Abstract
Aims
This study investigated the pharmacokinetics of cyclophosphamide (CP) and its main metabolite 4-hydroxycyclophosphamide (4-OH-CP) in patients with breast cancer undergoing high dose chemotherapy prior to autologous stem cell transplantation. An enzyme turn-over model was also developed to study the time course of cyclophosphamide induction.
Methods
Fourteen patients received a combination of CP (6 g m−2), thiotepum (500 mg m−2) and carboplatin (800 mg m−2) as a 96 h infusion. Plasma concentrations of CP and 4-OH-CP were determined with h.p.l.c. and a pharmacokinetic and enzyme turn-over model applied to data using NONMEM.
Results
CP plasma concentrations were described by a two-compartment model with a noninducible and an inducible pathway, the latter forming 4-OH-CP. In the final enzyme model, CP affects the amount of enzymes by increasing the enzyme production rate. CP concentrations decreased during the infusion with no subsequent change in 4-OH-CP concentrations. CP inducible and noninducible clearance were estimated to 1.76 l h−1 (90% C.I. 0.92–2.58) and 1.14 l h−1 (0.31–1.85), respectively. The induction resulted in an approximately doubled CP clearance through the inducible pathway at the end of treatment. The model predicted the enzyme turn-over half-life to be 24 h.
Conclusions
The presented mechanism-based enzyme induction model where the pharmacokinetics of the inducer and the enzyme pool counterbalance each other successfully described CP autoinduction. It is reasonable to believe that CP affects its own elimination by increasing the enzyme production rate and thereby increasing the amount of enzyme by which CP is eliminated.
Keywords: 4-hydroxycyclophosphamide, autoinduction, breast cancer, cyclophosphamide, enzyme induction model, pharmacokinetics
Introduction
Cyclophosphamide (CP) is an alkylating pro-drug used in chemotherapy of various malignancies [1]. Cyclophosphamide is inactive until it undergoes hepatic transformation to form 4-hydroxycyclophosphamide (4-OH-CP), which accounts for about 95% of the elimination [2]. 4-OH-CP equilibrates with the ring-opened aldophosphamide, which undergoes chemical decomposition to yield the alkylating metabolite phosphoramide mustard and the urotoxic metabolite acrolein [3]. Phosphoramide mustard is considered to be the ultimate alkylating metabolite. 4-OH-CP enters the cells and allows the polar metabolite phosphoramide mustard to be formed intracellular where cytotoxic activation takes place [4]. The 4-hydroxylation of CP yielding 4-OH-CP is mediated by cytochrome P450 (CYP) 2B6, 3A4 and 2C9 [2, 5, 6]. 4-OH-CP is detoxified by aldehyde dehydrogenase to yield the inactive carboxyphosphamide or oxidized to form 4-ketocyclophosphamide [7–10]. Deschloroethylcyclophosphamide and chloroacetaldehyde are formed through inactivation of CP by side-chain oxidation mediated by CYP3A4 [2, 11, 12].
CP pharmacokinetics are characterized by a low total clearance (5.4 l h−1) which classifies CP as a low extraction drug [1]. Repeated administration or continuous infusion of CP to cancer patients over a period of several days resulted in increased total clearance but with no alteration in volume of distribution or renal clearance and hence a decreased elimination half-life due to its low liver extraction [13–16]. CP induces CYP2C and CYP3A4 in human hepatocytes [17]. The time dependent clearance of cyclophosphamide and ifosfamide has previously been modelled [18, 19]. However, the mechanism underlying this auto-induction has not been studied in vivo. A greater understanding of the time-course and mechanism of this autoinduction would assist in optimizing dosing schedules and avoiding potential drug–drug interactions.
In the present study, a model was developed to simultaneously describe CP and 4-OH-CP pharmacokinetics as well as the time-course and mechanism of CP induction after continuous infusion of CP for 96 h to breast cancer patients.
Methods
Patients
Fourteen females with diagnosis breast cancer stage III or IV undergoing autologous stem cell transplantation were included in the study. The patients were aged 33–58 years and had normal haematopoietic, cardiac, pulmonary, renal and hepatic functions. The patients were informed and consent was obtained from each patient before enrolment into the study. The study was approved by the local ethics committee of the Karolinska Institute, Stockholm, Sweden.
Drugs
Three to four weeks after harvesting the stem cells, the patients received a combination of cyclophosphamide (6 g m−2, Sendoxan®, ASTA Medica), thiotepum (500 mg m−2, Thiotepa®, Wyeth Lederle) and carboplatin (800 mg m−2, Paraplatin®, Bristol-Myers Squibb). The three drugs were administered simultaneously as a 96 h infusion prior to autologous stem cell transplantation.
Blood sampling
Through a central venous catheter, blood was drawn at 0, 10, 30, 60 min and 24, 48, 72, 96 h after start of the infusion. Blood samples were again collected 5, 20, 30, 60 min and 2, 4, 6, 12 and 18 h after the end of the infusion. Blood was collected into cold heparinized tubes and immediately chilled on ice. The blood samples were centrifuged for 3 min at 3000 rev min−1 and plasma was harvested. To stabilize 4-OH-CP, 0.5 ml of plasma from each sample was transferred into a prechilled tube containing 1 ml of acetonitrile, vortexed and centrifuged for 3 min at 3000 rev min−1. The supernatant was harvested and stored at −70° C.
