Abstract
Aims
The present pharmacokinetic study was undertaken to determine the dose proportionality of three different doses of budesonide—400 μg, 800 μg or 1600 μg administered twice daily by a dry-powder inhaler (Turbuhaler®) in adult patients with mild asthma.
Methods
A total of 38 patients received budesonide by inhalation, 13 received 400 μg twice daily, 12 received 800 μg twice daily and 13 received 1600 μg twice daily. Mean FEV1 at inclusion was 3.4, 4.0 and 3.9 l min−1 in the three groups, respectively. Blood samples were taken after a single dose, and after 3 weeks of daily treatment, for pharmacokinetic evaluation. Plasma concentrations of budesonide were determined by liquid chromatography plus mass spectrometry.
Results
Eleven evaluable patients remained in each dose group. Mean time to peak budesonide plasma concentration (tmax) was short (0.28–0.40 h) and did not differ between treatment groups. Budesonide concentrations declined rapidly thereafter, indicating efficient pulmonary absorption and rapid elimination with a half-life of approximately 3 h. Cmax was 1.4(2.0) nmol l−1 (single (repeated) doses), 2.6(3.6) nmol l−1 and 5.4(6.4) nmol l−1 after 400, 800 and 1600 μg twice daily, respectively. The corresponding results for the area under the plasma concentration vs time curve (AUC) were 271(325), 490(628) and 915(1096) nmol l−1 min. Ninety percent confidence intervals for pairwise dose-normalized Cmax and AUC comparisons between groups were large but contained unity in all cases, thus indicating dose-proportional pharmacokinetics. Regression on analysis supported these findings. Mean AUC after repeated doses (AUC(0,12 h,RD)) was on average 23% higher than the mean AUC after single doses (AUC(0,∞,SD)(P = 0.04) with no significant differences between doses, indicating slight accumulation following bid dosing.
Conclusions
In this relatively small study, budesonide inhaled via Turbuhaler® appeared to have dose-proportional pharmacokinetics, both within and above the clinically recommended dose range for asthmatic patients.
Keywords: budesonide, pharmacokinetics, Turbuhaler®
Introduction
Over the last four decades, glucocorticosteroids (GCS) have been the most effective therapy available for the maintenance treatment of asthma. Unfortunately, oral administration of GCS is often associated with a high incidence of systemic side effects. Inhaled GCS have a better ratio of local to systemic effects than those given orally. This is because the inhaled drug acts locally, so a lower dose can be employed, which reduces systemic exposure and results in fewer side effects [1]. As a consequence of the improved safety profile, inhaled steroids are now commonly used across a wide spectrum of asthma severity.
Budesonide was developed to further enhance topical anti-inflammatory effects while minimizing the systemic effects observed with other GCS. Both preclinical and clinical studies with inhaled budesonide have demonstrated favourable ratio between topical anti-inflammatory activity and systemic GCS effect over a wide range of doses [2–7]. After inhalation via Turbuhaler®, an inspiratory flow-driven, metered dose, dry powder inhaler, budesonide lung deposition is about 30% of the labelled dose and the systemic absorption of the swallowed fraction is low [8]. Budesonide does not undergo oxidative or reductive metabolism in the lungs [9] and the low systemic activity of orally ingested budesonide is mainly a result of its rapid first pass hepatic transformation (approximately 90%) into metabolites with low intrinsic GCS activity [10, 11].
Previous pharmacokinetic studies have shown that, as a result of better lung targeting, peak plasma concentration and systemic exposure was approximately doubled when budesonide [8] and terbutaline [12] were inhaled from Turbuhaler® as compared with the same drugs inhaled via a pressurized metered dose inhaler (pMDI). For terbutaline, this 2:1 relationship in lung deposition was coupled with increased clinical efficacy [12]. Hence, both the drug and the device used for their administration appear to have an influence on treatment outcome in asthma [13–16].
The aim of the current study was to investigate the dose-proportionality of the pharmacokinetics of budesonide administered by Turbuhaler®, an inspiratory flow-driven, metered dose, dry powder inhaler in adult patients and to determine the plasma levels of budesonide after repeated administration. The study also investigated pharmacodynamic parameters but these are reported elsewhere [17].
