Abstract
Aims
Following intravenous administration of its prodrug, L-758,298, we assessed the pharmacodynamics of L-754,030, a novel and highly selective NK1 receptor antagonist, by examining systemic haemodynamics and the blood flow responses to intra-arterial substance P infusion.
Methods
Sixteen healthy male volunteers participated in a double-blind, randomised, placebo controlled crossover trial of L-758 298. Forearm blood flow was measured using venous occlusion plethysmography during intrabrachial substance P infusion (0.125–128 pmol min−1). In part 1, eight subjects received substance P infusions before and during placebo, 0.25 mg, 1 mg or 5 mg of L-758 298. In part 2, eight subjects received substance P infusions 24 h after placebo or 1.43 mg of L-758 298.
Results
L-758 298 caused dose dependent inhibition of substance P induced vasodilatation (P < 0.001). Placebo adjusted differences (95% CI) in baseline forearm blood flow, mean arterial pressure and heart rate showed no relevant changes with 5 mg of L-758 298 (>1400–fold shift in substance P response): 0.00 (−0.49 to +0.49) ml 100 ml−1 min−1, 1.0 (−3.2 to +5.2) mmHg and 1.9 (−5.9 to +9.7) beats min−1, respectively. Twenty-four hours after 1.43 mg of L-758,298, there was ~34–fold shift in response to substance P induced vasodilatation (P < 0.008) at plasma L-754 030 concentrations of 2–3 ng ml−1. L-758 298 was generally well tolerated without serious adverse events.
Conclusions
Substance P induced forearm vasodilatation is mediated by the endothelial cell NK1 receptor in man but endogenous substance P does not appear to contribute to the maintenance of peripheral vascular tone or systemic blood pressure.
Keywords: substance P, neurokinin 1 receptor, endothelium, haemodynamics, blood flow
Introduction
Substance P is a widely distributed endecapeptide which is found principally in the neural tissue of the central, peripheral and enteric nervous systems [1–4]. The physiological functions of substance P include neurotransmission in primary sensory neurones with particular involvement in nociception and emesis. In addition to functioning as a neurotransmitter, it also acts as an inflammatory mediator [5–7] and neurohumoral regulator [1, 8].
Substance P is a member of the tachykinin family of peptides and acts through stimulation of the neurokinin receptors, having a particularly high affinity for the type 1 (NK1) receptor [9]. When given intra-arterially, substance P is a potent vasodilator [10–12] through an endothelium dependent mechanism [13] which is partly mediated by nitric oxide release [14, 15]. In animal studies, this response is induced via stimulation of the endothelial cell NK1 receptor [9] although, to date, this has not been confirmed in vivo in man. Substance P is found in perivascular neural tissue [16] and has been postulated to play a role in the regulation of vascular tone [17, 18].
Antagonism of the NK1 receptor has potentially diverse therapeutic indications such as in the treatment of pain, inflammation and emesis [19]. L-754 030 (2-(R)-(1-(R) −3,5-bis (trifluoromethyl) phenylethoxy)-3-(S)- (4-fluoro) phenyl-4-(3-(5-oxo-4H-1,2,4-triazolo)methyl morpholine; also known as MK-869) is a long acting, highly selective, competitive NK1 receptor antagonist with poor solubility in aqueous solution. L-754 030 is more selective for the NK1 than the NK3 (3000–fold) or the NK2 and other G-protein linked receptors and ion channels (>50 000–fold) [20]. N-phosphorylation of L-754 030 produces L-758 298, a prodrug which is readily soluble in aqueous solutions. L-758 298 undergoes rapid in vivo conversion to L-754 030 and thereby provides a prodrug which can be administered intravenously.
The primary aims of the present study were: first, to determine the ability of L-754 030 to inhibit substance P induced vasodilatation during and 24 h after intravenous administration of L-758 298; second, to confirm that substance P induced vasodilatation is mediated via the endothelial cell NK1 receptor in man; and third, to determine whether endogenous substance P regulates peripheral vascular tone or blood pressure in man. An additional important aim of the study was to evaluate the tolerability of single intravenous doses of L-758 298 in healthy male volunteers.
Methods
Subjects
Healthy nonsmoking men aged between 18 and 45 years participated in a series of studies which were undertaken with the approval of the Lothian Research Ethics Committee and the written informed consent of each subject. None of the subjects was taking regular medications, or received vasoactive or nonsteroidal anti-inflammatory drugs in the week before each phase of the study, and all abstained from alcohol for 24 h and from food and caffeine-containing drinks for at least 9 h before each study. All studies were performed in a quiet, temperature controlled room maintained at 23.5–24.5° C.
