Introduction
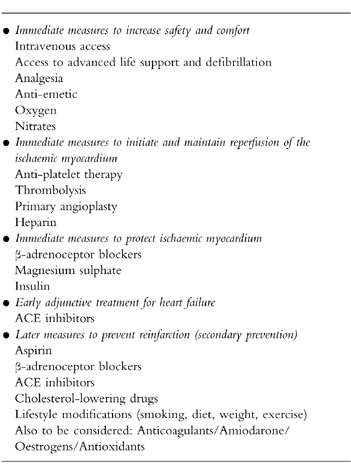
Ischaemic heart disease remains the commonest cause of death in the UK. Among its various manifestations, acute myocardial infarction continues to present a particular challenge to emergency health services. This case will be used to illustrate some of the therapeutic advances that have been made in the management of myocardial infarction over recent years and will highlight some of the areas that remain controversial. The review will concentrate on the immediate and early management priorities that arise within the first day of admission to hospital (Table 1).
Table 1.
Therapeutic interventions in acute myocardial infarction.
Case presentation
A 57 year old man presented to the Accident and Emergency Unit of his local district general hospital complaining of 2 h of central ‘crushing’ chest pain radiating to the neck and shoulders. This had begun shortly after dinner and was more severe than any pain he had previously experienced. His wife explained that for the last 4 months he had been having episodes of ‘indigestion’ at work which were only partially relieved by self-administered antacid preparations. They had first rung their General Practitioner but he had advised them to call for an ambulance urgently. The past medical history included a diagnosis of hypertension 8 years earlier and a hernia repair 3 years earlier. He was also diagnosed as having a duodenal ulcer at endoscopy 2 years earlier but this had responded well to a course of cimetidine. At the time of admission his only relevant drug history was nifedipine 20 mg twice daily. The family history included the sudden death of his father aged 55 years (cause unknown). It was also known that his mother was hypertensive, his elder brother had angina and that his paternal grandmother had type 2 diabetes mellitus. He was married with two sons and worked as a bus driver. He was a regular smoker (20 per day) and consumed approximately 16 units of alcohol per week.
On examination he looked grey and unwell, and was sweating. There was an early lower segment corneal arcus. The blood pressure was 138/94 mmHg, pulse 96 regular and the jugular venous pressure was not elevated. The apex beat was undisplaced but thrusting in character and the heart sounds were normal. The peripheries were cold to the touch but there was no ankle oedema. The foot pulses on the left were absent and there was a femoral bruit on the left. He was mildly tachypnoeic but the chest was clear on auscultation. There was an old herniorrhaphy scar visible in the right groin.
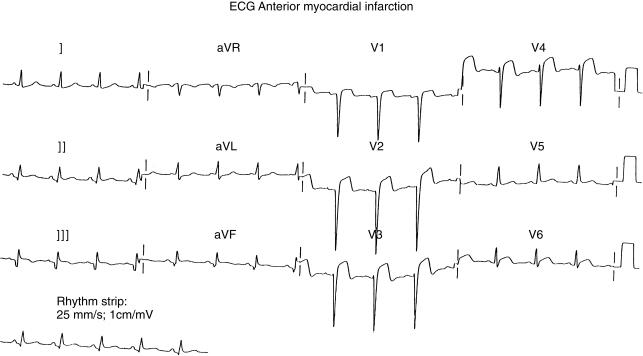
The initial electrocardiogram demonstrated sinus rhythm and 4 mm of ST elevation in leads V2 to V5 (Figure 1). The diagnosis of acute anterior myocardial infarction was made.
Figure 1.
The electrocardiogram taken on presentation to the Accident and Emergency Unit.
The results of the initial blood tests were:
Hb 13 4 g l−1WCC 7.4×109 l−1Platelets 345×109 l−1
Na+ 138 mmol l−1
K+ 4.2 mmol l−1
Urea 4.5 mmol l−1
Creatinine 95 μmol l−1
Glucose 7.6 mmol l−1
CK 160 iu l−1
LDH 580 iu l−1
Immediate management priorities
The immediate concerns for a patient with suspected myocardial infarction should be their safety and comfort. Intravenous access must be available for effective administration of emergency drug therapy followed by rapid transfer to an area with a high level of supervision and resuscitation facilities. Increasing the comfort of the patient includes administration of oxygen, analgesia, anti-emetics and nitrates. An ECG should be arranged rapidly.
Supervision and monitoring
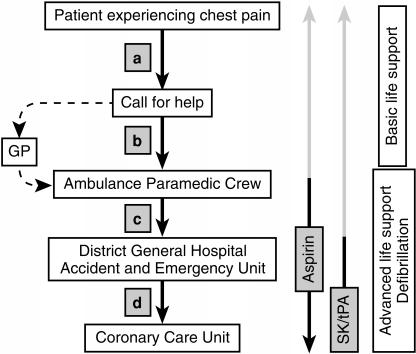
Assessment and treatment should not be delayed: acute myocardial infarction is a medical emergency where minutes can make a difference. Progress of the index case through these stages of care is illustrated in Figure 2. Rapid movement through the care pathway is essential because a malignant ventricular arrhythmia is a major and reversible cause of death in the early hours after the onset of infarction. The long-term prognosis will depend on the salvage of myocardium, particularly with thrombolysis. However, the effectiveness of thrombolysis is greatest if it is given early. Bringing emergency diagnosis and drug treatment to the patient in order to reduce delays in treatment has been explored [1–3].
Figure 2.
The progress of the typical patient with myocardial infarction through the care pathway. Time delays to effective therapy are (a) time for recognition of the potential seriousness of symptoms by the patient, (b) arrival of help with advanced life support experience, (c) transfer to hospital, and (d) triage and transfer from the Accident and Emergency Unit to the Coronary Care Unit. Aspirin is available in most homes and can easily be administered at an early stage. Thrombolytic therapy is largely restricted to hospital usage although recent trials suggest that it can be successfully used in the community [1–3].
Oxygen
Arterial hypoxaemia is a frequent occurrence during the first 24 h post-myocardial infarction and is more common after the use of opiate analgesia [4]. The largest controlled trial of oxygen therapy in 157 patients with uncomplicated myocardial infarction failed to demonstrate any differences in mortality, arrhythmias or analgesic usage [5]. However, this was carried out prior to the introduction of reperfusion therapy for the ischaemic myocardium. The efficiency of reperfusion might be influenced by the oxygen content in the blood. In support of this view, decreased infarct size and increased left ventricular ejection fraction have been demonstrated with high inspired oxygen concentration after reperfusion in a canine model of myocardial infarction [6]. Therefore it seems prudent that oxygen therapy should be given routinely within the first 24 h after an acute myocardial infarction.
Analgesia and anti-emetics
The pain of myocardial infarction is usually severe and requires potent opiate analgesia. Intravenous diamorphine 2.5–5 mg (repeated as necessary) is the drug of choice and is not only a powerful analgesic but also has a useful anxiolytic effect. The use of opiates may be associated with nausea and vomiting which can be prevented with anti-emetic drugs such as cyclizine 50 mg or metoclopramide 10 mg given intravenously. Opiate analgesia may also be associated with arterial hypoxaemia (see above). For some patients the psychological stress of a coronary event and the environment of a coronary care unit can provoke severe anxiety; the use of diazepam 2–5 mg orally as an anxiolytic and hypnotic may be useful.
Nitrates
Nitrates reduce myocardial workload and hence myocardial oxygen demand by reducing preload (venodilatation) and afterload (reduced peripheral resistance and blood pressure). They may also improve myocardial blood flow by coronary vasodilatation although this has been much debated. Nitrates have been available for over a hundred years and are still the first-line drugs for relieving acute ischaemic pain. In the context of acute myocardial infarction many patients derive some pain relief from glyceryl trinitrate administered sublingually (0.5 mg), as buccal tablets (Suscard® 3–5 mg) or via intravenous infusion. A major question is whether in addition to giving symptomatic relief, nitrates might also improve prognosis if given routinely in the early post-infarct period. This issue has been addressed in three studies.