Methods
Subjects
Adult patients diagnosed as having asthma were enrolled into the study at five centres in the USA. Patients of either sex were eligible for inclusion if they were aged between 18 and 65 years, and had a forced expiratory volume in 1 s (FEV1) of ≥65% of the predicted value. In addition, the patients had to have the ability to use Turbuhaler® and be able to achieve an inspiratory flow rate of ≥50 l min−1 through the device.
All patients had to give their written, informed consent to participate in the study and be willing and able to complete a daily diary card. Female patients had to give a negative serum pregnancy test, and unless they were surgically sterile or postmenopausal, had to be using a medically acceptable contraceptive technique, or other contraceptive methods involving oestrogen or progestogen.
Patients were excluded if they had a history of carcinoma, or of multiple drug allergies or hypersensitivity to corticosteroids, or had any other significant disease or major physical impairment. Patients were not included if their asthma had required hospitalization, or if they had suffered a significant chest or upper respiratory tract infection, in the previous 4 weeks. Also excluded were patients receiving other steroids or who had received any form of steroid therapy in the previous 6 months; who had received investigational drugs in the previous 4 weeks; or who would be starting immunotherapy.
Study design
The study was of a double-blind, randomized, parallel group, multicentre design. Patients initially underwent a 1 week screening period followed by a 1 week baseline period. During this time medical histories were recorded, blood samples taken for laboratory testing, lung function measured, and the patient’s ability to use Turbuhaler® and comply with the dosage regimen were assessed. Patients attended the clinic for assessment at the end of each of these weeks (visits 1 and 2).
Patients who successfully completed these visits were randomly allocated to receive double-blind treatment with either 400 μg, 800 μg or 1600 μg budesonide (Pulmicort®, Astra Draco, Lund, Sweden) twice daily from identical inhaler devices for 6 weeks. Each dose from the inhaler consisted of two metered inhalations of study medication. Budesonide is a mixture of two epimers, 22R and 22S. The three batches of Pulmicort Turbuhaler used in the present study had identical epimer ratios (57.7:42.3, 22R:22S).
Concurrent treatment with cromolyn sodium, nedocromil sodium, adrenaline, β-adrenoceptor blockers, parenteral β-adrenoceptor agonists, or combinations of expectorants or sedatives with bronchodilators was not permitted. Inhaled β-adrenoceptor agonists other than albuterol sulphate were also not allowed.
Clinical assessments
Clinical assessments were performed on the first day of treatment and 3 weeks after the start of treatment. In addition, as part of the pharmacodynamic evaluation, clinical assessments were performed at 6 and, if necessary, 8 weeks after start of treatment. After taking the study drug, patients were instructed to gargle with water and wash out their mouths without swallowing in order to avoid possible local side effects.
Plasma budesonide
Blood samples (20 ml) were taken via an indwelling intravenous catheter 10, 20, 40 and 60 min, and 2, 4, 6, 9 and 12 h after administration of the first dose of study medication. This dosing and sampling procedure was repeated after 3 weeks of study treatment. Blood samples taken at each time point were inverted four times and centrifuged immediately at 1500 g for 10 min. Plasma samples from two vacuum tubes were removed by pipette, placed into one 10 ml polystyrene tube and after careful mixing dispensed into two different polystyrene tubes, each containing at least 3.5 ml of plasma, and labelled with the patient’s identifying number. Samples were stored frozen at −20° C until the analyses could be performed.
The levels of budesonide in plasma were determined at Astra Draco AB, Department of Bioanalytical Chemistry. The assay was based on a combination of liquid chromatography and mass spectrometry (LC–MS) [18]. The lower limit of quantification (LOQ) for plasma budesonide was 0. 1 nmol l−1 (43 ng l−1). During the study the precision, expressed as coefficient of variation (CV), was 16% at LOQ and better than 5% at higher concentrations as measured from the QC samples. The accuracy was better than 3%.
Safety assessments
Patients were instructed to record any adverse event on their diary cards and were questioned in a general manner at every visit about possible adverse experiences. Adverse events reported were graded as mild, moderate or severe and their relationship to study medication assessed.