Cyclophosphamide assay
CP was determined using a previously described h.p.l.c.-method with some modifications [20]. The chromatographic system consisted of a Shimadzu LC-10 AD pump, a Spectromonitor 3100 LDC Analytical u.v.-detector (195 nm) and a Beckman ODS Ultrasphere 5 μm (4.6 mm×25 cm) column. The mobile phase consisted of a 2 mm phosphate buffer (pH 4.0) and acetonitrile (80:20). The flow rate was 1.0 ml min−1. Plasma (1 ml) was mixed with 2 ml of water and 50 μl internal standard (ifosfamide). After vortexing, the mixture was slowly passed through a C-18 column (SepPak Cartage), that previously had been activated with 10 ml methanol, dried with 10 ml air and conditioned with 10 ml distilled water. Plasma proteins were removed with 20 ml distilled water and thereafter the column was dried with air. CP and the internal standard were eluted with 2 ml methanol. The methanol was evaporated under a N2 stream (40° C) and the residue was dissolved in 100 μl distilled water and 40 μl toluene, vortexed and centrifuged for 5 min at 3500 rev min−1 75 μl of the aqueous phase was extracted with 2.5 ml dichloromethane and 2 ml of the dichloromethane phase was transferred to a new tube and evaporated (N2, 40° C). The residue was reconstituted in 100 μl mobile phase and 30 μl was injected onto the chromatographic system. The calibration curve was linear within the range 3–160 μg ml−1 (correlation coefficient of 0.9987). The precision of the analytical method was 4.8% (C.V.) at the 5 μg level (n = 8) and 7.5% at 150 μg level (n = 8). The detection limit of cyclophosphamide using this method was measured to 0.3 μg (C.V. = 5.2% and n = 8).
4-Hydroxycyclophosphamide assay
4-OH-CP was measured with a reversed-phase h.p.l.c. method described by Johansson et al. [21]. To the supernatant (1 ml) obtained from mixing plasma with acetonitrile, 70 μl of hydrochloric acid (1 m) and 200 μl of 2,4-dinitrophenylhydrazine (3.8 mg ml−1 in acetonitrile) were added. After vortexing and heating for 5 min at 50° C, 40 μl was injected onto the h.p.l.c. column. The calibration curve ranged between 22 and 2180 ng ml−1 (correlation of coefficient of 0.9976). The precision of the analytical method was 6.2% (C.V.) at the 50 ng level (n = 8) and 9.5% at 2000 ng level (n = 8). The detection limit of 4-hydroxycyclophosphamide using the adapted method was measured to 15 ng (C.V. = 6.8% and n = 8).
Data analysis
Models were fitted to all pharmacokinetic data (i.e. both cyclophosphamide and 4-hydroxycyclophosphamide) from all patients simultaneously, using the first-order method (FO) without or with centreing in the nonlinear mixed effects modelling program NONMEM, version V [22]. FO without centreing is the standard method in NONMEM, whereas centreing may offer less biased population average parameter estimates in the case the random effects model is misspecified. For computer-intensive situations the centreing option allows the use of a conditional estimation method to be implemented with less computational burden than the standard first-order conditional estimation method (FOCE). Compartmental models were used to describe drug disposition and elimination was assumed to take place from the central compartment. Linear and nonlinear elimination components in single- or multicompartment models were tried. A change in enzyme turn-over was used as a mechanistic model to address the induction phenomena. Linear or nonlinear dependence of the formation or elimination of the enzyme on the concentration of CP or 4-OH-CP were tested. Models where induction took place at different steps in the CP metabolic degradation scheme were tried. As an empirical model for changes in clearance, a linear increase in clearance with time was also explored. Discrimination between hierarchical models was based on the objective function value and graphical analyses of residual and predictions. For model diagnostics, the program Xpose, version 2 [23] was used. Confidence intervals for parameters of final model were obtained by the likelihood profile method [22]. The concentration ratio of 4-hydroxycyclophosphamide/cyclophosphamide at 24, 48, 72 and 96 h after start of the infusion was calculated.