ISIS-4 examined the use of a slow-release isosorbide mononitrate given acutely (30 mg) and then daily (60 mg) for 28 days compared with standard treatment in 58 050 patients [7]. There was a small non-significant reduction in mortality at 5 weeks (7.34%vs 7.54%) and at 1 year among patients allocated the nitrate compared with control. Also, there was a significant reduction in early deaths on days 0–1 in the nitrate group when compared with the control groups (1.77%vs 2.16%). However, there was no overall advantage from the use of nitrates in those patients who received early treatment, who had heart failure or who had not already received nitrates. Discontinuation of the study drug due to severe hypotension occurred significantly more frequently in the nitrate-treated group (8.1%vs 6.7%). GISSI-3 examined the use of a 24 h intravenous infusion of glyceryl trinitrate (GTN) followed by 6 weeks of transdermal GTN in almost 20 000 patients but showed only a small non-significant trend towards improved survival [8]. A similar trend was observed among 4000 patients randomised to 2 weeks of the nitric oxide donor molsidomine or placebo in the ESPRIM study [9]. Taken together, these studies suggest that, although routine use of nitrates does not confer a long-term survival advantage, their early use appears to be safe and well-tolerated for patients with ischaemic pain or left ventricular failure.
The patient was rapidly transferred to the Coronary Care Unit arriving within 20 min of presentation at the A&E unit. His pain was much eased by intravenous diamorphine and buccal glyceryl trinitrate. His blood pressure was now 128/84 mmHg and pulse 88 beats min−1in sinus rhythm with occasional ventricular premature beats. A repeat ECG on arrival in the coronary care unit was unchanged. The registrar in charge of Coronary Care considered the further management.
Immediate measures to salvage threatened myocardium
Long-term prognosis for patients after myocardial infarction depends on their residual cardiac function [10]. It is now accepted that myocardial infarction usually results from thrombus in a diseased atherosclerotic coronary artery [11]. A close relationship has been demonstrated between early patency of the infarct-related coronary artery, myocardial salvage and survival [12, 13]. Therefore, early recanalisation of the thrombosed coronary artery is of paramount importance in the salvage of threatened ischaemic myocardium and, by extension, improvement in the quality and length of survival. The three major therapies available for this purpose are anti-platelet drugs, thrombolytic agents and primary coronary angioplasty.
Aspirin
Aspirin has potent anti-platelet effects as a result of the inhibition of platelet cyclo-oxygenase. Daily doses of at least 75 mg can produce almost complete enzyme inhibition in less than 1 h. The duration of this effect is limited by the turnover of circulating platelets. The impact of 160 mg enteric-coated aspirin in suspected acute myocardial infarction was examined in 17 187 patients in the ISIS-2 study [14]. After 1 month of treatment there was a 23% reduction in vascular death in those patients allocated to aspirin compared with placebo, equivalent to the prevention of about 25 early deaths per 1000 patients treated. Aspirin also prevented 10 non-fatal reinfarctions and 3 non-fatal strokes per 1000 patients treated and was not associated with any excess bleeding. The benefit was apparent regardless of the time of first administration up to 24 h after onset of pain. Long-term follow-up showed that the mortality benefit is maintained for at least 4 years [15].
The Anti-Platelet Trialists Collaboration [16] meta-analysis examined nine trials involving a total of about 20 000 patients (including those from the ISIS-2 trial) (Table 2). There was a 29% reduction in the risk of suffering a vascular event, representing 38 events prevented per 1000 patients treated. This included a reduced risk of non-fatal reinfarction (12 prevented per 1000 treated), non-fatal stroke (2 per 1000) and vascular death (24 per 1000). The effects were additional to those of treatment with fibrinolytics or anticoagulants and unaffected by the delay to starting treatment. One important mode of action of aspirin was to prevent the excess of reinfarctions that normally follow recanalisation of the infarct-related artery, presumably as unstable plaques rethrombose [14].
Table 2.
The impact of anti-platelet therapy upon early outcome (1 month) after suspected or definite acute myocardial infarction. Total of 18,773 patients in nine trials most of whom came from the ISIS-2 study given 1 month of 160 mg aspirin. Data derived from the Anti-platelet Trialists Collaboration [16].
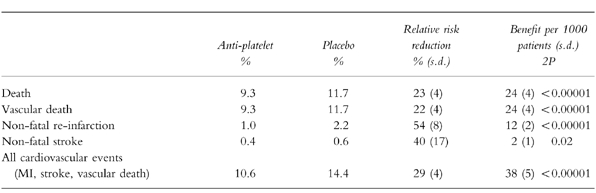
These data suggest that aspirin 150–300 mg should be given to patients with suspected acute myocardial infarction as soon as possible following the event. The tablet should be chewed or dispersed in water to achieve a quick onset of its anti-platelet action. If the final diagnosis of acute myocardial infarction is confirmed (or another acute coronary syndrome is suspected) the patient should continue to receive 150 mg aspirin daily.
Aspirin should be avoided in patients who have had a previous serious allergic reaction, recent gastrointestinal bleeding or recent intracranial haemorrhage. Insufficient patients have been randomised to different anti-platelet regimens to draw any definitive conclusions about superiority of any particular agent. Indirect comparisons of various drugs suggest that all produce similar relative risk reductions of 20–30% for a subsequent vascular event [16]. When there is a clear contra-indication to aspirin, an alternative antiplatelet drug should be used eg. dipyridamole or clopidogrel [17].
Thrombolysis
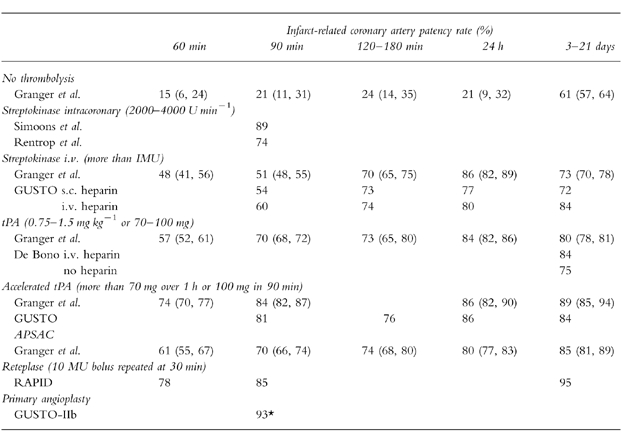
Early studies of streptokinase (SK) infused directly into infarct-related coronary arteries suggested that recanalization could be achieved in approximately 80% of cases [18, 19]. However coronary angiography is impractical for routine clinical usage. After intravenous SK the coronary artery patency at 90 min was 54% rising to 73% at 180 min, compared with 21% and 24% without thrombolysis (Table 3) [20, 21]. A meta-analysis of early small trials suggested that intravenous thrombolysis could reduce mortality by about a quarter [25]. However, the true benefits of thrombolysis only became apparent in the 1980s, when several mega-trials assessed mortality as the primary end-point [14, 26, 27].
Table 3.
Infarct-related coronary artery patency rates after intravenous thrombolysis strategies. The patency rate at time 0 was 20% (16–24) estimated by Granger et al. [21]. Data have been adapted from Rentrop et al. [18], Simoons et al. [19], the GUSTO Angiographic Investigators [20], Granger et al. [21], GUSTO-IIb [22], De Bono et al. [23] and Smalling et al. [24]. Values where applicable are mean and 95% confidence intervals. Patency is equivalent to TIMI grades 2 or 3. *The median time from randomization to baloon inflation in the GUSTO-IIb study was 78 min.