Routine haematology and blood chemistry and urine testing were performed at visits 1, 2 and 5, and at visit 6, if necessary. Serum pregnancy tests were carried out at visits 1 and 5. Morning plasma cortisol levels were measured at visits 2 and 5.
All patients underwent a full physical examination at visits 1 and 5, vital signs were recorded at visits 1 and 5, and a 12-lead electrocardiogram was obtained at visit 1.
Patient compliance and withdrawal
Patients’ compliance with treatment was assessed by the use of diary cards and by recording the quantity of double-blind study medication used.
Patients were withdrawn from the study if they experienced intolerable adverse events, worsening of asthmatic symptoms, or if exclusion criteria or concurrent disease developed. Patients could be withdrawn from the study at the discretion of the investigator.
Ethical considerations
The protocol procedures and consent form were approved by the appropriate Institutional Review Board before initiation of the study. The study was performed in accordance with the Declaration of Helsinki and in compliance with the appropriate US Federal regulations.
Data analysis
Data were analysed using GAUSS (Aptech Systems Inc.) version 3.0. The overall purpose of the data analysis was to support the statement that kinetics of budesonide are linear, i.e. that any saturation or other nonlinear process in absorption, distribution or elimination is negligible. To prove linearity is not possible, what is possible is to disprove it by proving that some requirement of linearity is not fulfilled. Two such requirements are that area under the curve from 0 to infinity after a single dose (AUC(0,∞, SD)) is equal to AUC over the dosing interval in steady state (AUC(0,12 h, RD)), and that AUC and maximum plasma concentration (Cmax) are proportional to the dose given.
The ratio AUC(0,∞, SD)/AUC(0,12 h, RD) discussed above should not be confused with the corresponding ratio obtained after single dose only, which is the accumulation ratio (Racc). This accumulation ratio assumes linearity for its proper interpretation.
In order to compute AUC(0,∞, SD), a terminal elimination rate of budesonide was estimated using linear regression on visually determined points, on log-linear plasma concentration vs time curve. This procedure was followed for data obtained after a single dose and after repeated dosing. Pre-dose values at the first dose were deemed to be zero. AUC was calculated using the trapezoidal rule between 0 and the last measurement above the LOQ, plus the monoexponential extrapolated area using the terminal elimination rate estimate. For the single-dose data, AUC was extrapolated to infinity; for the data following repeated dosing AUC was calculated to 12 h after dosing, the curve was extrapolated only if the budesonide concentration fell below the LOQ within this period. If no predose sample was taken at steady state or if the value was below the LOQ, the budesonide level at the start of treatment was estimated by backward linear extrapolation.
Statistically the equality of AUC after a single dose and after 3 weeks of treatment was tested using a t-test on the difference of log AUCs (AUC(0,∞) for single dose and AUC(0,12 h) for repeated dose), motivated by what is standard in bioavailability studies. Dose proportionality was tested by an analysis of variance (anova on dose-normalized log AUCs, with dose and centre as factors. Centre was incorporated since this is standard statistical practise in a multicentre study. Single dose data and steady state data were analysed separately. Budesonide treatments were compared pairwise and the results are presented after exponentiation back to the original scale to produce an estimate of pairwise quotients of adjusted geometric means and the corresponding 90% confidence intervals. Cmax was analysed in similar manner. tmax was analysed by Wilcoxon nonparametric test using Hodge–Lehmann estimators for confidence intervals. In addition, a regression analysis of individual data based on the model Y=A D^b, where Y is either AUC or Cmax computed either from single dose or repeated dose data and using metered doses, and b is the slope. Dose-proportionality means that b=1.
Results
Patients
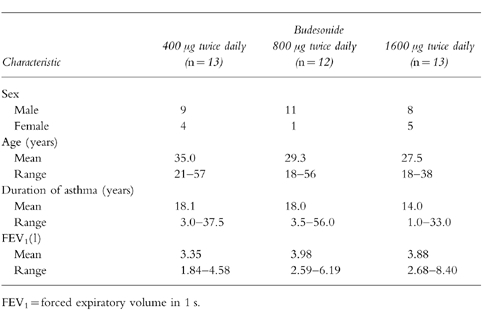
A total of 38 patients, from five centres in the USA, were randomised to double-blind treatment with budesonide: 13 to the 400 μg twice daily group, 12 to the 800 μg twice daily group and 13 to the 1600 μg twice daily group.