Drug administration
The brachial artery of the nondominant arm was cannulated with a 27-standard wire gauge steel needle (Cooper’s Needle Works Ltd, Birmingham, UK) under 1% lignocaine (Xylocaine; Astra Pharmaceuticals Ltd, Kings Langley, UK) local anaesthesia. The cannula was attached to a 16-gauge epidural catheter (Portex Ltd, Hythe, UK) and patency maintained by infusion of saline (0.9%: Baxter Healthcare Ltd, Thetford, UK) via an IVAC P1000 syringe pump (IVAC Ltd, Basingstoke, UK). The total rate of intra-arterial infusions was maintained constant throughout all studies at 1 ml min−1. Synthetic pharmaceutical-grade substance P (Clinalfa AG, Läufelfingen, Switzerland) of ≥95% purity, was administered following dissolution in saline.
Matched placebo and L-758 298 (Merck Research Laboratories, West Point, USA) were reconstituted with 0.9% saline in glass vials containing 50 mg of mannitol alone or 6 mg of L-758 298 and 50 mg of mannitol, respectively. The 5 mg initial dose of L-758 298 was chosen to exceed the ID90 of 50 μg kg−1 in the guinea pig sensorotoxin-induced systemic vascular leak model (Merck Research Laboratories, Terlings Park, UK). Cannulae were inserted into the veins of the antecubital fossae of both arms. L-758 298 was administered into the dominant arm via a 19-G cannula (Wallace Y-Can; Wallace Ltd, Colchester, UK) and venous samples were withdrawn from the nondominant arm via a 17-G cannula (Venflon; BOC Ohmeda AB, Helsingborg, Sweden).
Measurements
Blood flow was measured in the dominant and nondominant forearms by venous occlusion plethysmography using mercury-in-silastic strain gauges which were applied to the widest part of the forearm [21]. Since forearm length between the occlusion and collecting cuff is constant, volumetric changes are directly proportional to circumferential changes measured by the strain gauge [21, 22]. During measurement periods, the hands were excluded from the circulation by rapid inflation of wrist cuffs to a pressure of 220 mmHg using E20 Rapid Cuff Inflators (D. E. Hokanson Inc, Washington, USA). Upper arm cuffs were inflated intermittently to 40 mmHg pressure for 10 s in every 15 s to achieve venous occlusion and obtain plethysmographic recordings. Analogue voltage output from an EC-4 strain gauge Plethysmograph (D. E. Hokanson) was processed by a MacLab® analogue-to-digital converter and Chart® v3.3.8 software (AD Instruments Ltd, Castle Hill, Australia) and recorded onto a MacIntosh Classic II computer (Apple Computers Inc, Cupertino, USA). Calibration was achieved using the internal standard of the plethysmograph.
Blood pressure was monitored in the dominant arm at intervals throughout each study using a semiautomated noninvasive oscillometric sphygmomanometer (Takeda UA 751, Takeda Medical Inc, Tokyo, Japan) [23].
Blood (10 ml) was withdrawn from the nondominant arm before and after the incremental infusion of substance P and admixed with 1 ml of 1% disodium EDTA. Blood samples were placed on ice before being centrifuged at 2000 g for 30 min. Plasma was frozen and stored at −80° C prior to assay. Plasma L-754 030 concentrations were determined using high performance liquid chromatography and mass spectrometry using an internal standard (L-752 611). The assay was validated over a concentration range of 1–500 ng ml−1 with a limit of detection at 1 ng ml−1 and a coefficient of variation of <9%.
Tolerability assessments were made before, during and following completion of the study and included: clinical examination, repeated questioning for symptoms, clinical chemistry screen (liver enzymes, bilirubin, electrolytes, urea, creatinine, protein and albumin), haematology screen (full blood and differential count), urinalysis, 12-lead electrocardiogram.
