of CYP2C19.

Inhibition with selective chemical inhibitors and monoclonal antibodies
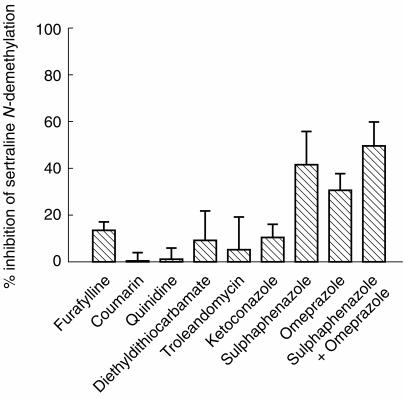
Omeprazole (100 μm) and sulphaphenazole (20 μm) caused a mean 30.4% (P < 0.01) and 41.5% (P < 0.01) reduction in the formation of N-demethylsertraline, respectively, at a substrate concentration of 50 μm. Furafylline, ketoconazole and diethyldithiocarbamate inhibited this reaction to a minor extent. No effect was observed for troleandomycin, quinidine and coumarin. The addition of omeprazole (100 μm) plus sulphaphenazole (20 μm) resulted in maximal inhibition of 49.3% (P < 0.01) for this metabolic pathway (Figure 3).
Figure 3.
Effects of selective cytochrome P450 inhibitors, furafylline (20 μm), coumarin (200 μm), quinidine (10 μm), diethyldithiocarbamate (20 μm), troleandomycin (50 μm), ketoconazole (1.0 μm), sulphaphenazole (20 μm), omeprazole (100 μm), and sulphaphenazole (20 μm) plus omeprazole (100 μm) on the formation of N-demethylsertraline in human liver microsomes (n = 4, three EMs: HL-A, HL-B, HL-F; one PM: HL-I) at a substrate concentration of 50 μm sertraline. The values are the mean inhibition percentage (±s.d).
Omeprazole was only a weak inhibitor of the high-affinity site of N-demethylsertraline formation (Figure 4a). Although inhibition was relatively weak, it was greater at the low (5 μm) substrate concentration than at the high (50 μm) substrate concentration when 25 μm of omeprazole was added.
Figure 4.
Effects of omeprazole (left, Figure 4a) and sulphaphenazole (right, Figure 4b) on the formation of N-demethylsertraline in human liver microsomes (n = 3, three EMs: HL-A, HL-B, HL-F). The substrate (sertraline) concentration used in Figure 4(a) and (b) was 5 and 50 μm sertraline, respectively. The values are the mean inhibition percentage (±s.d).
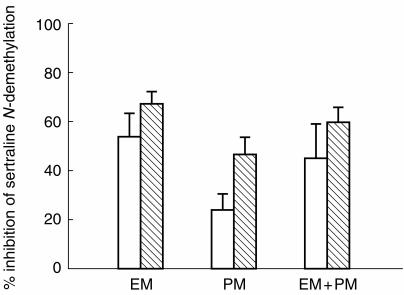
Of the five antibodies tested, only anti-CYP2C8/9/19 substantially inhibited the formation of N-demethylsertraline in human liver microsomes (Figure 5). No appreciable effect was seen for anti-CYP1 A2, 2 A6, 2D6 and 3 A4/5 even at a ratio of antibody:microsomal protein of 1:5. Inhibition by anti-CYP2C8/9/19 using a ratio of 1:5 antibody:microsomal protein was about the same as that found with a ratio of 1:10 in all six livers tested at both low (5 μm) and high (50 μm) substrate concentrations. Thus we assumed that the maximal inhibitory effect was achieved at a ratio of 1:5. At the high (50 μm) substrate concentration, the addition of anti-CYP2C8/9/19 resulted in a greater inhibition than at low (5 μm) substrate concentration, both with EM (67.7%vs 54.0%, P < 0.05) and PM (46.6%vs 24.3%, P < 0.01) liver microsomes (Figure 6). Inhibition of N-demethylsertraline formation by the anti-CYP2C8/9/19 antibody for the EM microsomes (54.0% at 5 μm; 67.7% at 50 μm) was greater than that for the PM microsomes (24.3%, 46.6%), both at the low (P < 0.01) and high (P < 0.01) substrate concentrations (Figure 6).