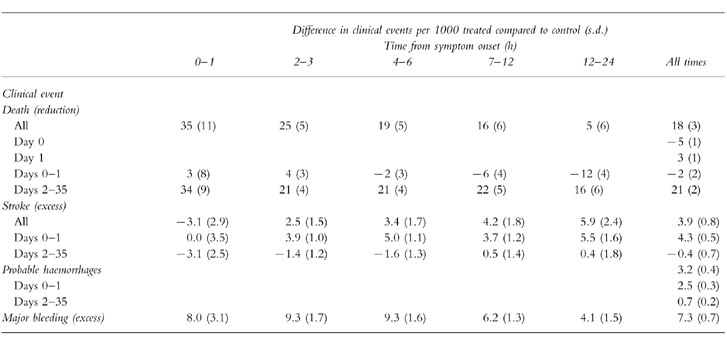
In ISIS-2 17 187 patients, presenting up to 24 h after the onset of ‘suspected’ myocardial infarction, were randomised to receive either a one hour intravenous infusion of 1.5 MU of SK or placebo [14]. At 35 days vascular mortality was reduced by 25% in patients treated with SK. Thrombolysis and aspirin had an additive effect reducing vascular mortality at 35 days by 42% (95% CI 34, 50%) compared with no treatment. The overall benefit of thrombolysis in acute myocardial infarction has been assessed in a meta-analysis of 58 600 patients enrolled into controlled trials which each randomised at least 1000 patients within 24 h of the onset of symptoms [13]. Thrombolysis produced a 18% reduction total mortality at 1 month from 11.5% to 9.6% (OR 0.82, 95% CI 0.77, 0.87) (Table 4). This is equivalent to 18 lives saved per thousand patients treated. In addition, the reduction in mortality at 1 month is sustained to at least 4 years [15]. However, thrombolysis was associated with a small increase in mortality on days 0–1.
Table 4.
The impact of thrombolytic therapy upon outcome after acute myocardial infarction. Data from 58,600 patients randomized into nine trials of 1,000 or more patients where thrombolysis was administered within 24 h. Day 0 was the day of randomization. Major bleeding includes non-cerebral bleeding that required blood transfusion or was life-threatening. Data derived from the Fibrinolytic Therapy Trialists’ Collaborative Group [13].
Who should receive thrombolysis?
Subgroup analysis has helped to define those patients who were most likely to benefit from thrombolysis (Table 5) [13]. Considering the ECG at presentation there was clear evidence for reduced mortality in patients with anterior ST segment elevation (25%), inferior ST segment elevation (11%), other combinations of ST segment elevation (23%) or with bundle branch block (25%). However, patients with ST segment depression had an increase in mortality of 13% (99% CI −12%, +44%). There were too few deaths among patients with T-wave inversion (‘other abnormalities’) to be certain of outcome; these patients showed a trend to a reduction in mortality (−9%, 99% CI −29%, +17% increase). The relative reduction in mortality for treatment of anterior ST elevation was statistically similar to that following inferior ST elevation, however, the absolute benefit is much greater because of higher absolute risk. For patients who have a good history of acute myocardial infarction, but do not have either ST segment elevation or bundle branch block, the best policy is to repeat the ECG and only to treat if definite abnormalities develop.
Table 5.
Absolute reduction in 35 day mortality associated with the use of thrombolytic therapy in patients with ‘suspected’ acute myocardial infarction. Adapted from the Fibrinolytic Therapy Trialists’ Collaborative Group [13].
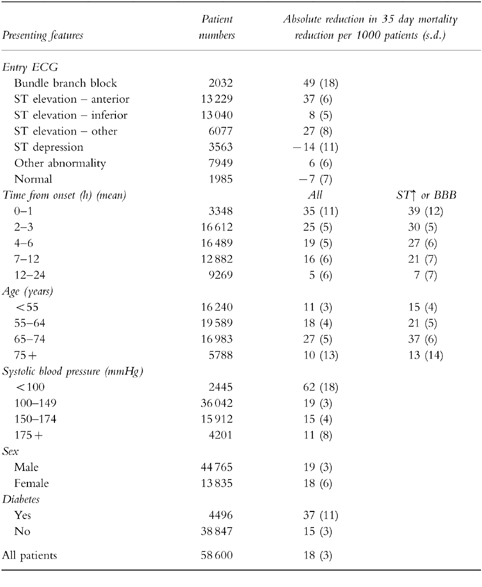
Meta-analysis of the trials showed a reduction in 35 day mortality in both young and elderly patients. In patients with ST segment elevation or bundle branch block, numbers of lives saved per 1000 patients treated for various age groups less than 55, 55–64, 65–74 and 75 years or above were 15, 21, 37 and 13 respectively [13]. There was an excess of early strokes and deaths on days 0–1 among elderly patients. However, age alone should not be a contra-indication to thrombolysis.
In the presence of hypotension (systolic blood pressure <100 mmHg) and/or tachycardia (heart rate > 100 beats min−1) a greater absolute reduction in mortality was demonstrated (62 and 33 lives saved per 1000 treated respectively). Gender or history of prior myocardial infarction did not affect the outcome after thrombolysis while diabetic patients had greater absolute benefit than non-diabetics (Table 5).
Time-dependence of benefit from thrombolysis
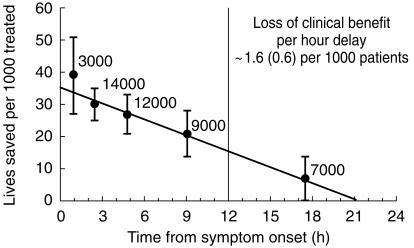
The extent of the benefit from thrombolysis is strongly dependent on the delay between symptom onset and administration of thrombolytic therapy. In ISIS-2, patients randomised between 0–4, 5–12 and 13–24 h after the onset of pain had a 35%, 16% and 21% reduction in vascular death, respectively [14]. The Fibrinolytic Therapy Trialists’ overview of the nine largest trials involving 45 000 patients who presented with ECG changes of either ST-segment elevation or bundle branch block suggested progressive loss of benefit with increasing delay to treatment (Figure 3) [13]. It was estimated that for each hour delay 1.6 fewer lives were saved per 1000 patients treated. It has been suggested that the benefit of thrombolysis is particularly striking if it is administered within the first hour (‘the golden hour’), possibly resulting in as many as 65 lives saved per 1000 patients treated [28]. This observation came only from GISSI-1 and was not observed in other trials. The overview strongly supported the use of thrombolytic therapy in patients with ECG abnormalities up to 12 h after the onset of symptoms. Too few patients were randomised between 13 and 24 h to draw any firm conclusions about the risk:benefit ratio after 12 h. The diminution of effectiveness of delayed thrombolysis was due to excess mortality on days 0–1 although the cause of this is unknown.
Figure 3.
The time-dependence of the mortality benefit of thrombolysis. Absolute number of lives saved per 1000 patients with ST elevation or bundle branch block treated with thrombolytic therapy plotted from the time of onset of symptoms. Adapted from Fibrinolytic Therapy Trialists Collaborative Group overview [13].
The association between early administration of thrombolysis and the greatest clinical benefit stimulated trials with early administration by ambulance-based physicians, trained ambulance nurses or paramedics [1–3, 29]. The advantages of this general approach are unproven.
Which thrombolytic?
A pooled analysis of 14 124 patients from 58 studies compared angiographic patency following different thrombolytic drugs. The patency rate at 90 min following standard intravenous tissue plasminogen activator (tPA) (70%; 95% CI 68, 72%) was significantly better than that of 51% (95% CI 48, 55%) achieved with SK [21]. However, the angiographic assessments for tPA and SK at 120–180 min after treatment did not differ (Table 3). This compares with patency rates of 20% throughout the first 24 h without thrombolysis. In five studies involving 1183 patients reocclusion was significantly more common with tPA (13.4%, 95% CI 11, 16%) than with the other thrombolytic agents (8.0%, 95% CI 3, 10%) [21]. A randomised angiographic trial of 652 patients given tPA with or without intravenous heparin suggested that reocclusion could be prevented by anticoagulation [23]. Indeed, the GUSTO angiographic study, in which intravenous heparin was given routinely, suggested much lower re-occlusion rates of 6.4% for SK and 5.9% for accelerated tPA [20].