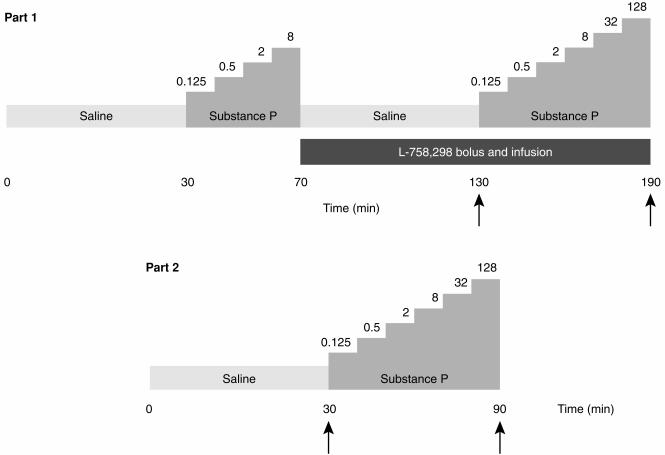
Study design (Figure 1)
Figure 1.
Schematic of protocol. Doses of substance P given in pmol min−1. Arrows indicate blood sampling time points for the estimation of plasma L-754 030 concentrations. Part 2 infusions were commenced 24 h after 1.43 mg of intravenous L-758 298 administration.
Subjects attended at 09.00 h, rested recumbent throughout each study and intravenous cannulae were inserted into each arm. Strain gauges and cuffs were applied, and the brachial artery of the nondominant arm cannulated. Saline was infused into the arterial cannula for the first 30 min to allow for equilibration. Forearm blood flow was measured for 3 min beginning at 23, 13 and 3 min before commencing substance P infusions. Throughout all studies, substance P was infused for 10 min at each dose. Forearm blood flow measurements were made from 3 to 6 min of each infusion period.
Screening
Before inclusion in the main study, subjects received intra-arterial infusions of substance P at 0.125, 0.5, 2, 8 and 32 pmol min−1 [24]. To reduce overall variability in the responses, subjects were recruited to the main study if 2 pmol min−1 of substance P increased forearm blood flow by ≥100% and ≤500%.
Part 1—Maintenance L -758 298 infusion
On each occasion, eight subjects received incremental intra-arterial infusions of substance P at 0.125, 0.5, 2 and 8 pmol min−1 followed by 60 min of saline. A second infusion of substance P was then administered at 0.125, 0.5, 2, 8, 32 and 128 pmol min−1 [24].
At the beginning of the intervening 60 min saline infusion, subjects received a double-blind, randomised intravenous infusion of either L-758 298 or placebo: (Table 1). An intravenous bolus (two-thirds of the total dose over 20 min) was given followed by a continuous maintenance infusion (one third of the total dose over the subsequent 100 min) throughout the second challenge of intra-arterial substance P administration. From earlier phase I pharmacokinetic studies in man (Merck; data on file), this dosage regimen was predicted to produce a stable plasma concentration of L-754 030 during the second incremental infusion of intra-arterial substance P. Plasma samples to measure L-754 030 concentrations were taken 60 and 120 min after the start of the intravenous infusion of L-758 298 or placebo. In the first week, subjects received a total dose of 5 mg of L-758 298 or placebo. The next dose administered in the second week would be increased to 20 mg if the PD100 (defined below) was increased by <40-fold by 5 mg of L-758 298 or reduced to 1 mg if >40-fold (based on data from 6 subjects). The same criteria were applied to determine whether the final dose (80 mg or 0.25 mg) of L-758 298 was to be administered, or the study completed after two doses of L-758 298 and placebo (based on data from four subjects; see Table 1). If the third dose was not to be given then this would be replaced by placebo and the study terminated after 3 weeks. To maintain double-blind randomization, all data analysis and dosage decisions were made at the central co-ordinating centre (Merck Research Laboratories, Terlings Park, UK) independent of the investigators.
Table 1.
Schematic of drug allocation.
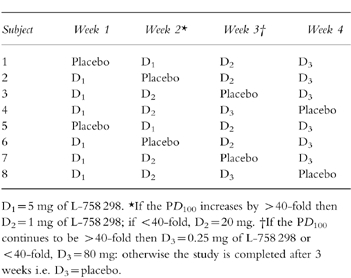
Part 2–24 h post L -758 298 infusion
Eight further subjects attended on two occasions, 1 week apart, and received an intravenous infusion of either L-758 298 or placebo over 30 min in a double-blind randomised manner. Subjects returned 24 h later to receive infusions of intra-arterial substance P at 0.125, 0.5, 2, 8, 32 and 128 pmol min−1 [24]. Plasma samples to measure L-754 030 concentrations were taken 24 and 25 h after the start of the intravenous infusion of L-758 298 or placebo. The dose of L-758 298 was derived from the pharmacodynamic and pharmacokinetic data of part 1 and was chosen to produce an approximately 40–fold increase in the PD100 24 h after L-758 298 administration. L-754 030 has a plasma half-life of 15±4 h in man.
