Abstract
Aims
To test the hypothesis that inhibition of cytochrome P450 2D6 (CYP2D6) by quinidine increases the antitussive effect of dextromethorphan (DEX) in an induced cough model.
Methods
Twenty-two healthy extensive metaboliser phenotypes for CYP2D6 were studied according to a double-blind, randomised cross-over design after administration of: (1) Placebo antitussive preceded at 1 h by placebo inhibitor; (2) 30 mg oral DEX preceded at 1 h by placebo inhibitor (DEX30); (3) 60 mg oral DEX preceded at 1 h by placebo inhibitor (DEX60); (4) 30 mg oral DEX preceded at 1 h by 50 mg oral quinidine sulphate (QDEX30). Cough frequency following inhalation of 10% citric acid was measured at baseline and at intervals up to 12 h. Plasma concentrations of DEX and its metabolites were measured up to 96 h by h.p.l.c.
Results
Inhibition of CYP2D6 by quinidine caused a significant increase in the mean ratio of DEX to dextrorphan (DEX:DOR) plasma AUC(96) (0.04 vs 1.81, P < 0.001). The mean (±s.d.) decrements in cough frequency below baseline over 12 h (AUEC) were: 8% (11), 17% (14.5), 25% (16.2) and 25% (16.9) for placebo, DEX30, DEX60 and QDEX30 treatments, respectively. Statistically significant differences in antitussive effect were detected for the contrasts between DEX60/placebo (P < 0.001; 95% CI of difference +80, +327) and QDEX30/placebo (P < 0.001, +88, +336), but not for DEX30/placebo, DEX30/DEX60 or DEX30/QDEX30 (P = 0.071, −7, +241; P = 0.254, −37, +211; P = 0.187, −29, +219, respectively).
Conclusions
A significant antitussive effect was demonstrated after 60 mg dextromethorphan and 30 mg dextromethorphan preceded by 50 mg quinidine using an induced cough model. However, although the study was powered to detect a 10% difference in cough response, the observed differences for other contrasts were less than 10%, such that it was possible only to imply a dose effect (30 vs 60 mg) in the antitussive activity of DEX and enhancement of this effect by CYP2D6 inhibition.
Keywords: antitussive effect, CYP2D6, dextromethorphan, genetic polymorphism
Introduction
Dextromethorphan, a codeine analogue devoid of opiate side-effects, is widely available over-the-counter as a cough suppressant. Its efficacy has been confirmed in both clinical cough [1–5] and in experimental cough challenge studies [6, 7]. Metabolism of dextromethorphan is largely by CYP2D6-mediated O-demethylation to dextrorphan, which then undergoes glucuronide formation [8]. N-demethylation to 3-methoxymorphinan also occurs, largely by CYP3A4 but with contributions from CYPs 2C9 and 2C19 [9–12]. Both dextrorphan and 3-methoxymorphinan are further metabolized to 3-hydroxymorphinan, by CYPs 3A4 and 2D6, respectively [13]. It is well established that the overall disposition of dextromethorphan is highly dependent upon CYP2D6 activity, which varies widely due to genetic polymorphism, and which can be inhibited selectively and significantly by a small dose of quinidine [14–16].
Capon et al. [17] attempted to establish differences in experimental cough suppression by dextromethorphan in normal subjects as a function of CYP2D6 phenotype (extensive metabolisers (EM), genetically poor metabolisers (PM), and PM phenocopies produced by coadministration of quinidine). However, the study was inconclusive because of insufficient statistical power. The volunteers were not screened for their ability to respond to the tussive challenge with capsaicin, and the sensitivity of the method was not validated by assessing a dose–response relationship. In the present study, we have compared the antitussive effect (citric acid challenge) of dextromethorphan at two dose levels (30 and 60 mg) and after inhibition of CYP2D6 by coadministration of quinidine, using a panel of subjects selected to be sensitive to the cough challenge. The study was powered to detect a 10% difference in the efficacy of cough suppression between treatment groups.