ISIS-3 compared thrombolysis with SK (1.5 MU infused over 1 h), tPA (duteplase, 0.6 MU kg−1 infused over 4 h) or anisoylated plasminogen-streptokinase activator complex (APSAC, 30 U over 3 min) [30]. Mortality after 5 weeks was not significantly different between those assigned to SK or tPA (10.6%vs 10.3%), even if treatment was given within 6 h of symptom onset. Although fewer reinfarctions occurred after tPA (3.47%vs 2.93%), it caused significantly more strokes (1.04%vs 1.39%), most of which occurred on days 0–1 and were attributable to cerebral haemorrhage (0.24%vs 0.66%). APSAC showed similar efficacy to SK. SK was associated with significantly more allergy (3.6%vs 0.8%) and hypotension (11.8%vs 7.1%) than tPA but significantly fewer extracranial bleeds (4.5%vs 5.2%). GISSI-2 compared SK (1.5 MU) with tPA (alteplase, 100 mg over 3 h) [31]. While mortality at 35 days was not significantly different (9.2%vs 9.6%) tPA again produced a significant excess of strokes (0.9%vs 1.3%).
Results from these two similar trials allow powerful conclusions to be drawn about thrombolysis with SK or tPA. Mortality at 35 days was similar with the two agents, although there were fewer reinfarctions with tPA (5 per 1000 patients treated). However, there was an excess of 4 strokes per 1000 patients treated.
Angiographic studies suggested that an accelerated (or ‘front-loaded’) delivery of tPA (at least 70 mg in the first hour) followed by intravenous heparin might further improve infarct artery patency [32] (Table 3). In a pooled analysis, the 60 and 90 min patency rates for the standard tPA regimen were 57% and 70% compared with 74 and 84% with an accelerated regimen [21]. Addition of heparin to tPA improved long-term patency of the infarct-related coronary artery. An angiographic assessment at 48–120 h after tPA in 324 patients assigned heparin (5000 units followed by 1000 units h−1 until angiography) showed 83.4% patency compared with 74.7% in 320 given placebo (OR for patency 0.66, 95% CI 0.47, 0.93) [23].
GUSTO-1 compared three thrombolytic regimens in patients presenting within 6 h of symptoms. An accelerated tPA regimen (15 mg bolus+0.75 mg kg−1 over 30 min+0.5 mg kg−1 over 60 min with intravenous heparin) was compared with SK (1.5 MU over 1 h followed by subcutaneous heparin 12 500 units twice daily) [33]. The results indicated that tPA with heparin reduced 30 day mortality compared with SK with heparin (7.2%vs 6.3%; OR 0.87, 95% CI 0.78, 0.97), equivalent to nine lives saved per 1000 patients treated. However, the accelerated tPA regimen increased strokes from 1.22% to 1.55% and haemorrhagic strokes from 0.49% to 0.72% (OR 1.31 95% CI 1.02, 1.67) equivalent to an excess of two strokes per 1000 patients treated. Subgroup analyses suggested that the advantage of tPA over streptokinase was most evident for anterior infarcts and in patients younger than 75 years, although the benefit was lost if treatment was delayed by more than four hours from onset of pain. Left ventricular function was better preserved in patients receiving tPA [20].
Some authorities argue that the trials of accelerated or standard tPA regimens should not be analysed separately and that conclusions should only be drawn from pooled results since the trials are not statistically heterogeneous [34] (Table 6). Pooled results from all the comparative trials of tPA with SK suggest a significant reduction in non-stroke deaths amongst tPA treated patients of 4.9 per 1000 patients treated, but 95% confidence intervals are wide (1, 9). Compared with SK, tPA increases the risk of stroke by 2.9 per 1000 patients treated, 95% CI 2, 4) [34–36]. Therefore, it has been estimated that tPA produces an overall non-significant benefit in the combined endpoint of death or stroke (1.6 fewer per 1000 patients treated) [34]. The cost of tPA compares unfavourably with SK, and its use is therefore often restricted to selected groups. These include patients who have previously received SK and are likely to have formed anti-SK antibodies (decreasing its potential efficacy) and those patients who have most to gain from more successful reperfusion i.e. younger patients with large anterior infarctions.
Table 6.
Randomized studies comparing thrombolysis in suspected acute myocardial infarction with streptokinase or tPA based thrombolytic regimens. Adapted from Collins et al. [34]. Figures for treatment regimens in trials are percentages. The bottom row of each trial compares tPA treatment with streptokinase (SK) treatment in terms of clinical events per 1000 patients treated (s.d.). Weighted average of trials are based on the sample size.
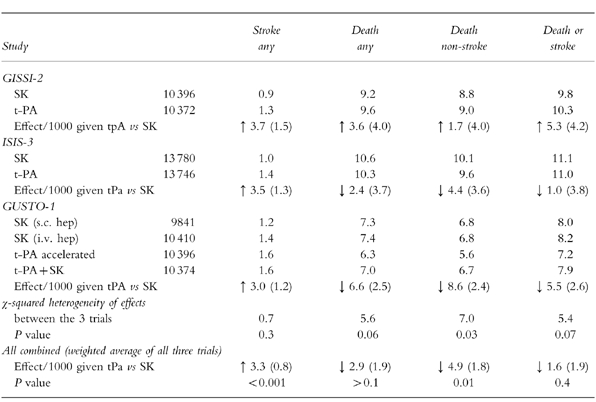
It is difficult to prove statistical superiority of one agent against another without studying large numbers of patients. For this reason newer more simply administered thrombolytic agents are being tested in smaller ‘equivalence trials’. The INJECT trial of 6000 patients showed that the double bolus 10 MU reteplase was equivalent to streptokinase with regard to mortality (9.5%vs 9.0%) and stroke rates (1.00%vs 1.23%) [37]. GUSTO-3 compared accelerated tPA with reteplase and found similar 30 day mortality (7.2%vs 7.4%) and stroke rates (1.83%vs 1.67%) [38].
Despite the evidence, the use of thrombolysis in Europe remains sub-optimal: a survey in 1993 and 1994 found that a third of patients with acute myocardial infarction and clear indications for thrombolysis were still left untreated. Women and the elderly were particularly disadvantaged [39].
Primary angioplasty
In experienced centres immediate (primary) percutaneous transluminal coronary angioplasty of the infarct-related artery offers a higher rate of reperfusion than is currently possible with thrombolytic therapy (Table 3). However, the procedure requires dedicated investigators and nursing staff and is generally restricted to tertiary centres with surgical facilities. A number of randomised studies have compared primary angioplasty with thrombolysis. Two initial studies suggested that early mortality could be significantly reduced from 6.4% to 2.5% [40, 41]. However, those studies enjoyed the availability of anaesthetic support for haemodynamically unstable patients and in 5% of cases angioplasty was thought to be inappropriate and the patients referred for immediate coronary artery bypass surgery. A pooled analysis of 1145 patients randomised to primary angioplasty or thrombolysis following acute myocardial infarction in 7 trials between 1986 and 1993 was recently reported [42]. The data suggested that for those patients treated with angioplasty there was a significant reduction in total mortality at 6 weeks (OR 0.56, 95% CI 0.33, 0.94) and death or non-fatal reinfarction (OR 0.53, 95% CI 0.35, 0.80). However, one third of these patients were followed to 1 year when the early benefits had disappeared.