Figure 5.
Effects of specific monclonal antibodies to cytochrome P450 on the formation of N-demethylsertraline in human liver microsomes at a substrate concentration of 50 μm sertraline. The immunoinhibition studies for anti-CYP1A2, 2A6, 2D6 and 3A4/5 were performed with three liver microsomal preparations (n = 3, two EMs: HL-A, HL-B; one PM: HL-I), but those for anti2C8/9/19 were performed with six liver microsomal preparations (n = 6, three EMs: HL-A, HL-B, HL-C; three PMs: HL-G, HL-H, HL-I). The values are the mean inhibition percentage (±s.d).
Figure 6.
The mean maximum inhibitory effect of anti-CYP2C8/9/19 on the formation of N-demethylsertraline in EM (HL-A, HL-B, HL-C) and PM (HL-G, HL-H, HL-I) microsomes at low (5 μm, □) and high (50 μm,  ) substrate concentrations. The values are the mean inhibition percentage (±s.d).
) substrate concentrations. The values are the mean inhibition percentage (±s.d).
Discussion
We observed biphasic enzyme kinetics for the formation of N-demethylsertraline from sertraline in EM microsomes. These data indicate clearly that at least two enzymes are involved in this reaction which possess high-and low-affinity components. N-Demethylation exhibited monophasic enzyme kinetics in liver microsomes from PMs and lacked the high-affinity component. The mean Km value (1.9 μm) for the high-affinity component in EM liver microsomes is almost the same as a previously reported Ki value (2.0 μm) for the inhibition of S-mephenytoin 4′-hydroxylation, a CYP2C19 substrate, by sertraline. This finding together with the data from the genotyped livers suggest that the high-affinity component for sertraline N-demethylase is CYP2C19. Since the intrinsic clearance of the high-affinity component is 2.7 times that of the low-affinity, CYP2C19 is probably the major enzyme contributing to sertraline N-demethylation in vivo.
Recently, omeprazole has been proposed to act as a potent selective inhibitor of CYP2C19 activity [23]. At a concentration of 10 μm omeprazole strongly inhibits the formation of both 4′-hydroxymephenytoin from S-mephenytoin (>90%) [23] and cycloguanil from proguanil (47%) in vitro [24], two reactions catalysed by CYP2C19. When assessing the inhibitory potency of omeprazole on sertraline N-demethylation, a low substrate concentration (5 μm) was chosen since CYP2C19 is the high-affinity site of this reaction. However, in our experiments omeprazole at 25 μm, the highest concentration within ‘the window of selectivity’ [23] inhibited the high-affinity N-demethylation of sertraline (5 μm) by only 27%. We estimate that at this substrate concentration the high-affinity CYP2C19 activity represents about 45% of the total activity. Thus omeprazole appears to abolish only about half the CYP2C19 activity at this concentration. This can be explained by the lower affinity of omeprazole for CYP2C19 (Km = 6.0 μm) [25] than that of sertraline (Km = 1.9 μm, present data). When incorporating these values into the Michaelis-Menten equation for competitive inhibition, a decrease in the high-affinity N-demethylase activity of 20–30% would be predicted. Thus, our experiments demonstrate that the use of omeprazole as a selective inhibitor of CYP2C19 may be limited when it is employed to assess the contribution of CYP2C19 to the metabolism of a drug with very high-affinity for this enzyme. Sulphaphenazole (20 μm), a selective and potent inhibitor of CYP2C9 [20, 21, 26] inhibited sertraline N-demethylation by up to 41.5% at a 50 μm substrate concentration, indicating the involvement of CYP2C9. The occurrence of significant inhibition by anti-CYP2C8/9/19 in PM livers also supports the involvement of a CYP2C isoform other than CYP2C19. Since the high-affinity component of sertraline N-demethylation is absent in liver microsomes from PMs with respect to CYP2C19, CYP2C9 is likely to be the low affinity site of enzyme activity. Omeprazole also inhibits CYP2C9 activity [23], which is consistent with the finding that omeprazole resulted in a concentration-dependent decrease in N-demethylsertraline formation above the 25 μm ‘selectivity window’ threshold.