Methods
Subjects
Inclusion criteria for the study were EM phenotype for CYP2D6 (determined using debrisoquine) [18], age 18–45 years, a normal ECG, and FEV1>80% predicted value. In addition, the subjects were required to exhibit a cough response between 7 and 14 coughs when screened on two occasions at least 5 days apart (interoccasion difference < 40%; intraoccasion difference < 20%). At the first screening, a baseline cough response was established. On the second occasion, the cough challenge was administered at baseline and every hour for 3 h. Exclusion criteria were smoking, asthma or other significant respiratory or systemic illness, respiratory tract infection or acute cough in the previous month.
Twenty-two subjects (12 male, 10 female, mean age 24 years), fulfilling the criteria, were recruited to the study. They gave written informed consent to the investigation, which was approved by the South Sheffield Research and Ethics Committee.
Drugs
Dextromethorphan was given orally as a 60 ml solution containing 30 or 60 mg dextromethorphan hydrobromide, made from a stock solution (13.5 mg ml−1; Boots Tusana Cough Syrup). A placebo solution with similar taste was prepared by the Pharmacy Department of the Royal Hallamshire Hospital. Quinidine sulphate (50 mg) and quinidine placebo were administered in the form of identical white capsules.
Study design
Subjects received four treatments at least 5 days apart according to a randomised (Latin Square) crossover, double-blind design. Each treatment was commenced at 07.30 h. The treatments were: (1) dextromethorphan placebo, preceded at 1 h by quinidine placebo; (2) dextromethorphan 30 mg (DEX30), preceded at 1 h by quinidine placebo; (3) dextromethorphan 60 mg (DEX60), preceded at 1 h by quinidine placebo; (4) dextromethorphan 30 mg, preceded at 1 h by quinidine sulphate 50 mg (QDEX30).
The cough challenge was performed at t = −1 h (immediately before giving placebo inhibitor or active quinidine), t = 0 h (immediately before dosing with active or placebo dextromethorphan) and at 1, 2, 4, 6, 8, 10 and 12 h after dosing with active or placebo dextromethorphan. Cough response at baseline was required to be within 40% of that at initial screening.
Reference data obtained on the screening days showed that the average interoccasion cough response was 10.3 coughs (or 123 coughs over 12 h), with a coefficient of variation (CV) of 13%. Using equation 1 [19], it was estimated that the use of 22 subjects in a parallel comparison would provide a power of 90% to detect a 10% change in cough response (12 coughs over 12 h) between treatment groups at α = 0.025.
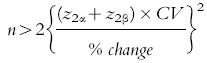 |
1 |
(where: n = number of subjects; z2α and z2β = the standardized normal deviate exceeded (in either direction) with probabilities of 2α and 2β, respectively; α and β = one side probability of type I and type II errors, respectively; and CV = coefficient of variation).
The power of the study to detect a similar change from multiple comparisons of the four treatments was estimated to be at least 80% (using the most conservative assumption that α errors are additive).
Cough challenge
A 10% w/v solution of citric acid was prepared by diluting a stock 1 m solution with 0.9% saline, and 3 ml was placed in a nebulizing chamber. The subjects were required to inhale the nebulized solution over 1 s using a breath-activated dosimeter driven by compressed air (Dosimeter MB3, Mefar Elletromedicale). Five inhalations were performed at 1 min intervals over 5 min. The number of coughs was counted following each inhalation and summed for the total 5 min period.
Blood sampling, drug and metabolite assay
Peripheral venous blood samples (10 ml) were taken though an indwelling Teflon cannula (Wallace Y-Can, Simcare Ltd, W. Sussex UK) at baseline and 0.5, 1, 2, 3, 4, 8, 12, 24, 48, 72 and 96 h after administration of dextromethorphan. The plasma was separated by centrifugation and stored at −20° C until assay. Dextromethorphan (DEX), dextrorphan (DOR) (free and conjugated), 3-methoxymorphinan and 3-hydroxymorphinan (free and conjugated) were measured by the method of Chen et al. [20]. Intra-assay coefficients of variation for the analytes at 2.5 ng ml−1 were between 6 and 13%. Limits of quantification were 0.3 ng ml−1 for dextrorphan and 3-hydroxymorphinan, and 0.5 ng ml−1 for dextromethorphan and 3-methoxymorphinan.