Data analysis and statistics
Plethysmographic data were extracted from the Chart data files and forearm blood flows were calculated for individual venous occlusion cuff inflations by use of a template spreadsheet (Excel v5.0; Microsoft Inc, USA). Recordings from the first 60 s after wrist cuff inflation were not used, because of the instability in blood flow that this causes [25]. Usually, the last five flows recorded in each 3 min measurement period were calculated and averaged for each arm. Basal blood flow was taken to be that recorded immediately before drug infusion. To reduce the variability of substance P blood flow responses, the ratio of flows in the two arms was calculated for each time point, in effect using the dominant arm as a contemporaneous control for the nondominant arm [21]. Plethysmographic data were compiled by blinded investigators.
Percentage change in forearm blood flow from baseline was calculated using the ratios of flows in the two arms for each of the substance P infusions. Natural logarithm transformations of the substance P doses were used to estimate individual PD100 values using linear regression techniques. The PD100 was defined as the interpolated or extrapolated dose of substance P which provokes a 100% increase in forearm blood flow. To determine whether there was a within day difference in PD100 following placebo, an analysis of variance (anova) with the terms ‘subjects’ and ‘time’ (predose and postdose) was used to analyse the natural logarithm transformed PD100 data from the placebo treatment group in part 1.
The influence of each dose of L-758 298 in parts 1 and 2 on the forearm blood flow response to substance P were evaluated relative to placebo. In these analyses, the PD100 after administration of a placebo dose was used as the control for assessment of the fold shifts in PD100 due to the administration of the various doses of L-758 298. An anova with the terms ‘subject’ and ‘treatment’ was used to analyse the natural logarithm transformed PD100 data from the treatments in part 1. An anova appropriate for a two period crossover study was used to analyse the natural logarithm transformed PD100 data from the treatments in part 2. These anova analyses were used to estimate the geometric means, their ratios and 95% confidence intervals for the geometric mean ratio for PD100.
Mean arterial pressure, heart rate and blood flow data were examined, where appropriate, by multifactorial anova with repeated measures and paired Student’s t-test. All results are expressed as mean±s.e. mean. Statistical significance was taken at the 5% level.
Results
Twenty-two healthy male volunteers were required to identify 16 subjects who met the entry criteria: forearm blood flow increased by >500% in two subjects and <100% in four subjects. The 16 volunteers subsequently recruited were aged 30±2 years (range 20–40 years) and weighed 76±3 kg (range 61–95 kg).
There were no significant baseline differences in forearm blood flow, blood pressure or heart rate between any parts of the study.
Part 1
Substance P caused dose-dependent vasodilatation in the nondominant arm (P < 0.001; anova) during the first incremental infusion of substance P which was not significantly different between screening or the 4 separate study days.
L-758 298 was administered in three descending doses: 5, 1 and 0.25 mg. During placebo and L-758 298 infusion, there were no significant changes in blood pressure, heart rate, or blood flow in the dominant forearm (Table 2). Placebo adjusted differences (95% CI) in dominant forearm blood flow, mean arterial pressure and heart rate with 5 mg of L-758 298 were 0.00 (−0.49–0.49) ml 100 ml−1 min−1, 1.0 (−3.2–5.2) mmHg and 1.9 (−5.9–9.7) beats min−1, respectively. Plasma concentrations of L-754 030 were not significantly different immediately before and after the second incremental infusion of substance P (Table 2).
Table 2.
Mean arterial pressure (MAP), heart rate (HR), forearm blood flow and plasma L-754 030 concentrations during substance P challenges in parts 1 and 2 (see Figure 1).