The treatment groups were similar with respect to age, sex distribution, duration of asthma and lung function at baseline. These demographic characteristics are summarized in Table 1.
Table 1.
Demographic characteristics of patients. FEV1 was measured at recruitment.
Of the 38 patients who started study medication, 34 completed 6 weeks of treatment. Compliance was monitored throughout the study. One patient each in the budesonide 400 μg twice daily and 800 μg twice daily groups, and two patients from the budesonide 1600 μg twice daily group withdrew from the study. Both patients from the budesonide 1600 μg twice daily group withdrew because of adverse experiences (one patient: stomach pain, coughing, sore throat, hoarseness, nasal congestion; other patient: menorrhagia); the remainder were withdrawn due to poor compliance or failure to fulfil inclusion criteria. In addition, one patient from the budesonide 400 μg twice daily group was unevaluable for pharmacokinetic analysis. Thus, a total of 33 patients, 11 in each treatment group, were included in the pharmacokinetic analyses.
Two patients (one receiving 800 μg twice daily and one 1600 μg twice daily) took terfenadine as required during budesonide treatment. Otherwise no patient used any of the known inhibitors or inducers of CYP3 A throughout the study.
Pharmacokinetic evaluation
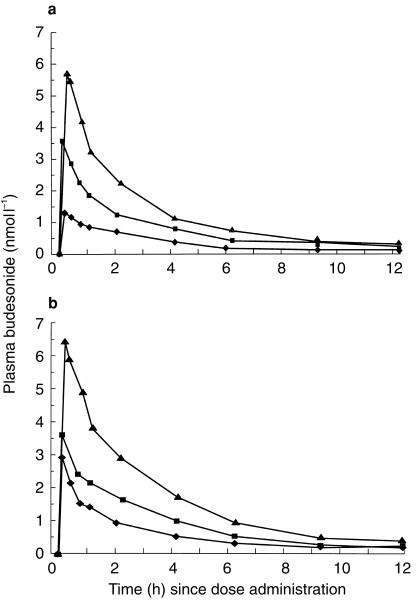
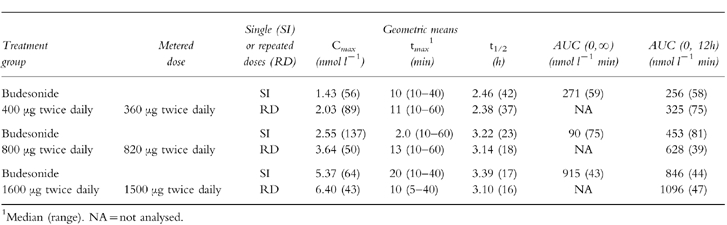
Mean budesonide plasma concentrations after the first dose and after 3 weeks of treatment with daily doses of budesonide are shown in Figure 1. Table 2 summarizes derived descriptive pharmacokinetic variables. All three groups showed a rapidly attained peak and then a steady reduction in budesonide levels from the peak values, with a plasma half-life of approximately 3 h, both after a single dose and after repeated dosing.
Figure 1.
Plasma concentrations (mean) of budesonide after the first dose (single dose) (a) and after 3 weeks of treatment (repeated dosing) (b) with 400 μg twice daily, 800 μg twice daily and 1600 μg twice daily via Turbuhaler®.
Table 2.
Descriptive pharmacokinetic parameters: geometric means and (within brackets) coefficient of variation (%).
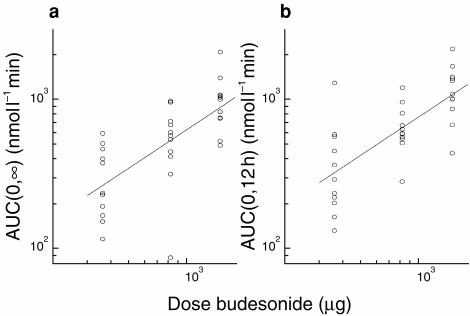
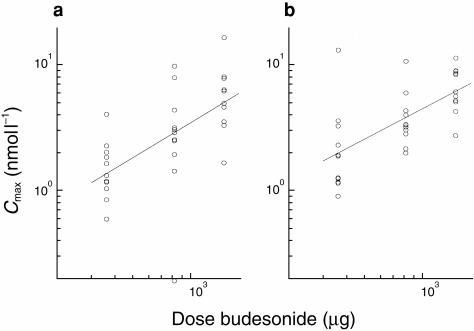
Mean AUC and Cmax increased with increasing dose, after both a single dose and after repeated dosing, as illustrated in Figures 2 and 3, respectively.