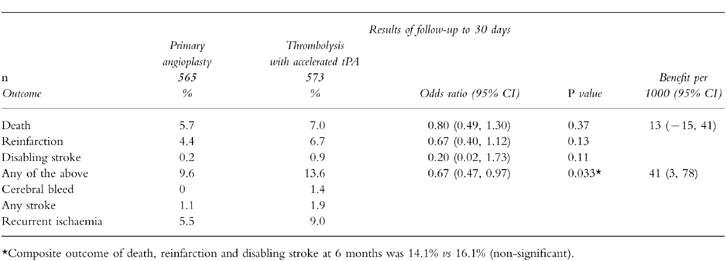
The largest study reported so far has been the GUSTO-IIb which enrolled 1138 patients presenting within 6 h of the onset of symptoms [22]. They were randomised to reperfusion with an accelerated tPA regimen, or immediate angioplasty. Primary angioplasty achieved normal coronary flow in 74% of patients, while this was achieved in only 54% of patients receiving accelerated tPA [20]. At 30 days, there were trends towards reductions in the endpoints of death (5.7 vs 7.0%), reinfarction (4.5 vs 6.5%) and disabling stroke (0.2 vs 0.9%) in the angioplasty group and there was a significant reduction in combined primary endpoints (9.6 vs 13.7%) (Table 7). Calculations from this study suggest that it would take 100 primary angioplasties to prevent one death, two reinfarctions, and one cerebral bleed at a cost of one non-cerebral bleed. These results were less promising than those of earlier studies. Furthermore, a retrospective analysis of experience of over 1000 primary angioplasties in Seattle between 1988 and 1994 showed no benefit in comparison to 2000 matched patients receiving thrombolysis [43].
Table 7.
Results of the GUSTO-IIb randomised trial of primary angioplasty vs thrombolysis in acute myocardial infarction. Data derived from [22].
Percutaneous transluminal coronary angioplasty is an alternative to thrombolysis only in experienced, well-organized centres, preferably with stand-by surgical support. There are inherent delays and risks in transferring patients from centres without angioplasty facilities. Angioplasty may be a more attractive option in young patients with large anterior infarcts for whom there is an absolute contra-indication to thrombolysis. Primary angioplasty may also be an attractive option to ‘rescue’ patients where thrombolysis has failed to provide reperfusion [42].
The coronary care unit registrar recognised the potential danger associated with aspirin and thrombolytic therapy in a relatively young man with a previous history of duodenal ulceration. However, he concluded that the ulcer had been successfully treated and that there was little symptomatic evidence that the ulcer was ‘active’. The potential benefits of salvaging left ventricular myocardium and prolonging survival of this patient far outweighed any risk of gastro-intestinal bleeding. While limited evidence supports the contention that tPA followed by intravenous heparin may be a more effective thrombolytic regimen than SK, the registrar chose to administer SK, the unit’s standard thrombolytic for infarcts where there is no clear contra-indication. After chewing a 300 mg tablet of aspirin, a 1 h intravenous infusion of 1.5 MU SK was started. Although there was a tertiary cardiology centre 5 miles away, it was considered that the delays inherent in arranging a hospital transfer would jeopardise further the myocardium and would be very unlikely to be offset by any early survival gains that might result from primary angioplasty.
Shortly after beginning SK infusion the patient’s blood pressure fell transiently. The infusion was continued and completed, despite frequent ventricular premature beats and two brief episodes of ventricular tachycardia. At this stage it was noted that the raised anterior ST segments had partially resolved. The registrar now considered other therapeutic measures which might be appropriate at this stage.
Other early measures
Although the immediate priority in managing acute myocardial infarction is thrombolysis and reperfusion of the myocardium, a variety of other drug therapies such as heparin, β-adrenoceptor blockers, magnesium and insulin might also be considered in the early hours.
Heparin
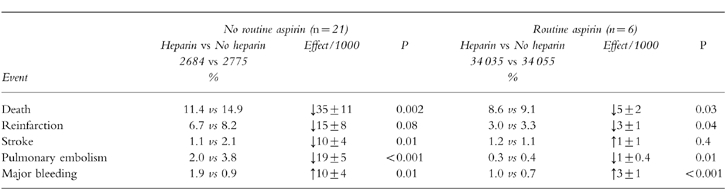
Randomised placebo-controlled trials of heparin (in the absence of treatment with aspirin and a thrombolytic) suggested that it reduced death, reinfarction, stroke and pulmonary embolism at the cost of more major bleeds (Table 8) [44]. The question of whether aspirin and a thrombolytic should be given alone or in combination with heparin was addressed in about 68 000 patients, most of whom were studied in GISSI-2 and ISIS-3 [30, 31]. The heparin regimen was 12 500 units twice-daily subcutaneously for a week beginning 4–12 h after thrombolysis. Heparin reduced immediate mortality by 5 deaths per 1000 patients [44]; however the benefit was lost by 6 months [30]. The use of heparin also produces an excess of major bleeds and strokes (Table 8).
Table 8.
A meta-analysis of 26 trials involving the use of heparin anticoagulation after acute myocardial infarction. Events refer to those occurring before hospital discharge. Most of the patients (62,067) in the aspirin plus heparin vs aspirin alone group come from GISSI-2 [31] and ISIS-3 [30] where the regimen was 12,500 units subcutaneously twice daily. Data has been derived from Collins et al. [34]. Values are mean±s.d. The trials that did not use aspirin used thrombolysis in 14% patients while those using aspirin used thrombolysis in 93% cases.
More intensive intravenous regimens of heparin (24 000 or 25 000 units for 2–3 days) have been studied in about 1300 patients; heparin had no effect on death or reinfarction and produced more strokes (1.8%vs 0.6%) [45]. In GUSTO-1, 20 000 patients who were thrombolysed with streptokinase were randomised to either subcutaneous heparin or 48 h of intravenous heparin (5000 unit bolus followed by 1000 units per hour adjusted to give a aPTT 60–85 s) [33]. Compared with subcutaneous heparin, intravenous heparin was associated with more deaths (0.9 per 1000 patients treated), reinfarctions (7.2), haemorrhagic strokes (1.1) and major bleeds (2.6). Indirect comparison of the uses of intravenous heparin with placebo after SK is gained from GISSI-2 and ISIS-3, which used the same subcutaneous heparin regimen as GUSTO-1. This analysis suggests that the use of intravenous heparin is associated with fewer deaths (1 per 1000 patients treated), more reinfarctions (4), more haemorrhagic strokes (2) and more major bleeds (6) [34]. Thus there is no advantage to the routine use of heparin after thrombolysis with SK. Although there are no randomised clinical outcome trials comparing tPA alone with the addition of heparin, improved coronary artery patency with intravenous heparin has resulted in widespread adoption of this regimen [23].
β-adrenoceptor blockers
β-adrenoceptor blockers reduce myocardial oxygen demand as a result of reductions in blood pressure and heart rate; they also have anti-arrhythmic properties [46]. The Metoprolol in Acute Myocardial Infarction (MIAMI) trial randomized 5,778 patients within 24 h of symptom onset to metoprolol 15 mg i.v. followed by 200 mg daily by mouth for 2 weeks or placebo. There was a non-significant 13% reduction in death at 15 days in the metoprolol-treated group (4.3%vs 4.9%) [47].
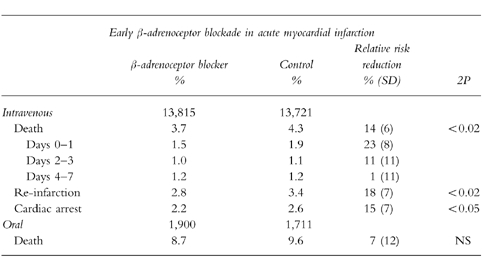
The larger ISIS-1 trial in 16 027 patients admitted within 12 h of a suspected myocardial infarction examined the impact of atenolol 5–10 mg i.v. followed by 100 mg orally daily for 7 days. Patients we excluded if they had contra-indications to β-adrenoceptor blocker therapy including bradycardia or heart failure [48]. Oral atenolol was taken by 35% of treated group and by 25% of the control group at discharge. Vascular mortality was lower at 7 days in patients taking atenolol (3.9%vs 4.6%) and at 1 year (10.7%vs 12.0%). The benefits of atenolol were manifest primarily during days 0–1 and were particularly apparent in patients aged 65 years or over, those with a systolic blood pressure of >160 mmHg, in diabetics and in those with large infarcts. Atenolol was deleterious in patients with systolic blood pressure <120 mmHg and those with cardiogenic shock. There were no significant differences in the frequency of in-hospital cardiac arrhythmias although complete heart block was increased among patients with pre-existing first degree heart block.