Results
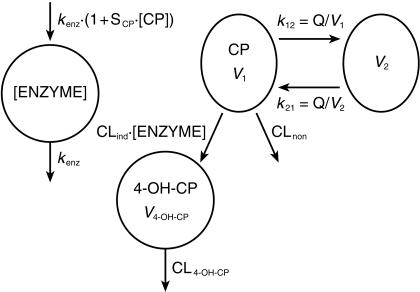
On average, 15 and 13 samples per patient were available for analysis of CP and 4-OH-CP concentrations, respectively. The combined CP and 4-OH-CP data were described by the model outlined in Figure 1. It contains the drug model (right) and the enzyme model (left). CP plasma concentration drives the enzyme production rate, which then affects the clearance of CP. CP was described by a two-compartment model with two elimination pathways, one inducible (CLind) and one uninducible (CLnon). The inducible pathway forms 4-OH-CP, which is described by a one-compartment model with uninducible elimination (CL4-OH-CP). CP autoinduction was modelled with an enzyme turn-over model, under the assumption that CP increases the production rate of enzyme in a linear fashion, where SCP is the slope of linear function for induction by CP. kenz is the rate constant for first-order degradation (time−1) of the enzyme pool. To normalize the enzyme concentration to unity at baseline, the zero-order production rate of enzyme was set to kenz(amount×time−1). Alternative models that gave a worse fit to data as judged by the objective function value and goodness-of-fit criteria were: (i) models with CP being eliminated by a single pathway, inducible or uninducible, (ii) a one-compartment model for CP, (iii) models with 4-OH-CP being eliminated by an inducible pathway, or (iv) 4-OH-CP increasing the enzyme production rate instead of CP, (v) models where the clearance was not related to CP concentration. Models that described the data as well as the chosen model, but seemed less plausible were: (i) an induction model with CP affecting the rate of enzyme degradation instead of increasing enzyme production, and (ii) a model where 4-OH-CP is formed by the uninduced pathway. More complex alternative models that did not describe data better than the chosen model include: (i) a model where the influence of CP on enzyme production is described by an Emax type relationship rather than a linear function, (ii) models where either the inducible or the uninducible elimination of CP is described by Michaelis-Menten kinetics.
Figure 1.
Schematic presentation of the two-compartment cyclophosphamide model, one-compartment 4-hydroxycyclophosphamide model and one-compartment enzyme model. The enzyme model is linked to the drug model by cyclophosphamide concentrations stimulating the enzyme production rate and enzyme amount affecting the cyclophosphamide clearance.
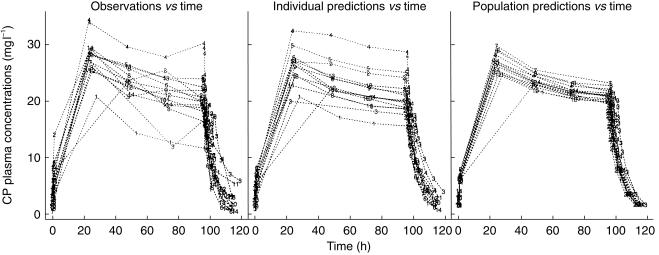
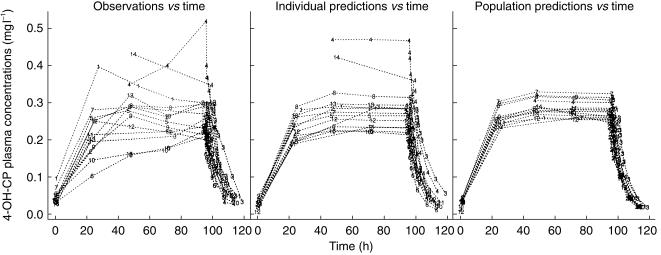
CP concentrations decreased during the 96 h infusion in all subjects (Figure 2). There was no change in 4-OH-CP plasma concentrations with time during steady-state (Figure 3). There was no change in the concentration ratio of 4-hydroxycyclophosphamide/cyclophosphamide over time. The ratio was 0.0104± 0.0028 (mean±s.d.), 0.0113±0.0040, 0.0135±0.0052 and 0.0107±0.0016 at 24, 48, 72 and 96 h after start of infusion, respectively. The decrease of 4-OH-CP paralleled that of CP, indicating 4-OH-CP to be formation rate limited. The half-life of 4-OH-CP could therefore not be estimated but is expected to be shorter than the elimination half-life of CP.
Figure 2.
Observed and population predicted plasma concentrations of cyclophosphamide vs time in breast cancer patients receiving a 96 h cyclophosphamide infusion (6 g m−2) in combination with thiotepa (500 mg m−2) and carboplatin (800 mg m−2).
Figure 3.
Observed and population predicted plasma concentrations of 4-hydroxycyclophosphamide vs time in breast cancer patients receiving a 96 h cyclophosphamide infusion (6 g m−2) in combination with thiotepa (500 mg m−2) and carboplatin (800 mg m−2).
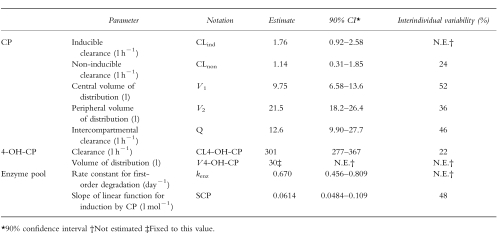
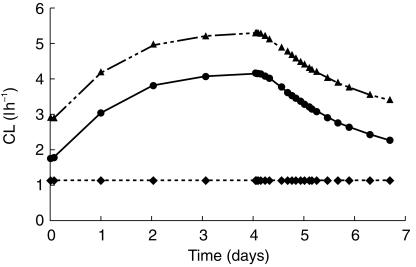
The estimated model parameters were similar for the two estimation methods (FO with and without centreing). The estimates of the former method are presented in Table 1. The two elimination pathways of CP were determined to be of similar magnitude in the uninduced state. However, the imprecision in CLind and CLnon estimates were rather large, due to a high negative correlation between the parameter estimates. At the end of treatment, CLind had increased two-fold and the model predicted 75% of the dose to be eliminated through the inducible pathway in a typical individual (Figure 4). Estimated interindividual variability in clearance was relatively low, 24% in CLnon and not significantly different from zero in CLind. However, the magnitude of induction differed as a consequence of rather high interindividual variability in SCP.