Figure 2.
Individual values and regression lines fitted to log data for AUC (nmol l−1 min) after single and repeated dosing of 400 μg (metered dose 360 μg), 800 μg (820 μg) and 1600 μg (1500 μg).
Figure 3.
Individual values regression lines fitted to log data for Cmax (nmol l−1) after single and repeated dosing of 400 μg (metered dose 360 μg), 800 μg (820 μg), and 1600 μg (1500 μg).
Single vs repeated dosing
Mean budesonide plasma concentrations after repeated dosing were higher than those recorded after the first dose (Figure 2 and Table 2). The geometric mean of AUC-ratios following repeated doses (RD) vs single doses (SD), i.e. AUC(0,12 h, RD/AUC(0,∞, SD), was 1.23 (90% confidence interval: 1.04–1.44). This difference was statistically significant (P = 0.04). The corresponding Cmax-ratio was 1.34 (90% confidence interval: 1.01–1.78), which was not statistically significant. There was no evidence of any dose effect in these parameters: AUC(0,12 h, RD)/AUC(0,∞, SD) ratios at 400, 800 and 1600 μg twice daily were 1.20, 1.28 and 1.20, respectively; the corresponding Cmax-ratios were 1.41, 1.42 and 1.19. The observed accumulation ratio (Racc) upon repeated dosing, i.e. AUC(0,∞, SD)/AUC(0,12 h, RD) was also higher than expected: observed Raccs at 400, 800 and 1600 μg twice daily were 1.27, 1.39 and 1.30, respectively, which can be compared with expected Raccs (i.e. AUC(0,∞, SD)/AUC(0, 12h, RD)) of 1.06, 1.08 and 1.08.
Dose proportionality
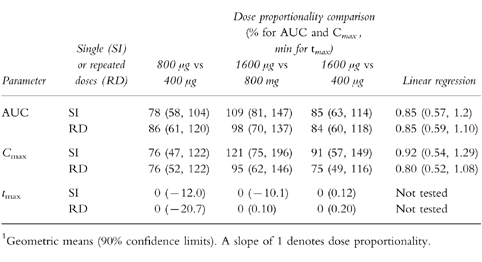
Pairwise comparisons of tmax, AUC and Cmax, normalized o the metered dose (the amount of drug leaving the drug reservoir), did not result in any significant differences among the three budesonide treatment groups, although the 90% confidence intervals were large (Table 3). In addition, the 90% confidence intervals of regression slopes include unity. Taken together, the data do suggest that plasma budesonide concentrations are proportional to the administered dose.
Table 3.
Dose proportionality comparisons of pharmacokinetic parameters, based on metered doses and expressed as geometric means and (within brackets) 90% confidence limits. Pairwise comparisons of Cmax and AUC were tested by anova on dose-normalized log data. A Wilcoxon test was used for pairwise comparisons of tmax. Dose proportionality was also tested by linear regression of log data.
Safety
The majority of patients in all treatment groups experienced adverse events during the double-blind treatment period and the overall incidence was similar in all groups. Most of these events occurred in the respiratory system—mainly pharyngitis and rhinitis—or in the body as a whole, primarily headaches. Adverse events that the investigators believed were possibly or probably related to study medication were recorded for four patients from the budesonide 400 μg twice daily group, two from the 800 μg twice daily group and six from the 1600 μg twice daily group. Only two patients, both from the budesonide 1600 μg twice daily group, discontinued the study because of adverse events: in one patient these were stomach pain, coughing, sore throat, hoarseness and nasal congestion, in the other menorrhagia. None of the adverse events reported was considered to be serious.