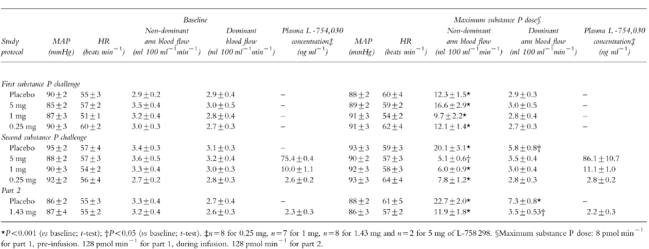
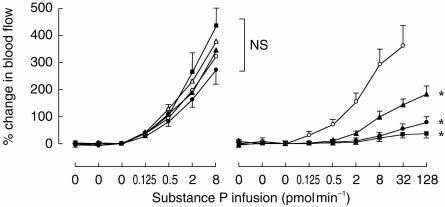
In comparison with the response to the first incremental infusion, the response to the second infusion of substance P was significantly different following placebo (P < 0.006) and all doses of L-758 298 in a dose dependent manner (Table 2; Figure 2)). The geometric mean PD100 (95% CI) increased by 1.85-fold (1.27–2.86) during placebo infusion. The influence of each dose of L-758 298 on the forearm blood flow response to substance P was examined relative to placebo. The geometric mean PD100 (95% CI) increased by 30–fold (9–99) with 0.25 mg, 319–fold (98–1044) with 1 mg and >1400–fold with 5 mg of L-758 298 (P < 0.001 for all).
Figure 2.
Part 1: Forearm blood flow responses to intrabrachial substance P infusion before (left panel) and during placebo or L-758 298 infusion (right panel). Mean±s.e.mean. ○ Placebo; ▴ 0.25 mg, • 1 mg and ▪ 5 mg of L-758 298. *P < 0.001; L-758 298 vs placebo, anova.
Forearm blood flow in the dominant arm increased at doses ≥32 pmol min−1 of substance P only during placebo (P < 0.02 vs baseline; paired t-test). Heart rate and blood pressure did not change significantly (Table 2).
Part 2
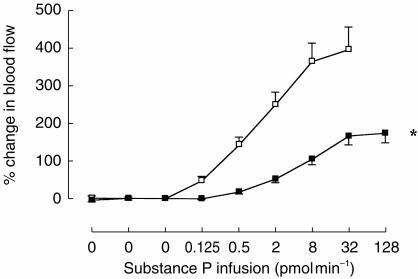
Following placebo infusion, substance P again caused dose-dependent vasodilatation in the nondominant arm (P < 0.001; anova: Figure 3). However, 24 h following intravenous administration of 1.43 mg of L-758 298, substance P induced vasodilatation was significantly inhibited (Table 2; Figure 3). The geometric mean PD100 (95% CI) was increased by 34–fold (4–299; P < 0.008). The PD100 and plasma L-754 030 concentrations (Table 2) were similar to those obtained with 0.25 mg of L-758 298 in part 1.
Figure 3.
Part 2: Forearm blood flow responses to intrabrachial substance P infusion. (Mean±s.e.mean.) Placebo; 1.43 mg of L-758,298*P < 0.001; L-758 298 vs placebo, anova.
Forearm blood flow in the dominant arm increased at doses ≥32 pmol min−1 following placebo (P < 0.02 vs baseline; paired t-test) and at 128 pmol min−1 following 1.43 mg of L-758 298 (P=0.03 vs baseline; paired t-test). Heart rate and blood pressure did not change significantly (Table 2).
Concentration-response relationship
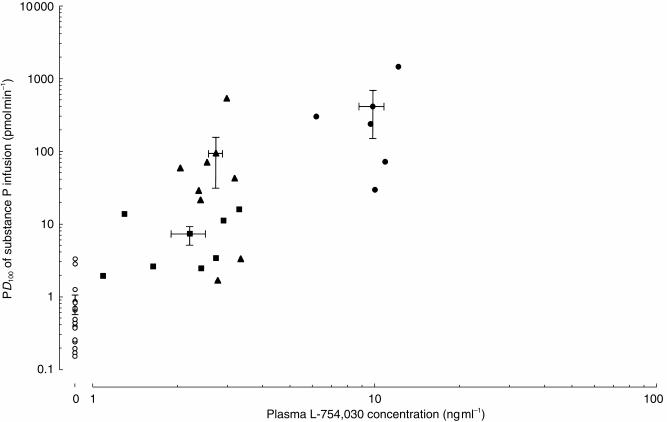
The logarithm of the mean plasma L-754 030 concentrations significantly correlated with the logarithm of the PD100 of the rate substance P infusion (r=0.62, P=0.003; Figure 4).
Figure 4.
Concentration–response relationship between the plasma concentration of L-754 030 and the PD100 values for the rate of substance P infusion. Individual values shown with mean±s.e.mean. Three points with indeterminate PD100 values >1454 are not shown. Placebo; ○ 0.25 mg, ▴ 1 mg and ▪ 24 h after 1.43 mg of L-758 298 r=0.62, P=0.003.