Table 1.
Estimated model parameters from plasma cyclophosphamide (CP) and 4-hydroxycyclophosphamide (4-OH-CP) data in breast cancer patients receiving a 96 h cyclophosphamide infusion (6 emsp14;g m−2) in combination with thiotepa (500 mg m−2) and carboplatin (800 mg m−2).
Figure 4.
Model predicted total clearance (▴), inducible clearance (•) and noninducible clearance (♦) of cyclophosphamide in breast cancer patients receiving a 96 h cyclophosphamide infusion (6 g m−2) in combination with thiotepa (500 mg m−2) and carboplatin (800 mg m−2).
While the apparent CL of 4-OH-CP (CL4-OH-CP) was well estimated, the apparent volume of 4-OH-CP (v4-OH-CP) could not be estimated (Table 1). Therefore it was fixed to 30 l in the estimation of the other CP and 4-OH-CP pharmacokinetic parameters. To assess the influence of v4-OH-CP on other parameters, a sensitivity analysis was performed where it was fixed to 10 and 100 l. This resulted in only minor changes of the estimates of other parameters.
The model predicted the half-life of the enzyme to be 1.0 day, a value that was relatively well determined at steady-state (Table 1). Addition of interindividual variability in this parameter did not improve the fit. In the final model, CP steady-state concentrations of 16 and 32 mg l−1 are predicted to double and treble the enzyme amount compared with the uninduced state, respectively.
Discussion
The results from this study clearly show that cyclophosphamide (CP) undergoes auto-induction with an increase in clearance and as a result decreasing plasma concentrations despite a constant rate of administration. Our results are in agreement with earlier studies where repeated administration or continuous infusion of CP resulted in increased CP clearance [13–15, 24, 25] but no alteration in volume of distribution [13, 16] or renal clearance of CP [15]. CP autoinduction has previously been modelled as a time-dependent clearance [18]. However, a mechanistic pharmacokinetic model for the in vivoautoinduction where the concentration of the inducer is counter-balanced by the enzymatic activity has not previously been presented.
Induction may result as an effect of increased enzyme synthesis (increased transcription or translation) or decreased enzyme degradation (enzyme stabilization). Whatever the mechanism, all will result in an increased amount of enzyme also referred to as the enzyme pool. Induction can also be due to an increased activity of the existing enzyme pool. It has been shown that CP is capable of inducing mRNA levels of CYP2C8, CYP2C9 and CYP3A4, but not CYP2B6 in human hepatocytes [17] and we therefore chose a model where CP affects the production rate and not the change in degradation rate. CP plasma concentrations drive the enzyme pool which then affects the clearance of CP (Figure 1). The advantage of this model is that the kinetics of CP are included. We assumed that the increase in the enzyme pool leads to a proportional increase in CP clearance. A linear relationship between CP and induction is not realistic for very high concentrations of CP, but in the studied interval it described data as well as an Emax type relationship. For the sake of parsimony, we chose the former model. This type of counter-balanced model was first described by Scheyer et al. [26] although the population parameters were not estimated but abstracted from multiple studies.
A model that includes some precursor which leads to the expression of the induced enzyme and where the effect of the inducer lies on the precursor could be a possible mechanism. The mechanism(s) by which phenobarbitone and phenobarbitone-like inducers increase the expression of CYP2A6, CYP2B6, CYP2C and CYP3A4 are still poorly understood, but they act primarily at the level of transcription to induce CYP450 activity [17, 27, 28]. No phenobarbitone receptor has yet been found and it is difficult to believe the existence of a receptor due to the structure diversity between the different phenobarbitone-like inducers. It is therefore more likely that the mechanism proceeds via an indirect mechanism like interaction with an endogenous inducer or repression of a precursor [27]. In this study, no data on gene transcription, translation or fractional turnover rate of the enzyme pool was available. With the assumption that the rate constant governing the formation of the enzyme pool from the precursor is fast, this step can be omitted. Therefore in our model, CP is affecting the synthesis rate by a direct mechanism where kenz reflects the rate-limiting step.
When the elimination of the inducer is rapid with respect to the fractional turnover, an approximation is often made and the kinetics of the inducer are not included in the induction model. The effect of pentobarbitone on nortriptyline metabolism is a recent example of this [29]. However, in the case of CP where the elimination constant is less than kenz (0.670 day−1), the induction model can not be simplified. Therefore the model presented here which includes the concentration of the inducer is in favour when the kinetics of the inducer may in part be rate limiting. There are other models which include the kinetics of the inducer [30, 31] but they do not include a negative feed-back where the plasma concentrations of the inducer and the enzyme pool counterbalance each other.