Since the mean maximum inhibition of sertraline (50 μm) N-demethylation by anti-CYP2C8/9/19 was about 60% in liver microsomes from all 6 livers tested, at least one or more other enzymes must catalyse sertraline N-demethylation. The present study shows that CYP1A2, 2A6, 2D6, 2E1 and 3A4/5 are not involved. In PMs with respect to CYP2C19, inhibition of sertraline (50 μm) N-demethylation by anti-CYP2C8/9/19 is about 50%, suggesting that the unidentified enzymes make an almost equal contribution to sertraline N-demethylation in those with an absence of CYP2C19 activity.
In summary, we conclude that the polymorphic CYP2C19 enzyme is the high-affinity CYP isoform catalysing sertraline N-demethylation in human liver microsomes, and that CYP2C9 is one of the low-affinity components responsible for this metabolic pathway.
Acknowledgments
This study was supported by China Medical Board of New York (grant no. 92–568) and National Natural Scientific Foundation of China (grant no. F39330230). We are very grateful to Ms. Yi-Qing Chen and Mr Xiao-Ping Wu (Environmental Protection Monitoring Center of Hunan Province, Changsha, Hunan) for their technical assistance in the GC-analysis of sertraline and demethylsertraline, and to Dr Richard Weinshilboum and Ms Carol Szumlanski (Department of Pharmacology, Mayo Clinic, USA) for critical reading of this manuscript.
References
- 1.Heym J, Koe BK. Pharmacology of sertraline: a review. J Clin Psychiatry. 1988;49(Suppl):40–45. [PubMed] [Google Scholar]
- 2.Murdoch D, McTavish D. Sertraline, a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992;44:604–624. doi: 10.2165/00003495-199244040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Brøsen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S. Fluvoxamine is a potent inhibitor of cytochrome CYP1A2. Biochem Pharmacol. 1993;45:1211–1214. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen BB, Mäenpää J, Pelkonen O, et al. Selective serotonin reuptake inhibitors and theophylline metabolism in human liver microsomes: potent inhibition by fluvoxamine. Br J Clin Pharmacol. 1995;39:151–159. doi: 10.1111/j.1365-2125.1995.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effects of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi K, Yamamoto T, Chiba K, Tani M, Ishizaki T, Kuroiwa Y. The effects of selective serotonin reuptake inhibitors and their metabolites on S-mephenytoin 4′-hydroxylase activity in human liver microsomes. Br J Clin Pharmacol. 1995;40:481–485. doi: 10.1111/j.1365-2125.1995.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu ZH, Xie HG, Zhou HH. In vivo inhibition of CYP2C19 but not CYP2D6 by fluvoxamine. Br J Clin Pharmacol. 1996;42:518–521. doi: 10.1046/j.1365-2125.1996.45319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmider J, Greenblatt DJ, von Moltke LL, Karsov D, Shader RI. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol. 1997;44:495–498. doi: 10.1046/j.1365-2125.1997.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Moltke LL, Greenblatt DJ, Cotreau-Bibbo MM, Harmatz JS, Shader RI. Inhibitors of alprazolam metabolism in vitro: effect of serotonin-reuptake-inhibitor antidepressants, ketoconazole and quinidine. Br J Clin Pharmacol. 1994;38:23–31. doi: 10.1111/j.1365-2125.1994.tb04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ring BJ, Binkley SN, Roskos L, Wrighton SA. Effect of fluoxetine, norfluoxetine, sertraline and demethylsertraline on human CYP3A catalyzed 1′-hydroxy midazolam formation in vitro. J Pharmacol Exp Ther. 1995;275:1131–1135. [PubMed] [Google Scholar]