A pooled analysis of studies examining early administration of β-adrenoceptor blockers after myocardial infarction estimated that mortality is reduced by 14% in the first week after an event [49] (Table 9). However, much of the information comes from pre-thrombolytic trials. In addition, the use of antiplatelet agents was low (e.g. 5% in the ISIS-1 trial). TIMI-II randomised 1 390 patients treated with aspirin and following thrombolysis to 15 mg metoprolol intravenously followed by oral metoprolol (50 mg twice daily increasing to 100 mg twice daily if tolerated) or to oral metoprolol starting on day 6 [50]. Although mortality in the two groups was similar, after the early intravenous regimen there were fewer non-fatal re-infarctions at 6 days (2.3%vs 4.5%) and at 42 days (3.9%vs 6.1%). There were also fewer recurrent myocardial ischaemic episodes by day 6 in the group assigned to early metoprolol (15.5%vs 21.2%) although this advantage was lost following the deferred administration of metoprolol. The effect of intravenous metoprolol was greatest in patients treated within two hours of the onset of symptoms and in those who were at lowest risk.
Table 9.
A pooled analysis of 28 randomized trials of early intravenous β-adrenoceptor blockade after suspected acute myocardial infarction involving 27,536 patients (adapted from ISIS-1 Collaborative Group [50]). Data for in-hospital mortality after early oral β-adrenoceptor blockade in 3611 randomized patients derived from Yusuf et al. [48].
Mild heart failure is not a contra-indication to β-adrenoceptor blockade; in fact these patients probably derive greatest benefit [48]. The European Society of Cardiology currently recommend that intravenous β-adrenoceptor blockade should be given to patients who are hypertensive prior to thrombolysis, or who are tachycardic in the absence of heart failure. β-adrenoceptor blockers should be avoided in patients with other known contra-indications such as asthma, a history of reversible airways disease, heart block or significant peripheral vascular disease.
Magnesium
Intravenous infusions of magnesium reduce peripheral vascular resistance while increasing cardiac output, with no increase in cardiac work. Magnesium may also increase the threshold for depolarisation of cardiac myocytes, thus reducing the likelihood of ventricular arrhythmias due to injury currents in ischaemic tissues. Furthermore, acute increases in extracellular magnesium concentrations reduce the potentially damaging influx of calcium ions into ischaemic and reperfused myocardial cells. Magnesium infusions initiated acutely after the onset of myocardial infarction might be expected to limit infarct size, reduce malignant arrhythmias and improve prognosis.
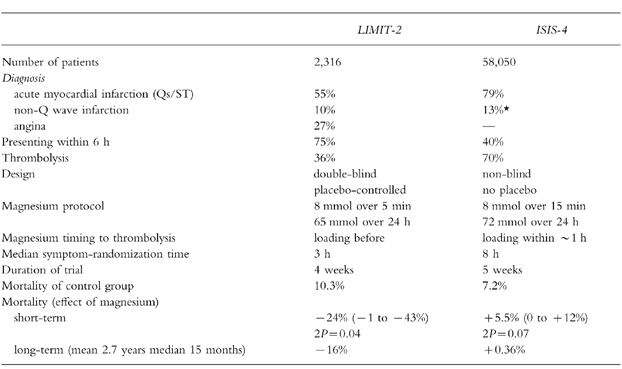
LIMIT-2 examined the impact of an acute bolus of 8 mmol magnesium sulphate injected over 5 min followed by 65 mmol infused over 24 h [51]. The study enrolled 2 316 patients with suspected myocardial infarction (over a third had a final diagnosis of a non-Q wave infarct or unstable angina); 36% received thrombolysis. The median time from symptoms to randomisation was 3 h. Magnesium reduced 28 day mortality by 24% (95% CI 1, 43%) and there was also a 25% (95% CI 7, 39%) reduction in early heart failure. Long-term follow-up to a mean of 2.7 years showed a 16% (95% CI 2, 29%) reduction in all-cause mortality in the magnesium-treated group [52].
ISIS-4 examined the impact of an acute bolus of 8 mmol magnesium sulphate injected over 15 min followed by 72 mmol infused over 24 h compared with open control in 58 050 patients with suspected myocardial infarction [7]. The median time from symptoms to randomisation was longer (8 h) and 70% of the patients received thrombolysis. At 5 weeks there were more deaths in the magnesium group (7.64%vs 7.24%) which was largely attributed to cardiogenic shock (1.62%vs 1.26%). Magnesium did not show a benefit in any subgroup, nor did it reduce malignant arrhythmias.
The disappointing results of ISIS-4 contrast with those of LIMIT-2 (Table 10). A potential explanation for the major disparity in these two trials was the timing of commencing magnesium infusion. Experimental evidence suggests that any myocardial protection offered by magnesium may be highly dependent on significantly elevated serum magnesium concentrations at the time of reperfusion [53]. In LIMIT-2, magnesium was given before thrombolysis in one third of patients. In ISIS-4, magnesium was initiated within 2 h after thrombolysis in about half of all treated patients. Since SK produces patency rates of at least 50% at 90 min, it is likely that the majority of patients in this study did not have elevated plasma magnesium concentrations during the early minutes of reperfusion. Therefore, the ISIS-4 trial may not have addressed the ‘magnesium hypothesis’ appropriately. The uncertainty will only be resolved if a further large controlled trial of early magnesium infusion is performed.
Table 10.
Trials of intravenous magnesium in acute myocardial infarction. Data have been derived from the ISIS-4 [7] and LIMIT-2 [53] studies. *This figure is derived by taking those with ST elevation at randomization from the figure of 92%‘confirmed’ infarctions in the trial.
Insulin
Diabetic patients have a significantly worse prognosis after myocardial infarction than those without diabetes, mainly because of greater left ventricular dysfunction and increased re-infarction rates [54–57]. This applies to both patients who are newly diagnosed on the coronary care unit and those already diagnosed [58]. Recent evidence from the DIGAMI study suggests that a more intensive approach to achieving metabolic control in patients presenting with hyperglycaemia may be beneficial [59]. Six hundred and twenty patients presenting within 24 h of a myocardial infarction with a blood glucose concentration greater than 11 mmol l−1 (whether known to have diabetes mellitus or not) were randomised to receive a glucose-insulin infusion followed by subcutaneous insulin four times daily for at least 3 months. Patients in the control group only received insulin if indicated by normal clinical practice. The mean time to randomisation was 13 h after the onset of symptoms. The target range plasma glucose was 7–10.9 mmol l−1, with 2 h monitoring of plasma glucose for at least 24 h. There were hypoglycaemic episodes in 15% of patients receiving intensive therapy. Mortality at 1 year was 29% lower in the intervention group than in the control group (95% CI 4%, 51%). After a mean follow-up of 3.4 years there was a 28% relative risk reduction in mortality in the glucose insulin treatment group (95% CI 8%, 45%) [60]. The benefit was particularly apparent in the low risk patients (age less than 70, no previous infarct, no heart failure) who were not previously using insulin (51% reduction, 95% CI 19, 70%). In this group the benefit was already apparent at the time of hospital discharge and continued to 5 years of follow-up. The results suggest that diabetic patients are likely to benefit from an early intensive approach to metabolic control although this is likely to pose logistic problems for many units.
The CCU registrar felt that on the basis of a lack of supporting evidence that he would not give intravenous heparin but elected to give twice daily subcutaneous injections of 5000 units for prophylaxis against deep vein thrombosis. The patient was to be prescribed atenolol 50 mg on the morning following admission with the intention of increasing the dosage to 100 mg if blood pressure and pulse rate allowed. On admission, the blood glucose was mildly elevated at 7.8 mmol l−1. In the absence of evidence supporting more intensive management, the blood glucose concentration was repeated the following day when it was normal. In addition to the above drugs the patient was prescribed 150 mg enteric coated aspirin daily and treatment with nifedipine was stopped.