Tolerability
L-758 298 was generally well tolerated by all the subjects, with no excess of adverse events (mild headaches and back pain) in comparison with placebo. There were no serious adverse events during the study and no clinically significant abnormalities detected on safety monitoring (urinalysis, haematology, clinical chemistry and electrocardiography).
Discussion
For the first time, we have shown that substance P induced forearm vasodilatation is inhibited by a selective NK1 receptor antagonist in vivo in man. During L-758 298 infusion, substance P induced forearm vasodilatation was inhibited in a dose dependent manner. At the highest dose of L-758 298, the vasodilator response to substance P was abolished at doses up to 8 pmol min−1, suggesting that substance P mediated vasodilatation is entirely dependent on the endothelial NK1 receptor. Moreover, persistent inhibition of substance P induced vasodilatation was present 24 h after L-758 298 infusion.
Despite previous proposals [17, 18], it would appear that substance P, acting via the NK1 receptor, does not play a role in the regulation of peripheral vascular tone or blood pressure. We observed no alterations in baseline forearm blood flow or systemic haemodynamics following L-758 298 infusion despite a greater than 1454-fold shift in the PD100 for substance P induced forearm vasodilatation. The 95% confidence intervals indicate that if substance P provides any contribution to basal peripheral vascular tone or systemic haemodynamics then it is rather small.
We have previously shown that repeated responses to substance P are reproducible and well tolerated [24]. In the current study, we have again seen good reproducibility of both within-day and between-day responses to intra-arterial substance P infusions. Systemic effects, such as increases in contralateral forearm blood flow, were also observed at substance P doses of ≥32 pmol min−1 without significant changes in heart rate or blood pressure. Moreover, these increases in contralateral forearm blood flow were also inhibited by L-758 298 infusion. Finally, L-758 298 infusion was generally well tolerated without any significant adverse events.
Study limitations
Because L-758 298 was administered systemically, there remains the possibility that compensatory mechanisms may have obscured a potential haemodynamic effect. Direct intra-arterial administration of an NK1 receptor antagonist would provide a more precise method of assessing the role of substance P in the regulation of vascular tone. However, L-758 298 is a prodrug which requires conversion by systemic hepatic phosphatases to the active form L-754 030 and its intra-arterial administration would therefore not result in local NK1 receptor antagonism.
In the present study, we did not assess the selectivity of L-754 030 for the NK1 receptor by comparing substance P induced vasodilatation with an alternative non-NK1 receptor mediated, endothelium-dependent vasodilator, such as bradykinin or acetylcholine, and this requires confirmation in future studies. However, L-754 030 has been shown to be highly selective for the NK1 receptor [20].
We conclude that substance P induced forearm vasodilatation is mediated by the endothelial cell NK1 receptor in man. Endogenous substance P does not appear to contribute to the maintenance of peripheral vascular tone or systemic blood pressure. In this study, intravenous L-758 298 was generally well tolerated with L-754 030 causing long lasting and potent NK1 receptor antagonism in man.
Acknowledgments
Dr David Newby was the recipient of a British Heart Foundation Junior Research Fellowship (FS/95009). Professor David Webb is supported by a Research Leave Fellowship from the Wellcome Trust (WT 0526330). We would like to acknowledge the assistance of Marvin Constanzer and Cynthia Chavez-Eng in performing the plasma assays for L-754 030.