Statistical analysis
Cough suppression was measured as the area under the decrement in cough response below baseline up to 12 h (AUEC), calculated using the linear trapezoidal rule. The area under the plasma concentration–time curve up to 12 h (AUC(0,12h)) of dextromethorphan was also calculated using the linear trapezoidal rule. Treatments were compared using the General Linear Model (GLM) of anova in SPSS v 7.5. Tukey’s post hoc test was used for multiple comparisons. Subgroup analysis of phenocopiers was performed similarly. The Wilcoxon signed rank test was used to compare metabolic ratios between treatment groups and the Monte Carlo method to obtain (asymptotic) significance levels and confidence intervals.
The effect of quinidine on cough was examined in each treatment arm using AUEC values from t = −1 h to t = 0 h (pre and post quinidine or placebo inhibitor administration).
Results
Summary pharmacokinetic parameters for each treatment are listed in Table 1. The median DEX:DOR plasma AUC(96) ratio after DEX30 was 0.02 (mean 0.04, range 0–0.18). DOR was calculated as free and conjugated compound. The metabolic ratio was increased significantly after administration of 50 mg quinidine (median 0.44, mean 89.9, P < 0.001, 95% CI of difference = +0.00, +0.03). Thus, all of the subjects phenocopied as ‘poor metabolisers’ with respect to CYP2D6 after quinidine administration, using the antimode of 0.126 for plasma DEX:DOR reported by Kohler et al. [21].
Table 1.
Pharmacokinetic parameters of DEX and its metabolites according to treatment group. [Data are median values; range is given in parenthesis. Cmax = maximum plasma drug concentration; tmax = time to reach Cmax = AUC(0, 96 h) = area under the plasma drug concentration-time from 0 h to 96 h; AUC (0, 12 h) = area under the plasma drug concentration-time curve from zero to 12 h]
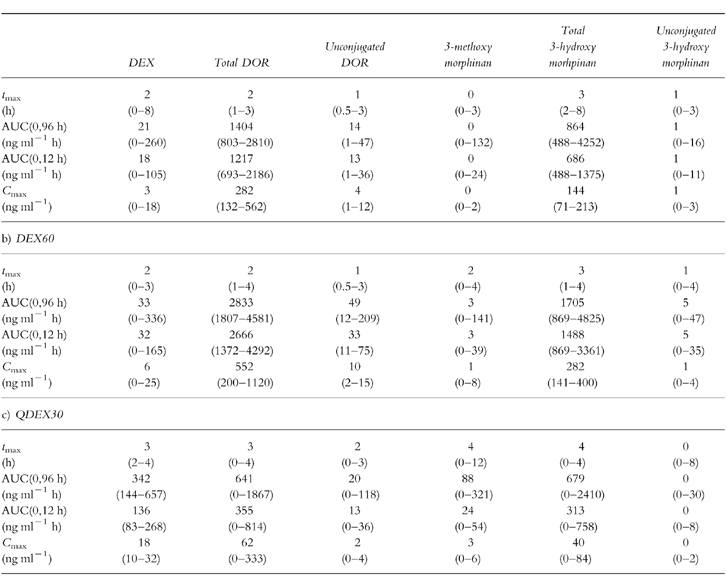
The median AUC(12) of DEX after DEX30 was increased significantly by 7.5-fold (P < 0.001; 95% CI of difference = +137, +94) and that of DOR AUC was decreased significantly by 3.4-fold (P < 0.001; 95% CI of difference = −534, −1242) by coadministration of quinidine (QDEX30); the median Cmax of DEX increased 6-fold (P < 0.001; 95% CI of difference = +10, +19) and the median value of tmax increased from 2 h to 3 h. CYP2D6 inhibition also resulted in a corresponding increase in 3-methoxymorphinan and lowering of 3-hydroxymorphinan concentrations. With regard to the comparison between DEX30 and DEX60, median values of both the AUC(0,12h) and Cmax of DEX were increased by only 1.7-fold (P = 0.06; 95% CI of difference = +42, −1 and P = 0.14; 95% CI of difference = +8, −0.9, respectively) as the dose was raised, while tmaxremained unchanged. The median plasma DOR AUC(12) increased by two-fold (P < 0.001; 95% CI of difference = +1830, +1122).