Discussion
Budesonide, administered by a dry-powder inhaler (Turbuhaler®) exhibited a linear increase in AUC and Cmax after both a single dose and after 3 weeks of repeated dosing. Dose-normalized data were consistent with the findings of a previous study on 24 healthy volunteers who received 800 μg of budesonide via Turbuhaler®, where a mean AUC of 604 nmol l −1 min and a mean Cmax of 3.5 nmol l−1 were found [8], suggesting that asthmatics, at least those with mild disease, have similar kinetics as healthy subjects. Also the rapidly attained tmax (<30 min) and the relatively short-terminal half-life (about 3 h) were consistent with previous findings in healthy subjects [8, 19].
The variability of obtained pharmacokinetic data was larger in the present study as compared with previous data in healthy subjects under strictly controlled conditions [8]. Variability in drug delivery as well as interindividual differences in uptake and metabolism may explain these findings. In healthy subjects, variability in drug delivery has been shown to be significantly less after inhalation via Turbuhaler® than after pMDI [8]. In the clinical setting, this difference might be even more accentuated [20]. In the present study, individual Turbuhaler® inhalers could not, for practical and technical reasons, be analysed concerning drug output. However, the utilized Turbuhaler® batches were characterized concerning the amount of drug leaving the drug reservoir (metered dose). Deviations from the labelled dose were 10% or less. These deviations were taken into consideration in the statistical analysis of dose proportionality.
Dose proportionality may be implied from the data provided. However, as a result of the interpatient variability, the data are only suggestive. In order to make firm conclusions of dose proportionality, a larger study would be needed. A cross-over rather than a parallel group design would most likely further improve the power of such a study.
The pharmacokinetics of inhaled budesonide after repeated dosing have not previously been reported. In this study, mean AUC and Cmax values after 3 weeks of repeated dosing were higher than after single doses. While Cmax can be expected to increase for a drug which partially remains in the body at the end of the dosing interval, an AUC-ratio (i.e. AUC(0,12 h, RD)/AUC(0,∞, SD) which is greater than unity is indicative of accumulation: observed accumulation ratios at the different doses (Racc) ranged between 1.27 and 1.39, which was greater than those expected from single dose data (expected Racc ranged between 1.06 and 1.08). The mean AUC-increase (estimated in this study to be 23%) is, however, lower than for the more lipophilic glucocorticoid fluticasone [21], but is consistent with the approximately 30% greater cortisol suppression noted after repeated dosing compared with a single dose [22]. Still, considering the short plasma half-life of budesonide, this accumulation was unexpected. That treatment itself would have this effect on the kinetics after repeated dosing cannot be ruled out. As this was not an efficacy study, lung function was not assessed following treatment. Overall lung deposition of budesonide from Turbuhaler® appears similar in patients with asthma [23] as in healthy subjects [8], although the regional deposition within the lung may be somewhat more central in asthmatics [23]. Hence, as the patients in our study had such mild disease at enrolment and as Turbuhaler® is such a robust delivery system even in severe asthma [24], it seems unlikely that treatment itself would have had any large effect on the plasma levels of budesonide. Intracellular fatty acid conjugation was recently reported as a mechanism by which budesonide might achieve a prolonged topical anti-inflammatory action [25, 26]. The overall extent of this rapidly occurring but reversible retention mechanism is not yet known, but could possibly contribute to the finding of slight accumulation after repeated dosing.
Budesonide consists of two epimers, 22R and 22S, which both have glucocorticoid activity [27]. However, the two epimers differ in their kinetics, so that epimer 22R has larger clearance and greater volume of distribution than epimer 22S. The elimination half-life appears the same for both epimers [28]. The bioanalytical LC–MS assay employed in the present study codetermines the two epimers, and the plasma concentration time data thus reflects the total plasma exposure of budesonide. The apparent dose linearity noted for the sum of the two epimers, suggest either that dose linearity actually prevails for each epimer or that the epimers differ in their kinetics at different doses in a counter-balanced fashion, in this way giving overall dose-linear kinetics. The latter cannot be ruled out, but seems unlikely. In either case, both epimers, in combination, determine the therapeutic ratio, and their joint kinetics are therefore clinically relevant to assess.