References
- 1.Hökfelt T, Elfvin L-G, Schultzberg M, Goldstein M, Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977;132:29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg J, Hökfelt T, Kewenter J, et al. Substance P-, VIP-, and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology. 1979;77:468–471. [PubMed] [Google Scholar]
- 3.Pernow B. Substance P. Pharmacol Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- 4.Aronin N, Coslovsky R, Leman SE. Substance P and neurotensin: their role in the regulation of anterior pituitary function. Ann Rev Physiol. 1986;48:537–549. doi: 10.1146/annurev.ph.48.030186.002541. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ, Belvisi MG, Rogers DF. Modulation of neurogenic inflammation: novel approaches to inflammatory disease. Trends Pharmacol Sci. 1990;11:185–189. doi: 10.1016/0165-6147(90)90112-l. [DOI] [PubMed] [Google Scholar]
- 6.Cappugi P, Tsampau D. Substance P provokes cutaneous erythema and edema through a histamine-independent pathway. Int J Dermatol. 1992;31:206–209. doi: 10.1111/j.1365-4362.1992.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith CH, Barker JN, Morris RW. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–3282. [PubMed] [Google Scholar]
- 8.Coiro V, Capretti L, Volpi R, et al. Stimulation of ACTH/cortisol by intravenously infused substance P in normal men: inhibition by sodium valproate. Neuroendocrinology. 1992;56:459–463. doi: 10.1159/000126262. [DOI] [PubMed] [Google Scholar]
- 9.Stjärne P, Rinder J, Delay-Goyet P. Effects of NK1 receptor antagonists on vasodilation induced by chemical and electrical activation of sensory C-fibre afferents in different organs. Acta Physiol Scand. 1994;152:153–161. doi: 10.1111/j.1748-1716.1994.tb09795.x. [DOI] [PubMed] [Google Scholar]
- 10.Löfström B, Pernow B, Wahren J. Vasodilating action of substance P in the human forearm. Acta Physiol Scand. 1965;63:311–324. doi: 10.1111/j.1748-1716.1965.tb04070.x. [DOI] [PubMed] [Google Scholar]
- 11.Eklund B, Jogestrand T, Pernow B. Effect of substance P on resistance and capacitance vessels in the human forearm. In: von Euler Pernow., editor. Substance P. New York: Raven Press; 1977. [Google Scholar]
- 12.McEwan JR, Benjamin N, Larkin S, Fuller RW, Dollery CT, MacIntyre I. Vasodilatation by calcitonin gene-related peptide and substance P. a comparison of their effects on resistance and capacitance vessels of human forearms. Circulation. 1988;77:1072–1080. doi: 10.1161/01.cir.77.5.1072. [DOI] [PubMed] [Google Scholar]
- 13.Gross DR, Fiscus RR, Arden WA, Maley RH, Lanzo S, Salley RK. Substance P induces biphasic endothelium-dependent relaxations in pig and rabbit carotid arteries. Neuropeptides. 1994;26:329–341. doi: 10.1016/0143-4179(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Cockcroft JR, Chowienczyk PJ, Brett SE, Ritter JM. Effect of NG-monomethyl-l-arginine on kinin-induced vasodilation in the human forearm. Br J Clin Pharmacol. 1994;38:307–310. doi: 10.1111/j.1365-2125.1994.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newby DE, Boon NA, Webb DJ. Comparison of forearm vasodilatation induced by substance P and acetylcholine: contribution of nitric oxide. Clin Sci. 1997;92:133–138. doi: 10.1042/cs0920133. [DOI] [PubMed] [Google Scholar]
- 16.Weihe E, Reinecke M, Opherk D, Forssman WG. Peptidergic innervation (substance P) in the human heart. J Mol Cell Cardiol. 1981;13:331–333. doi: 10.1016/0022-2828(81)90321-7. [DOI] [PubMed] [Google Scholar]
- 17.Crossman DC, Larkin SW, Fuller RW, Davies GJ, Maseri A. Substance P dilates epicardial coronary arteries and increases coronary blood flow in humans. Circulation. 1989;80:475–484. doi: 10.1161/01.cir.80.3.475. [DOI] [PubMed] [Google Scholar]
- 18.Quyyumi AA, Mulcahy D, Andrews NP, Husain S, Panza JA, Cannon RO. Coronary vascular nitric oxide activity in hypertension and hypercholesterolemia. Circulation. 1997;95:104–110. doi: 10.1161/01.cir.95.1.104. [DOI] [PubMed] [Google Scholar]
- 19.Navari RM, Reinhardt RR, Gralla RJ, et al. for the L-754,030 antiemetic trial group. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. N Engl J Med. 1999;340:190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 21.Webb DJ. The pharmacology of human blood vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 23.Wiinberg N, Walter-Larson S, Eriksen C, Nielsen PE. An evaluation of semi-automatic blood pressure manometers against intra-arterial blood pressure. J Ambulatory Monitoring. 1988;1:303–309. [Google Scholar]
- 24.Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NA, Webb DJ. Intra-arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. Br J Clin Pharmacol. 1997;43:493–499. doi: 10.1046/j.1365-2125.1997.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerslake D, McK McK. The effect of the application of an arterial occlusion cuff to the wrist on the blood flow in the human forearm. J Physiol (Lond) 1949;108:451–457. doi: 10.1113/jphysiol.1949.sp004348. [DOI] [PMC free article] [PubMed] [Google Scholar]