Comparisons of the cough response after quinidine (pre-DEX30) and each administration of placebo inhibitor (pre-placebo DEX, pre-DEX30 and pre-DEX60) did not detect any antitussive effect of quinidine (P = 0.36, 95% CI of difference = −15.7, +3.6; P = 0.99, −10.5, +8.8; P = 0.9, −7.2, +12.2, respectively).
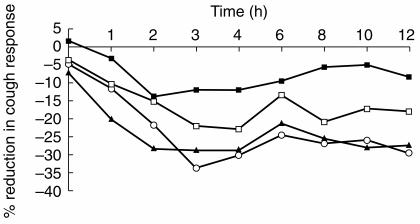
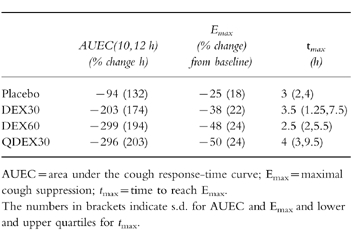
The time-courses of the mean cough response after each treatment are shown in Figure 1, and changes in integral (AUEC) values, maximal cough suppression (Emax) and the median times at which maximum effect occurred are listed in Table 2. The DEX60 and QDEX30 treatments produced a maximum response of 50% suppression compared with 25% after placebo. Changes in AUEC values after DEX60 and QDEX30 were similar (P = 0.998; 95% CI of difference = −116+131), and both were significantly different from that after placebo (P < 0.001; 95% CI of difference = +80, +327; P < 0.001; +88, +336, respectively). In contrast, the change in AUEC after DEX30 was not significantly different from that after placebo, DEX60 or QDEX30 (P = 0.071, 95% CI of difference = −7, +241; P = 0.254, −37, +211; P = 0.187, −29, +219, respectively).
Figure 1.
Mean cough response in the four treatment groups –▪– Placebo, –□– DEX 30, –▴– DEX60, –•– QDEX30
Table 2.
Mean cough response in the four treatment groups and median times at which maximum effect occurred.
Although all subjects phenocopied after quinidine based on a plasma DEX:DOR antimode of 0.126 [21], only 81% and 68% did so using alternative values of the antimode of 0.24 and 0.3 derived from urinary data [22, 23] applied to our plasma data. However, subgroup analysis of the cough response data for phenocopiers using the alternative antimodes did not alter the results of the statistical evaluation.
Discussion
We confirm the results of previous studies [14–16] showing that quinidine markedly inhibits the metabolism of dextromethorphan, causing a profound increase in the ratio of parent compound to metabolites. However, the pharmacodynamic changes accompanying these large pharmacokinetic differences were much smaller. Although a significant antitussive effect of 60 mg dextromethorphan was demonstrated using the citric acid cough challenge test, the study failed to show a significant effect of the 30 mg dose relative to placebo or a dose effect between 30 and 60 mg. This was despite a statistical power to detect a 10% difference, and the careful selection of subjects who produced a clear, reproducible cough response. The latter screening procedure eliminates 70–75% of individuals [24].
All of our subjects phenocopied with respect to CYP2D6 after administration of quinidine 50 mg, based on the reported antimode for plasma DEX:DOR [21]. In contrast, the median urinary DEX:DOR ratio in the subjects of the study by Capon et al. [17] after quinidine administration was 0.17, which is lower than the antimode of 0.24–0.3 reported to separate PM and EM phenotypes [22, 23].
Since we did not detect a statistically significant increase in antitussive effect on adding quinidine to dextromethorphan (DEX30 vs QDEX30), the central hypothesis that individuals genetically deficient in CYP2D6 activity or phenocopies produced by enzyme inhibition have a greater response was not proven directly. This mirrors the outcome of the study by Caponet al. [17]. Nevertheless, the addition of quinidine to 30 mg dextromethorphan did produce a significantly greater response compared to placebo whereas 30 mg dextromethorphan alone did not.
Nothing is known about the relative contributions of dextromethorphan and its metabolites to antitussive activity in humans, although Bragaet al. [25] found that dextrorphan had comparable activity to dextromethorphan using the citric acid model in guinea pigs.