In the applied model, CP affects the enzyme turn-over rate, wherefore only the enzyme pool is changed but not the time it takes to obtain a new steady-state with respect to the enzyme pool. The half-life of the induced enzyme pool was estimated to be 1.0 day, a value that was relatively well determined. If one assumes three half-lives of the induced enzyme to establish 90% of the new induced steady-state, it would take 3 days to establish CP steady-state plasma concentrations. Therefore the present 96 h infusion was sufficient to reach near steady state which is also seen in Figure 2.
CP is to a major extent eliminated through 4-hydroxylation by CYP2B6, CYP2C9 and CYP3A4 [2, 5, 6]. CP is also metabolized to deschloroethylcyclophosphamide by CYP3A4 [2, 11, 12]. It has been shown that CP is capable of inducing mRNA levels of CYP2C8, CYP2C9 and CYP3A4, but not CYP2B6 in human hepatocytes [17]. There are also in vivostudies showing the effect of CYP450 inducers on CP elimination as well as CP capable of inducing CYP450. Increased CP clearance has been reported in children who had received pretreatment with dexamethasone [25], a CYP3A4 inducer [32]. A decreased half-life of CP has also been seen after pretreatment with phenobarbitone [33], an effect that is likely to be due to induction of CYP2B6, CYP2C and CYP3A4 [27]. High dose CP administration to patients not only increased its own elimination but also that of dexamethasone [14], being eliminated by CYP3A4 [34]. One could therefore expect the enzyme induction by CP to cause an increased formation clearance of 4-OH-CP by inducing CYP2C9 and CYP3A4. However, Ren et al. [35] showed that deschloroethylcyclophosphamide formation clearance, mediated probably by CYP3A4 [2, 11, 12] was not affected by CP induction whereas 4-OH-CP formation clearance increased [35], indicating that the enzyme responsible for descloroethylcyclophosphamide formation is not induced by CP. One could speculate that CP maybe does not induce CYP3A4 or is a weak inducer of the enzyme even though an in vitrostudy has shown CP and ifosfamide to be inducers of CYP3A4 [36].
There are overall limited data on the turn-over of enzymes inducible by phenobarbitone-like compounds in man. The effect of pentobarbitone on nortriptyline metabolism was studied where the enzyme half-life was estimated to 140 h [29]. The autoinduction of another oxazaphosphorine, ifosfamide has been modelled and the enzyme half-life was estimated to be 2–6 h [19]. Carbamazepine induces CYP3A4 and a turn-over half-life of 85–806 h has been reported for the carbamazepine induced elimination of ethosuximide [37]. Autoinduction of methadone has been characterized and the turn-over of CYP3A4 was estimated to 94 h [38]. Even though carbamazepine and methadone induce CYP3A4 [39], it does not appear to induce the same enzymes as phenobarbitone [28]. In comparison, continuous infusion of CP results in a relatively high turn-over of the enzyme(s) by which it is metabolized. Reasons for different reports on enzyme half-lives in the literature could be that different enzyme models have been used, difficulty in estimating a true value, model miss-specification due to a cascade of events rather than a direct effect, sometimes multiple enzymes being induced or that different isoenzymes have been studied. The estimated enzyme half-life for CP in this study and previous report on ifosfamide [19], being shorter compared with the turn-over of CYP3A4 [29, 37], is likely to be due to CP inducing an isoenzyme other than CYP3A4 or multiple enzymes. The turn-over rate of CYP2B induction is known to be very fast [40, 41]. One could therefore speculate that CP and ifosfamide, apart from inducing CYP2C9, also induce CYP2B6 causing a rapid enzyme turn-over. However, in vitrodata have shown that CYP2B6 is not inducible by CP and ifosfamide [17].
In our study, the 4-OH-CP steady-state concentrations remained essentially constant despite induced formation of 4-OH-CP. A lack of simultaneous increase in 4-OH-CP steady-state concentrations despite induction of the formation and under the assumption of unchanged 4-OH-CP elimination clearance is expected since 4-OH-CP is the major metabolite also in the uninduced state [35]. When the fraction of formation from parent drug approaches unity, the metabolite concentration is principally determined by its own elimination clearance and becomes independent of the fraction of the parent compound converted to the metabolite [42]. This is also in agreement with both our results and those of Ren et al. [35] where the fraction of CP converted to 4-OH-CP only increased 16%. The results from Ren et al. [35] and the present study are also similar in that both studies predict that cyclophosphamide pharmacokinetics change due to increased formation of 4-OH-CP. However, they differ in that the result from the present study did not detect any pronounced increase in 4-OH-CP concentrations with increasing induction and it appears as if the differences between the studies occur in the elimination of 4-OH-CP. The increase in 4-hydroxycyclophosphamide concentrations in the study by Ren et al. [35] was explained as a decreased elimination of 4-OH-CP due to inhibition of aldehyde dehydrogenase-1, which also was confirmed with in vitrodata. The inhibition of aldehyde dehydrogenase-1 activity by CP is likely to be concentration-dependent and the plasma concentration of CP and 4-OH-CP were lower compared with the results from Ren et al. [35]. The concentrations obtained in our study might therefore not have been high enough to achieve sufficient inhibition which would explain the lack of increase in 4-OH-CP concentrations. The fact that our model did not contain the decreased 4-OH-CP elimination, observed by Ren et al. is directly supported by raw data from our study. The concentration ratio of 4-OH-CP/CP remained constant with time whereas, the AUC ratio of 4-OH-CP/CP increased two-fold from day 1 to day 2 in the study by Ren et al. [35]. There is also one major difference between the two studies in terms of study design. The patients were given fluconazole in the study by Ren et al. whereas CP was coadministered with thiotepum in the present study. Fluconazole is known to be a potent inhibitor of CYP2C9 and CYP3A4 [43–46] and thiotepum has been shown to inhibit CP metabolism in human liver microsomes [47]. It is reasonable to believe that coadministration of fluconazole or thiotepum with cyclophosphamide will result in changes in the metabolic pattern of cyclophosphamide and the results may therefore not be extrapolated to other situations due to involvement of multiple isoenzymes, different selectivity and unknown potency of the two inhibitors. Thus the difference in results lies in the different study conditions between the two studies or, less likely, the potential problems in the study of Ren et al. of calculating single day AUCs with multiple dosing.