- 11.Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. An overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokin. 1997;32(Suppl 1):1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ereshefsky L, Riesenman C, Lam YW. Antidepressant drug interactions and cytochrome P450 system. The role of cytochrome P450 2D6. Clin Pharmacokin. 1995;29(Suppl):10–18. doi: 10.2165/00003088-199500291-00004. [DOI] [PubMed] [Google Scholar]
- 13.Solai LK, Mulsant BH, Pollock BG, et al. Effect of sertraline on plasma nortriptyline levels in depressed elderly. J Clin Psychiatry. 1997;58:440–443. doi: 10.4088/jcp.v58n1006. [DOI] [PubMed] [Google Scholar]
- 14.Hamelin BA, Turgeon J, Vallee F, Belanger PM, Paquet F, LeBel M. The disposition of fluoxetine but not sertraline is altered in PMs of debrisoquin. Clin Pharmacol Ther. 1996;60:512–521. doi: 10.1016/S0009-9236(96)90147-2. [DOI] [PubMed] [Google Scholar]
- 15.de Morais SMF, Goldstein JA, Xie HG, et al. Genetic analysis of the S-mephenytion polymorphism in a Chinese population. Clin Pharmacol Ther. 1995;58:404–411. doi: 10.1016/0009-9236(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 16.von Bahr C, Groth CG, Jansson H, Lundgren G, Lind M, Glaumann H. Drug metabolism in human liver in vitro: Establishment of a human liver bank. Clin Pharmacol Ther. 1980;27:711–725. doi: 10.1038/clpt.1980.102. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with Folin phenol reagents. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Tremaine LM, Joerg EA. Automated gas chromatographic-electron-capture assay for the selective serotonin uptake blocker sertraline. J Chromatogr. 1989;496:423–429. doi: 10.1016/s0378-4347(00)82590-6. [DOI] [PubMed] [Google Scholar]
- 19.Schmider J, Greenblatt DJ, Harmatz JS, Shader RI. Enzyme kinetic modelling as a tool to analyse the behavior of cytochrome P450 catalyzed reactions: application to amitriptyline N-demethylation. Br J Clin Pharmacol. 1996;41:593–604. doi: 10.1046/j.1365-2125.1996.35717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton DJ, Wang RW, Lu AYH. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1995;23:154–158. [PubMed] [Google Scholar]
- 21.Eagling VA, Tjia JF, Back DJ. Differential selectivity of cytochrome P450 inhibitors against probe substrate in human and rat liver microsomes. Br J Clin Pharmacol. 1998;45:107–114. doi: 10.1046/j.1365-2125.1998.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson T, Miners JO, Veronese ME, Birkett DJ. Diazepam metabolism by human liver microsomes is mediated by S-mephenytoin hydroxylase and CYP3A isoforms. Br J Clin Pharmacol. 1994;38:131–137. doi: 10.1111/j.1365-2125.1994.tb04336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko JW, Sukhova N, Thacker D, Chen P, Flockhart DA. Evaluation of omeprazole and lansoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab Dispos. 1997;25:853–862. [PubMed] [Google Scholar]
- 24.Funck-Brentano C, Becquemont L, Lenevu A, Roux A, Jaillon P, Beaune P. Inhibition by omeprazole of proguanil metabolism: mechanism of the interaction in vitro and prediction of in vivo results from in vitro experiments. J Pharmacol Exp Ther. 1997;280:730–738. [PubMed] [Google Scholar]
- 25.Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T. Oxidative metabolism of omeprazole in human liver microsomes: Cosegrregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther. 1993;266:52–59. [PubMed] [Google Scholar]
- 26.Minors JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45(525–538):27. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]