Since quinidine markedly inhibits the conversion of dextromethorphan to dextrorphan, and a significant antitussive effect was demonstrated after QDEX30 but not after DEX30, this would suggest that the parent drug is the major active moiety in humans. However, a contribution of 3-methoxymorphinan to net activity cannot be excluded since plasma concentrations of this metabolite were highest after the QDEX30 treatment. The presence of either free or conjugated 3-hydroxymorphinan does not appear to contribute significantly to activity since plasma concentrations of these compounds were lowest after the QDEX30 treatment (Table 1c). The similarity in efficacy of DEX60 and QDEX30 despite higher plasma dextromethorphan concentrations associated with the latter (Table 1b, Table 1c) may reflect a saturation of the concentration-response relationship for parent drug or the differential concentrations of the metabolites (especially dextrorphan) following the two treatments. Further studies are in progress to elucidate the contribution of the metabolites of dextromethorphan to its antitussive effects, involving the development of a pharmacokinetic-pharmacodynamic model and the administration of dextrorphan per se.
In conclusion, we have shown that DEX60 and QDEX30 are both significantly active in comparison to placebo, but only trends were apparent with regard to DEX30 vs placebo and DEX60 and QDEX30 vs DEX30. Based on the variability of these data, and an average difference of 8% reduction in cough response (25% reduction by QDEX30 (or DEX60) vs 17% by DEX30), at least 96 subjects would be required to show a significant difference in effect between DEX30 and QDEX30 (or DEX60). Thus, any influence of CYP2D6 phenotype on the cough response to dextromethorphan is grossly overshadowed by variability in the pharmacodynamic end-point, at least with respect to experimental if not clinical cough.
References
- 1.Cass LJ, Frederik WS. Evaluation of a new antitussive agent. N Engl J Med. 1953;249:132–136. doi: 10.1056/NEJM195307232490402. [DOI] [PubMed] [Google Scholar]
- 2.Matthys H, Bleicher B, Bleicher U. Dextromethorphan and codeine: objective assessment of antitussive activity in patients with chronic cough. J Int Med Res. 1983;11:92–100. doi: 10.1177/030006058301100206. [DOI] [PubMed] [Google Scholar]
- 3.Cass LJ, Frederik WS. Quantitative comparison of cough suppressant effects of Romilar and other antitussive agents. J Lab Clin Med. 1956;48:879–885. [PubMed] [Google Scholar]
- 4.Aylward M, Maddock J, Davies D, Protheroe D, Leideman T. Dextromethorphan and codeine: comparison of plasma kinetics and antitussive effects. Eur J Resp Dis. 1984;65:283–291. [PubMed] [Google Scholar]
- 5.Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulmon Pharmacol. 1996;9:299–308. doi: 10.1006/pulp.1996.0039. [DOI] [PubMed] [Google Scholar]
- 6.Grattan TJ, Marshall AE, Higgins KS, Morice AH. The effect of inhaled and oral dextromethorphan on citric acid induced cough in man. Br J Clin Pharmacol. 1995;39:261–263. doi: 10.1111/j.1365-2125.1995.tb04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kartunnen P, Tukiainen H, Silvasti M, Kolonen S. Anti-tussive effect of dextromethorphan and dextromethorphan-salbutamol combination in healthy volunteers with artificially induced cough. Respiration. 1987;52:49–53. doi: 10.1159/000195303. [DOI] [PubMed] [Google Scholar]
- 8.Barnhart JW. The urinary excretion of dextromethorphan and three metabolites in dogs and humans. Toxicol Appl Pharmacol. 1980;55:43–48. doi: 10.1016/0041-008x(80)90218-5. [DOI] [PubMed] [Google Scholar]
- 9.Gorski JC, Jones DR, Wrighton SA, Hall SD. Characterisation of dextromethorphan N-demethylation by human liver microsomes—contribution of the cytochrome P450, 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;48:173–182. doi: 10.1016/0006-2952(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 10.Von Moltke LL, Greenblatt DJ, Grassi JG, et al. Revaluation of the specificity of dextromethorphan as an index substrate. Clin Pharmacol Ther. 1998;63:227. [Google Scholar]