There have been contradicting results regarding which isoenzymes that are the most dominant in the 4-hydroxylation of CP in vitro. Chang et al. showed that CYP2B6 was the major isoenzyme [5] while Ren et al. [2] demonstrated CYP2C9 and CYP3A4 to be the most important isoenzymes for the 4-hydroxylation of CP. However, with CP not being capable of inducing CYP2B6 [17] and with the fact that CYP2C9 and CYP3A4 are two of the most highly expressed enzymes in the liver [48, 49], these enzymes should be dominant in the 4-hydroxylation of CP during continuous infusion.
Inconsistent results regarding whether CP metabolism is saturated have been presented. Some studies have shown no saturation in CP metabolism at doses up to 3.5 g m−2 [50, 51] whereas Chan et al. detected saturated kinetics in some patients at a dose of 1 g m−2 [52]. Chen et al. [53] comodelled CP and 4-OH-CP whole blood concentrations after a 4 g m−2 90 min CP infusion. They proposed a one-compartment model with Michaelis-Menten saturable elimination for CP. With the data from this study, where it is evident that the induction occurs fast and has an enzyme turn-over half-life of 1.0 day, an alternative explanation for the observed data is auto-induction and not saturated elimination at high concentrations. Even if CP would exhibit saturated elimination, this effect would be apparent only initially during a constant infusion if subsequently superimposed by the greater changes caused by induction.
The presented enzyme turn-over model applied to data from this study agrees with CP affecting its own elimination by increasing the enzyme production rate and thereby increasing the amount of enzyme by which CP is eliminated. Continuous infusion of CP leads to an induced formation clearance of 4-OH-CP which will have less effect on the steady-state concentration of 4-OH-CP as the inducible pathway(s) is the major pathway(s) of CP elimination also before induction. The mechanism-based enzyme induction model applied herein, where the pharmacokinetics of the inducer and the enzyme pool counterbalance each other successfully described cyclophosphamide autoinduction and may be suitable to similar situations for studying the time course of enzyme induction.
Acknowledgments
This project was supported by grants from the Cancer Society in Stockholm (97: 116), the Swedish Cancer Society (4147–B98–61XAB) and Karolinska Institutet.
References
- 1.Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. doi: 10.2165/00003088-199120030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ren S, Yang J-S, Kalhorn TF, Slattery JT. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res. 1997;57:4229–4235. [PubMed] [Google Scholar]
- 3.Low JE, Borch RF, Sladek NE. Conversion of 4-hydroxycyclophosphamide to phosphoramide mustard and acrolein mediated by bifunctional catalysts. Cancer Res. 1982;42:830–837. [PubMed] [Google Scholar]
- 4.Colvin M, Brundrett RB, Kan MN, Jardine I, Fenselau C. Alkylating properties of phosphoramide mustard. Cancer Res. 1976;36:1121–1128. [PubMed] [Google Scholar]
- 5.Chang TKH, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochrome P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- 6.Chang TKH, Yu L, Goldstein JA, Waxman DJ. Identification of polymorphically expressed CYP2C19 and the wild type CYP2C9-Ile359 allele as low Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics. 1997;7:211–221. doi: 10.1097/00008571-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Connors TA, Cox PJ, Farmer PB, Foster AB, Jarman M. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochem Pharmacol. 1974;23:115–129. doi: 10.1016/0006-2952(74)90318-9. [DOI] [PubMed] [Google Scholar]
- 8.Fenselau C, Kan MNN, Rao SS, et al. Identification of aldophosphamide as a metabolite of cyclophosphamide in vitro and in vivo in humans. Cancer Res. 1977;37:2538–2543. [PubMed] [Google Scholar]
- 9.Domeyer BE, Sladek NE. Metabolism of 4-hydroxycyclophosphamide/aldophosphamide in vitro. Biochem Pharmacol. 1980;29:2903–2912. doi: 10.1016/0006-2952(80)90035-0. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl R. Aldehyde dehydrogenases and their role in carcinogenesis. Crit Rev Biochem Mol Biol. 1992;27:283–335. doi: 10.3109/10409239209082565. [DOI] [PubMed] [Google Scholar]