Learning points
The immediate priorities for a patient with suspected myocardial infarction are pain control and access to advanced life support facilities.
Survival after myocardial infarction is largely dependent on residual cardiac function and so the earliest therapeutic priority is salvage of threatened ischaemic myocardium. This can be achieved by the use of antiplatelet drugs, thrombolytic agents or primary angioplasty.
Aspirin should be given as soon as possible after the onset of symptoms and daily thereafter if it can be tolerated. Regular use of aspirin is associated with a reduction in mortality of about 25 lives per 1000 patients treated.
The benefits of thrombolysis are time-dependent and are unequivocal for patients presenting within 12 h with ST elevation or bundle branch block (greater than 20 lives saved per 1000 patients treated). No clear evidence of benefit exists for those who present later or with other kinds of ECG abnormality.
The principal danger of thrombolysis is intracranial haemorrhage which occurs in approximately 4 per 1000 patients treated. It is more common amongst the elderly, those with a high systolic blood pressure and those given tPA as a thrombolytic. Serious extra-cranial bleeding occurs in 7 per 1000 patients treated.
Comparative trials of thrombolytic agents suggest that tPA is more effective in achieving reperfusion than streptokinase and offers a small survival benefit but is associated with a significantly greater financial cost. Plasminogen activators should be used in patients previously exposed to streptokinase, which is immunogenic.
Primary angioplasty offers the most reliable method of achieving myocardial reperfusion but does not appear to confer long-term survival advantages over thrombolysis. Primary angioplasty is an important option in patients for whom there is an absolute contra-indication to thrombolysis or as ‘rescue’ for patients in whom thrombolysis has failed to provide reperfusion.
Heparin used routinely by the intravenous or subcutaneous routes does not improve survival after treatment with aspirin and thrombolysis with streptokinase.
Insulin therapy by ‘sliding scale’ should be considered to control hyperglycaemia in established and newly diagnosed diabetic patients.
Early intravenous β-adrenoceptor blockade reduces ischaemic episodes and improves survival but should be avoided in those who are hypotensive or in cardiogenic shock.
References
- 1.EMIP (European Myocardial Infarction Project) Subcommittee. Potential time saving with pre-hospital intervention in acute myocardial infarction. Eur Heart J. 1988;9:118–124. Coronary care unit admission/Oxygen/Analgesia/Nitrates. [PubMed] [Google Scholar]
- 2.Rawles J, et al. Halving of mortality at one year by domiciliary thrombolysis in the Grampian Region Early Anistreplase Trial (GREAT) J Am Coll Cardiol. 1994;23:1–5. doi: 10.1016/0735-1097(94)90494-4. [DOI] [PubMed] [Google Scholar]
- 3.Grijseels EWM, Bouten MJM, Lenderink T, et al. Pre-hospital thrombolytic therapy with either alteplase or streptokinase. Practical implications, complications and long-term results in 529 patients. Eur Heart J. 1995;16:1833–1838. doi: 10.1093/oxfordjournals.eurheartj.a060836. [DOI] [PubMed] [Google Scholar]
- 4.Wilson AT, Channer KS. Hypoxaemia and supplemental oxygen therapy in the first 24 hours after myocardial infarction: the role of pulse oximetry. J Roy Coll Phys Lond. 1997;31:657–661. [PMC free article] [PubMed] [Google Scholar]
- 5.Rawles JM, Kenmure ACF. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1:1121–1123. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly RF, Hursy TL, Parillo JE, Schaer GL. Effect of 100% oxygen administration on infarct size and left ventricular dysfunction in a canine model of myocardial infarction and reperfusion. Am Heart J. 1995;130:957–965. doi: 10.1016/0002-8703(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 7.ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised trial comparing captopril versus placebo, oral mononitrate versus placebo, and intravenous magnesium sulphate versus control among 58 043 patients with suspected acute myocardial infarction. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 8.Gruppo Italiano per lo Studio della Sopravvivenza nell-infarcto Miocardio. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 9.European Study of Prevention of Infarct with Molsidomine (ESPRIM) Group. The ESPRIM trial: short-term treatment of acute myocardial infarction with molsidomine. Lancet. 1994;344:91–97. [PubMed] [Google Scholar]
- 10.The Multicentre Postinfarction Research Group. Risk stratification and survival after myocardial infarction. New Engl J Med. 1983;309:331–336. doi: 10.1056/NEJM198308113090602. Aspirin and antiplatelet therapy. [DOI] [PubMed] [Google Scholar]
- 11.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. New Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 12.LATE Study Group. Late assessment of thrombolytic efficacy (LATE) study with alteplase 6–24 hours after onset of acute myocardial infarction. Lancet. 1993;342:759–766. [PubMed] [Google Scholar]
- 13.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 14.ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;ii:349–360. [PubMed] [Google Scholar]
- 15.Baigent C, Collins R for the ISIS Collaborative Group. ISIS-2: Four year mortality follow-up of 17 187 patients after fibrinolytic and anti-platelet therapy in suspected acute myocardial infarction. Circulation. 1993;88(Suppl I):I-291. [Google Scholar]
- 16.Antiplatelet Trialists Collaboration. Collaborative overview of the randomised trials of antiplatelet therapy—I: Prevention of death myocardial infarction and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 17.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 18.Rentrop KP, Feit F, Blancke H, et al. Effects of intacoronary streptokinase and intracoronary nitroglycerin infusion on angiographic patterns and mortality in patients with acute myocardial infarction. New Engl J Med. 1984;311:1457–1463. doi: 10.1056/NEJM198412063112301. Thrombolysis. [DOI] [PubMed] [Google Scholar]
- 19.Simoons ML, Serruys PW, van der Brand M, et al. Improved survival after early thrombolysis in acute myocardial infarction. A randomised trial by the Interuniversity Cardiology Institute in the Netherlands. Lancet. 1985;ii:578–582. doi: 10.1016/s0140-6736(85)90584-7. [DOI] [PubMed] [Google Scholar]
- 20.The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary artery patency, ventricular function and survival after acute myocardial infarction. New Engl J Med. 1993;329:1615–1622. doi: 10.1056/NEJM199311253292204. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, White HD, Bates ER, Ohman EM, Califf RM. A pooled analysis of coronary arterial patency and left ventricular function after intravenous thrombolysis for acute myocardial infarction. Am J Cardiol. 1994;74:1220–1228. doi: 10.1016/0002-9149(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 22.The GUSTO-IIb Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. New Engl J Med. 1997;336:1621–1628. doi: 10.1056/NEJM199706053362301. [DOI] [PubMed] [Google Scholar]
- 23.De Bono DP, Simoons ML, Tijssen J, et al. Effect of early intravenous heparin on coronary patency, infarct size, and bleeding complications after alteplase thrombolysis: results of a randomised double blind European Cooperative Study Group Trial. Br Heart J. 1992;67:122–128. doi: 10.1136/hrt.67.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smalling RW, Bode C, Kalbfleisch Kalbfleisch, et al. and the RAPID Investigators. More rapid, complete, and stable coronary thrombolysis with bolus administration of reteplase compared with alteplase infusion in acute myocardial infarction. Circulation. 1995;91:2725–2732. doi: 10.1161/01.cir.91.11.2725. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Collins R, Peto R, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: overview of the results on mortality, reinfarction and side-effects from 33 randomized controlled trials. Eur Heart J. 1985;6:556–585. doi: 10.1093/oxfordjournals.eurheartj.a061905. [DOI] [PubMed] [Google Scholar]