- 11.Von Moltke LL, Greenblatt DJ, Grassi JG, et al. Multiple human chromosomes contribute to biotransformation of dextromethorphanin vitro: role of CYP2C9, CYP2C19, CYP2D6, and CYP3A. J Pharm Pharmacol. 1998;50:997–1004. doi: 10.1111/j.2042-7158.1998.tb06914.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmider J, Greenblatt DJ, Fogelman SM, Von Moltke LL, Shader RI. Metabolism of dextromethorphan in vitro: involvement of cytochromes P450 2D6 and 3A3/4, with a possible role of 2E1. Biopharm Drug Dispos. 1997;18:227–240. doi: 10.1002/(sici)1099-081x(199704)18:3<227::aid-bdd18>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Jacq-Aigran E, Funck-Brentano C, Cresteil T. CYP2D6- and CYP3A-dependent metabolism of dextromethorphan in humans. Pharmacogenetics. 1993;3:197–204. doi: 10.1097/00008571-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen MD, Brosen K, Gram LF. A dose-effect study of the in vivo inhibitory effect of quinidine on sparteine oxidation in man. Br J Clin Pharmacol. 1990;29:299–304. doi: 10.1111/j.1365-2125.1990.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinn R, Brosen K, Gram LF, Haghfelt T, Otton SV. Sparteine oxidation is practically abolished in quinidine treated patients. Br J Clin Pharmacol. 1986;22:194–197. doi: 10.1111/j.1365-2125.1986.tb05250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Britto MR, Valderhaug KL, Wedlund PJ, Smith RA. Dextromethorphan: enhancing its systemic bioavailability by way of low-dose quinidine-mediated inhibition of cytochome P4502D6. Clin Pharmacol Ther. 1992;51:647–655. doi: 10.1038/clpt.1992.77. [DOI] [PubMed] [Google Scholar]
- 17.Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA. The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther. 1996;60:296–307. doi: 10.1016/S0009-9236(96)90056-9. [DOI] [PubMed] [Google Scholar]
- 18.Lennard MS, Silas JH, Smith AJ, Tucker GT. Determination of debrisoquine and its 4-hydroxy metabolite in biological fluids by gas chromatography with flame-ionization and nitrogen-selective detection. J Chromatogr. 1977;133:161–166. doi: 10.1016/s0021-9673(00)89216-x. [DOI] [PubMed] [Google Scholar]
- 19.Armitage P, Berry G. Statistical MethodsIn Medical Research. 2. Blackwell Scientific Publications; 1987. The planning of statistical investigations; p. 182. [Google Scholar]
- 20.Chen ZR, Somogyi AA, Bochner F. Simultaneous determination of dextromethorphan and 3 metabolites in plasma and urine using high-performance liquid-chromatography with application to their disposition in man. Ther Drug Monit. 1990;12:97–104. doi: 10.1097/00007691-199001000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Kohler D, Hartter S, Fuchs K, Siegarhart W, Hiemke C. CYP2D6 genotype and phenotyping by determination of dextromethorphan and metabolites in serum of healthy controls and of patients under psychotropic medication. Pharmacogenetics. 1997;7:453–461. doi: 10.1097/00008571-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Henthorn TK, Benitez J, Avram MJ, et al. Assessment of the debrisoquin and dextromethorphan phenotyping tests by Gaussian mixture distributions analysis. Clin Pharmacol Ther. 1989;45:328–333. doi: 10.1038/clpt.1989.36. [DOI] [PubMed] [Google Scholar]
- 23.Guttendorf RJ, Britto M, Blouin RA, Foster TS, John W, Pittman KA, et al. Rapid screening for polymorphisms in dextromethorphan and mephenytoin metabolism. Br J Clin Pharmacol. 1990;29:373–380. doi: 10.1111/j.1365-2125.1990.tb03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morice AH. Inhalation cough challenge in the investigation of the cough reflex and antitussives. Pulmon Pharmacol. 1996;9:281–284. doi: 10.1006/pulp.1996.0036. [DOI] [PubMed] [Google Scholar]
- 25.Braga PC, Fossati A, Vimercati MG, Caputo R, Guffanti EE. Dextrorphan and dextromethorphan: comparative antitussive effects on guinea pigs. Drugs Exp Clin Res. 1994;20:199–203. [PubMed] [Google Scholar]