- 11.Ruzicka JA, Ruenitz PC. Cytochrome P-450 mediated N-dechloroethylation of cyclophosphamide and ifosfamide in the rat. Drug Metab Dispos. 1992;20:770–772. [PubMed] [Google Scholar]
- 12.Bohnenstengel F, Hofmann U, Eichelbaum M, Kroemer HK. Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol. 1996;51:297–301. doi: 10.1007/s002280050201. [DOI] [PubMed] [Google Scholar]
- 13.Graham MI, Shaw IC, Souhami RL, et al. Decreased plasma half-life of cyclophosphamide during repeated high-dose administration. Cancer Chemother Pharmacol. 1983;10:192–193. doi: 10.1007/BF00255760. [DOI] [PubMed] [Google Scholar]
- 14.Moore MJ, Hardy RW, Thiessen JJ, Soldin SJ, Erlichman C. Rapid development of enhanced clearance after high-dose cyclophosphamide. Clin Pharmacol Ther. 1988;44:622–628. doi: 10.1038/clpt.1988.203. [DOI] [PubMed] [Google Scholar]
- 15.Fasola G, Lo Greco P, Calori E, et al. Pharmacokinetics of high-dose cyclophospahmide for bone marrow transplantation. Haematologica. 1991;76:120–125. [PubMed] [Google Scholar]
- 16.Sladek NE, Priest J, Doeden D, et al. Plasma half-life and urinary excretion of cyclophosphamide in children. Cancer Treat Rep. 1980;64:1061–1066. [PubMed] [Google Scholar]
- 17.Chang TKH, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation by primary human hepatocyte culture: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1953. [PubMed] [Google Scholar]
- 18.Chen T, Passos-Coelho J, Noe D, et al. Nonlinear pharmacokinetics of cyclophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Cancer Res. 1995;15:810–816. [PubMed] [Google Scholar]
- 19.Boddy AV, Cole M, Pearson ADJ, Idle JR. The kinetics of the auto-induction of ifosfamide metabolism during continous infusion. Cancer Chemother Pharmacol. 1995;36:53–60. doi: 10.1007/BF00685732. [DOI] [PubMed] [Google Scholar]
- 20.Hardy RW, Erlichman C, Soldin SJ. High-performance liquid chromatographic measurement of cyclophosphamide in serum. Ther Drug Monit. 1984;2:313–318. doi: 10.1097/00007691-198409000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M, Bielenstein M. Determination of 4-hydroxycyclophosphamide in plasma, as 2,4-dinitrophenylhydrazone derivative of aldophosphamide, by liquid chromatography. J Chromatogr. 1994;660:111–120. doi: 10.1016/0378-4347(94)00283-5. [DOI] [PubMed] [Google Scholar]
- 22.Beal SL, Sheiner LB. San Francisco: Project Group, University of California at San Francisco; 1998. NONMEM Users’Guides, NONMEM. [Google Scholar]
- 23.Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic-pharmacodynamic model building aid for NONMEM. Comp Meth Prog Biomed. 1998;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 24.Schuler U, Ehninger G, Wagner T. Repeated high-dose cyclophosphamide administration in bone marrow transplantation: exposure to activated metabolites. Cancer Chemother Pharmacol. 1987;20:248–252. doi: 10.1007/BF00570495. [DOI] [PubMed] [Google Scholar]
- 25.Yule SM, Boddy AV, Cole M, et al. Cyclophosphamide pharmacokinetics in children. Br J Clin Pharmacol. 1996;41:13–19. doi: 10.1111/j.1365-2125.1996.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 26.Scheyer RD, Cramer JA, Mattson RH. A pharmacodynamic approach to the estimate of carbamazepine autoinduction. J Pharm Sci. 1994;83:491–494. doi: 10.1002/jps.2600830409. [DOI] [PubMed] [Google Scholar]
- 27.Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlock JPJ, Denison MS. Cytochrome P450; structure mechanism, biochemistry. 2. Ortiz de Montellano P, New York and London: Plenum Press; 1995. Induction of cytochrome P450 enzymes that metabolize xenobiotics; pp. 374–378. [Google Scholar]
- 29.von Bahr C, Steiner E, Koike Y, Gabrielsson J. Time course of enzyme induction in humans: effect of pentobarbital on nortriptyline metabolism. Clin Pharmacol Ther. 1998;64:18–25. doi: 10.1016/S0009-9236(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 30.Abramson FP. Kinetic models of induction: I. Persistence of the inducing substance. J Pharm Sci. 1986;74:223–228. doi: 10.1002/jps.2600750302. [DOI] [PubMed] [Google Scholar]
- 31.Abramson F, Lutz M. The kinetics of induction by rifampin of alpha 1-acid glycoprotein and antipyrine clearance in the dog. Drug Metab Dispos. 1986;14:46–51. [PubMed] [Google Scholar]
- 32.Watkins PB, Wrighton SA, Maurel P, et al. Identification of an inducible form of cytochrome P-450 in human liver. Proc Natl Acad Sci U S A. 1985;82:6310–6314. doi: 10.1073/pnas.82.18.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jao JY, Jusko WJ, Cohen JL. Phenobarbital effects on cyclophosphamide pharmacokinetics in man. Cancer Res. 1972;32:2761–2764. [PubMed] [Google Scholar]