- 26.Gruppo Italiano per lo Studio della Streptochinasi nell’Infarcto miocardico (GISSI) Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;i:397–401. [PubMed] [Google Scholar]
- 27.The I.S.A.M. Study Group. A prospective trial of intravenous streptokinase in acute myocardial infarction (I.S.A.M.). Mortality, morbidity, and infarct size at 21 days. New Engl J Med. 1986;314:1465–1471. doi: 10.1056/NEJM198606053142301. [DOI] [PubMed] [Google Scholar]
- 28.Boersma E, Maas ACP, Deckers JW, Simmons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 29.Weaver WD, Cerquiera M, Hallstrom AP, et al. Pre-hospital initiated vs. hospital-initiated thrombolytic therapy. The Myocardial Infarction Triage and Intervention Trial. J Am Med Ass. 1993;270:1211–1216. [PubMed] [Google Scholar]
- 30.ISIS-3 Collaborative Group. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator versus anistreplase and of aspirin plus heparin versus aspirin alone among 41 299 cases of suspected acute myocardial infarction. Lancet. 1992;339:753–770. [PubMed] [Google Scholar]
- 31.The GISSI-2 International Study Group. GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12 490 patients with acute myocardial infarction. In-hospital mortality and clinical course of 20 891 patients with suspected acute myocardial infarction randomised between alteplase and streptokinase with or without heparin. Lancet. 1990;336:65–71. 71-75. [PubMed] [Google Scholar]
- 32.Carney R, Brandt T, Daley P, et al. Increased efficacy of rt-PA by more rapid administration: RAAMI Trial. J Am Coll Cardiol. 1992;20:17–23. doi: 10.1016/0735-1097(92)90131-6. [DOI] [PubMed] [Google Scholar]
- 33.The GUSTO Investigators. An international randomized trial comprising four thrombolytic strategies for acute myocardial infarction. New Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 34.Collins R, Peto R, Baigent C, Sleight P. Aspirin, heparin, and fibrinolytic therapy in acute myocardial infarction. New Engl J Med. 1997;336:847–860. doi: 10.1056/NEJM199703203361207. [DOI] [PubMed] [Google Scholar]
- 35.Gore JM, Granger CB, Simoons ML, et al. Stroke after thrombolysis: mortality and functional outcomes in the GUSTO-1 trial. Circulation. 1995;92:2811–2818. doi: 10.1161/01.cir.92.10.2811. [DOI] [PubMed] [Google Scholar]
- 36.Maggioni AP, Franzosi MG, Santoro E, et al. The risk of stroke in patients with acute myocardial infarction after thrombolytic and antithrombotic treatment. New Engl J Med. 1992;327:1–6. doi: 10.1056/NEJM199207023270101. [DOI] [PubMed] [Google Scholar]
- 37.International Joint Efficacy Comparison of Thrombolytics. Randomised double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. Lancet. 1995;346:329–336. [PubMed] [Google Scholar]
- 38.Topol E, Califf R, Ohman E, et al. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337:1118–1123. doi: 10.1056/NEJM199710163371603. [DOI] [PubMed] [Google Scholar]
- 39.European Secondary Prevention Study Group. Translation of clinical trials into practice: a European population-based study of the use of thrombolysis for acute myocardial infarction. Lancet. 1996;347:1203–1207. [PubMed] [Google Scholar]
- 40.Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. New Engl J Med. 1993;328:673–679. doi: 10.1056/NEJM199303113281001. Primary angioplasty. [DOI] [PubMed] [Google Scholar]
- 41.Zijlstra F, De Boer MJ, Hoorntje JCA, Reiffers S, Reiber JHC, Suryapranata H. A comparison of immediate angioplasty with intravenous streptokinase in acute myocardial infarction. New Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 42.Michels KB, Yusuf S. Does PTCA in acute myocardial infarction affect mortality and reinfarction rates? A quantitative overview (meta-analysis) of the randomized clinical trials. Circulation. 1995;91:476–485. doi: 10.1161/01.cir.91.2.476. [DOI] [PubMed] [Google Scholar]
- 43.Every NR, Parsons LS, Hlatky M, Martin JS, Weaver W for the Myocardial Infarction Triage and Intervention Investigators. A comparison of thrombolytic therapy with primary coronary angioplasty for acute myocardial infarction. New Engl J Med. 1996;335:1253–1260. doi: 10.1056/NEJM199610243351701. [DOI] [PubMed] [Google Scholar]
- 44.Collins R, MacMahon S, Flather M, et al. Clinical effects of anti-coagulant therapy in suspected acute myocardial infarction: systematic overview of randomised trials. Br Med J. 1996;313:652–659. doi: 10.1136/bmj.313.7058.652. Heparin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahaffey KW, Granger CB, Collins R, et al. Overview of randomised trials of intravenous heparin in patients with acute myocardial infarction treated with thrombolytic therapy. Am J Cardiol. 1996;77:551–556. doi: 10.1016/s0002-9149(97)89305-8. [DOI] [PubMed] [Google Scholar]
- 46.Kendall MJ, Lynch KP, Hjalmarson A, Kjekshus J. Beta-blockers and sudden cardiac death. Ann Int Med. 1995;123:358–367. doi: 10.7326/0003-4819-123-5-199509010-00007. β-adrenoceptor blockers. [DOI] [PubMed] [Google Scholar]
- 47.The MIAMI Trial Research Group. Metoprolol in Acute Myocardial Infarction (MIAMI). A randomised placebo-controlled international trial. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 48.The ISIS-1 (First International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;ii:57–66. [PubMed] [Google Scholar]
- 49.The TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. New Engl J Med. 1989;320:618–627. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 50.Beta-Blocker Pooling Project Research Group. The Beta-Blocker Pooling Project (BBPP): subgroup findings from randomised trials in post-infarction patients. Eur Heart J. 1988;9:8–16. [PubMed] [Google Scholar]
- 51.Woods KL, Fletcher S, Roffe C, Haider Y. Intravenous magnesium sulphate in suspected acute myocardial infarction: results of the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2) Lancet. 1992;339:1553–1558. doi: 10.1016/0140-6736(92)91828-v. Magnesium. [DOI] [PubMed] [Google Scholar]
- 52.Woods KL, Fletcher S. Long-term out come after intravenous magnesium sulphate in suspected acute myocardial infarction: the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2) Lancet. 1994;343:816–819. doi: 10.1016/s0140-6736(94)92024-9. [DOI] [PubMed] [Google Scholar]
- 53.Baxter GF, Sumeray MS, Walker JM. Infarct size and magnesium: insights into LIMIT-2 and ISIS-4 from experimental studies. Lancet. 1996;348:1424–1426. doi: 10.1016/s0140-6736(96)07281-9. [DOI] [PubMed] [Google Scholar]
- 54.Stone P, Muller J, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular dysfunction after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J Am Coll Cardiol. 1989;14:49–57. doi: 10.1016/0735-1097(89)90053-3. Diabetes mellitus. [DOI] [PubMed] [Google Scholar]
- 55.Jacoby R, Nesto R. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. J Am Coll Cardiol. 1992;20:736–744. doi: 10.1016/0735-1097(92)90033-j. [DOI] [PubMed] [Google Scholar]
- 56.Jaffe AS, Sparado JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J. 1984;108:31–37. doi: 10.1016/0002-8703(84)90541-6. [DOI] [PubMed] [Google Scholar]
- 57.Granger C, Califf R, Young S, et al. Outcome of patients with diabetes mellitus and acute myocardial infarction treated with thrombolytic agents. J Am Coll Cardiol. 1993;21:290–295. doi: 10.1016/0735-1097(93)90348-5. [DOI] [PubMed] [Google Scholar]
- 58.Oswald GA, Corcoram S, Yudkin JS. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction. Lancet. 1984;335:1264–1267. doi: 10.1016/s0140-6736(84)92447-4. [DOI] [PubMed] [Google Scholar]
- 59.Malmberg K, Ryden L, Efendic S, et al. Randomised trial of glucose-insulin infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 60.Malmberg K for the DIGAMI (Diabetes Mellitus; Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]