This pharmacokinetic study was performed as part of a clinical pharmacology investigation, where effects of budesonide and prednisone treatment on hypothalamus-pituitary-adrenal (HIPA) axis function were studied [17]. The three doses of budesonide were chosen to represent clinically recommended and anticipated doses: 800 and 1600 μg day−1 (400 and 800 μg twice daily), as well as a dose twice the proposed maximum recommended dose in the USA: 3200 μg day−1 (1600 μg twice daily). The primary pharmacodynamic endpoint was plasma cortisol levels following a 6-h challenge with cosyntropin. Budesonide caused a dose-related suppression of postcosyntropin plasma cortisol, but this effect was statistically significant vs placebo only after the highest dose (3200 μg day−1). Prednisone 10 mg day−1 affected postcosyntropin plasma cortisol to an extent corresponding with 5 mg day−1 of budesonide [17].
In conclusion, in this relatively small study, budesonide inhaled via Turbuhaler®, appeared to have linear pharmacokinetics, both within and up to twice the maximum of the clinically recommended dose range for asthmatic patients. The slight, but statistically significant, accumulation noted after repeated dosing may, at least partly, be explained by intracellular retention as fatty acid esters.
References
- 1.Toogood JH, Frankish CW, Jennings BH, et al. A study of the mechanism of the antiasthmatic action of inhaled budesonide. J Allergy Clin Immunol. 1990;85:872–880. doi: 10.1016/0091-6749(90)90071-b. [DOI] [PubMed] [Google Scholar]
- 2.Reed CE. Aerosol steroids as primary treatment of mild asthma. N Engl J Med. 1991;325:425–426. doi: 10.1056/NEJM199108083250610. [DOI] [PubMed] [Google Scholar]
- 3.Brattsand R, Thalén A, Roempke K, Källström Gruvstad L. New glucocorticoids with improved ratio between topical and systemic activities. Br J Pharmacol. 1982;77:4433P. [PubMed] [Google Scholar]
- 4.Ellul-Micallef R, Hansson E, Johansson SÅ. Budesonide: a new corticosteroid in bronchial asthma. Eur J Respir Dis. 1980;61:167–173. [PubMed] [Google Scholar]
- 5.Ellul-Micallef R, Hansson E, Johansson SÅ. Acute dose–response studies in bronchial asthma with a new corticosteroid, budesonide. Br J Clin Pharmacol. 1983;15:419–422. doi: 10.1111/j.1365-2125.1983.tb01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haahtela T, Jarvinen M, Kava T, et al. Comparison of a agonist, terbutaline, with an inhaled corticosteroid, budesonide in newly detected asthma. N Engl J Med. 1991;325:388–392. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- 7.Brattsand R, Pipkorn U. Glucocorticoids: experimental approaches. In: Kaliner MA, Barnes PJ, Persson CGA, editors. Asthma: its Pathology and Treatment. New York: Marcel Dekker Inc; 1991. pp. 667–709. [Google Scholar]
- 8.Andersson P, Ryrfeldt Å. Biotransformation of the topical glucocorticosteroids budesonide and beclomethosone 17α, 21-dipropionate in human liver and lung homogenate. J Pharm Pharmacol. 1984;36:763–765. doi: 10.1111/j.2042-7158.1984.tb04868.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahlberg E, Thalén A, Brattsand R, et al. Correlation between chemical structure, receptor binding, and biological activity of some novel, highly active 16-alpha, 17-alpha-acetyl substituted glucocorticoids. Mol Pharmacol. 1984;25:70–78. [PubMed] [Google Scholar]
- 10.Edsbäcker S, Jönsson S, Lindberg C, Ryrfeldt Å, Thaldén A. Metabolic pathways of the topical glucocorticoid, budesonide, in man. Drug Metab Dispos. 1983;11:590–596. [PubMed] [Google Scholar]