- 34.Gentile DM, Tomlinson ES, Maggs JL, Park BK, Back DJ. Dexamethasone metabolism by human liver in vitro. Metabolite identification and inhibition of 6-hydroxylation. J Pharmacol Exp Ther. 1996;277:105–112. [PubMed] [Google Scholar]
- 35.Ren S, Kalhorn TF, McDonald GB, et al. Pharmacokinetics of cyclophosphamide and its metabolites in bone marrow transplantation patients. Clin Pharmacol Ther. 1998;64:289–301. doi: 10.1016/S0009-9236(98)90178-3. [DOI] [PubMed] [Google Scholar]
- 36.Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction of oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- 37.Warren JW, Benmaman JD, Wannamaker BB, Levy RH. Kinetics of carbamazepine–ethosuximide interaction. Clin Pharamcol Ther. 1980;28:646–651. doi: 10.1038/clpt.1980.216. [DOI] [PubMed] [Google Scholar]
- 38.Rostami-Hodjegan A, Wolff K, Hay A, et al. Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Br J Clin Pharmacol. 1999;48:43–52. doi: 10.1046/j.1365-2125.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerling M, Lindström L, Bondesson U, Bertilsson L. Fluvoxamine inhibition and carbamazepine induction of the metabolism of clozapine: Evidence from a therapeutic drug monitoring service. Ther Drug Monit. 1994;16:368–374. doi: 10.1097/00007691-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Hardwick JP, Gonzalez FJ, Kasper CB. Cloning of DNA complementary to cytochrome P-450 induced by pregnenolone-16 alpha-carbonitrile. Characterization of its mRNA, gene, and induction response. J Biol Chem. 1983;258:10182–10186. [PubMed] [Google Scholar]
- 41.Ravishankar H, Padmanaban G. Turnover of messenger RNA, apoprotein and haem of cytochrome P-450b+e induced by phenobarbitone in rat liver. Biochem J. 1985;229:73–79. doi: 10.1042/bj2290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houston JB. Drug metabolite kinetics. Pharmacol Ther. 1982;15:521–552. doi: 10.1016/0163-7258(81)90056-5. [DOI] [PubMed] [Google Scholar]
- 43.Kunze KL, Wienkers LC, Thummel KE, Trager WF. Warfarin-fluconazole. I. Inhibition of the human cytochrome P450- dependent metabolism of warfarin by fluconazole: in vitro studies. Drug Metab Dispos. 1996;24:414–421. [PubMed] [Google Scholar]
- 44.Black DJ, Kunze KL, Wienkers LC, et al. Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos. 1996;24:422–428. [PubMed] [Google Scholar]
- 45.Kazierad DJ, Martin DE, Blum RA, et al. Effect of fluconazole on the pharmacokinetics of eprosartan and losartan in healthy male volunteers. Clin Pharmacol Ther. 1997;62:417–425. doi: 10.1016/S0009-9236(97)90120-X. [DOI] [PubMed] [Google Scholar]
- 46.Kaukonen KM, Olkkola KT, Neuvonen PJ. Fluconazole but not itraconazole decreases the metabolism of losartan to E-3174. Eur J Clin Pharmacol. 1998;53:445–449. doi: 10.1007/s002280050405. [DOI] [PubMed] [Google Scholar]
- 47.Anderson L, Chen T, Colvin O, et al. Cyclophosphamide ad 4-hydroxycyclophosphamide/aldophosphamide kinetics in patients receiving high-dose cyclophosphamide. Clin Cancer Res. 1996;2:1481–1487. [PubMed] [Google Scholar]
- 48.Romkes M, Faletto MB, Blaisdell JA, Raucy JL, Goldstein JA. Cloning and expression of complementary DNAs for multiple members of the human cytochrome P450IIC subfamily. Biochemistry. 1991;30:3247–3255. doi: 10.1021/bi00227a012. [DOI] [PubMed] [Google Scholar]
- 49.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 50.Brock N, Gross R, Hohorst H-J, Klein HO, Schneider B. Activation of cyclophosphamide in man and animals. Cancer. 1971;27:1512–1529. doi: 10.1002/1097-0142(197106)27:6<1512::aid-cncr2820270636>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson PM, O’Neill PA, Thatcher N, Lucas SB. Pharmacokinetics of high-dose cyclophosphamide in patients with metastatic bronchogenic carcinoma. Can Chem Pharmacol. 1983;11:196–199. doi: 10.1007/BF00254204. [DOI] [PubMed] [Google Scholar]
- 52.Chan KK, Hong PS, Tutsch K, Trump DL. Clinical pharmacokinetics of cyclophosphamide and metabolites with and without SR-2508. Cancer Res. 1994;54:6421–6429. [PubMed] [Google Scholar]
- 53.Chen T-L, Kennedy MJ, Anderson LW, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Met Dispos. 1997;25:544–551. [PubMed] [Google Scholar]