- 11.Thorsson L, Edsbäcker S, Conradson T-B. Lung deposition of budesonide from Turbuhaler is twice that from pressurised metered dose inhaler P-MDI. Eur Respir J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 12.Borgström L, Derom E, Ståhl E, Wåhlin-Boll E, Pauwels R. The inhalation device influences lung deposition and bronchodilating effect of terbutaline. Am J Respir Crit Care Med. 1996;153:1636–1640. doi: 10.1164/ajrccm.153.5.8630614. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla C, Lacronique J, Allaert FA, Godard P, Duroux P. A 3-month comparative dose-reduction study with inhaled beclomethasone dipropionate and budesonide in the management of moderate to severe adult asthma. Drug Invest. 1994;8:49–56. [Google Scholar]
- 14.Grahnén A, Eckenäs S-Å, Brundin RM, Ling-Andersson A. An assessment of the systemic activity of single doses of inhaled fluticasone propionate in healthy volunteers. Br J Clin Pharmacol. 1994;38:521–525. doi: 10.1111/j.1365-2125.1994.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selroos O, Backman R, Forsén KO, et al. Local side-effects during 4-year treatment with inhaled corticosteroids—a comparison between pressurized metered dose inhalers and Turbuhaler®. Allergy. 1994;49:888–890. doi: 10.1111/j.1398-9995.1994.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Agertoft L, Pedersen S. Importance of inhalation device on the effect of budesonide. Arch Dis Child. 1993;69:130–133. doi: 10.1136/adc.69.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson D, Kaiser H, Dockhorn R, et al. Effects of budesonide by means of Turbuhaler on HPA-axis in asthmatic subjects. J Allergy Clin Immunol. 1998;101:312–319. doi: 10.1016/S0091-6749(98)70241-6. [DOI] [PubMed] [Google Scholar]
- 18.Lindberg C, Blomqvist A, Paulson J. Determination of (22R,S) budesonide in human plasma by automated liquid chromatography/thermospray mass spectrometry. Biol Mass Spectrornetry. 1992;2:525–533. doi: 10.1002/bms.1200211102. [DOI] [PubMed] [Google Scholar]
- 19.Ryrfeldt Å, Andersson P, Edsbäcker S, et al. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis. 1982;63(Suppl 122):86–95. [PubMed] [Google Scholar]
- 20.Liard R, Aubier M, Zureik M, Neukirch F. Advantage of breath-actuated inhalers versus metered-dose inhalers. Eur Respir J. 1995;8:2195. [Google Scholar]
- 21.Thorsson L, Dahlström K, EdsBäcker S, Källén A, Wirén J-E. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lönnebo A, Grahnén A, Jansson B, Brundin RM, Ling-Andersson A, Eckernäs S-A. An assessment of the systemic activity of single and repeated doses of inhaled fluticasone propionate and budesonide in healthy volunteers. Eur J Clin Pharmacol. 1996;49:459–463. doi: 10.1007/BF00195931. [DOI] [PubMed] [Google Scholar]
- 23.Brown PH, Ning AC, Greening AP, McLean A, Crompton GK. Peak inspiratory flow through Turbuhaler in acute asthma. Eur Respir J. 1995;8:1940–1941. doi: 10.1183/09031936.95.08111940. [DOI] [PubMed] [Google Scholar]
- 24.Thorsson L, Kenyon C, Newman S, Borgström L. Lung deposition of budesonide in asthmatics: a comparison of different formulations. Int J Pharmaceut. 1998 l;68:119–127. [Google Scholar]
- 25.Wieslander E, Delander E-L, Järkelid L, Hjertberg E, Tunek A, Brattsand R. Pharmacologic importance of the reversible fatty acid conjugation of budesonide studied in a rat cell line in vitro. Am J Resp Cell Mol Biol. 1998;19:477–484. doi: 10.1165/ajrcmb.19.3.3195. [DOI] [PubMed] [Google Scholar]
- 26.Miller-Larsson A, Mattson H, Hjertberg E, Dahlbäck M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide: novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26(7):623–630. [PubMed] [Google Scholar]
- 27.Thalén A, Brattsand R, Gruvstad E. Synthesis and pharmacological properties of some 16alpha, 17alpha-acetals of 16alpha-hydroxyhydrocortisone, 16alpha- hydroxyprednisolone and fluorinated 16alpha-hydroxyprednisolones. Acta Pharm Suec. 1984;21:109–124. [PubMed] [Google Scholar]
- 28.Ryrfeldt Å, Edsbäcker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther. 1984;35:525–530. doi: 10.1038/clpt.1984.71. [DOI] [PubMed] [Google Scholar]