Abstract
Aims
A controlled-release gastrointestinal therapeutic system (GITS) formulation of doxazosin mesylate, a long-acting selective α1-adrenoceptor antagonist, was developed to enhance the pharmacokinetic profile and simplify the titration schedule by precisely controlling drug delivery rate, permitting an initial dose of 4 mg once daily, compared with standard doxazosin, which is initiated at 1 mg day−1 and titrated to a higher therapeutically effective dose. The aim of the present work was to evaluate the pharmacokinetics and bioavailability of doxazosin GITS with respect to the effect of food, age and gender, and multiple dosing. In addition, in vitro performance was assessed in conditions simulating the gastrointestinal environment.
Methods
A three-way crossover study in 24 subjects assessed the comparative bioavailability of doxazosin GITS under fed and fasting conditions and doxazosin standard under fasting condition. A multiple-dose, two-way crossover study in 35 subjects assessed the comparative pharmacokinetics and bioavailability of doxazosin GITS and doxazosin standard 4 and 8 mg upon multiple dosing. A multiple-dose, four-parallel–group study was conducted to determine the steady-state pharmacokinetics and bioavailability of doxazosin GITS 4 mg in 41 young and elderly male and female subjects. The release-rate profiles of doxazosin GITS were determined in artificial gastric fluid (pH =1.2), intestinal fluid (pH =7.5), and water. The effect of agitation on the dissolution characteristics of doxazosin GITS in artificial gastric fluid was studied at stirring rates of 50, 75, and 100 rev min−1.
Results
In vitro studies demonstrated that release rates for the GITS tablet are independent of pH in the range of 1.2 (gastric) to 7.5 (intestinal), and of stirring rates simulating gastrointestinal motility. Clinical pharmacology studies showed that doxazosin GITS had a lower maximum plasma concentration, prolonged time to reach maximum plasma concentration, and a higher minimum plasma concentration compared with doxazosin standard. Thus, the GITS formulation results in a more gradual absorption of doxazosin, and a reduced plasma doxazosin concentration peak-to-trough fluctuation ratio. The relative bioavailability of doxazosin GITS is approximately 60%. With a high-fat meal, the maximum plasma concentration and area under the concentration-time curve were 31% and 18% higher, respectively (P <0.05). Bioequivalence was established between the dose strengths of two 4 mg doxazosin GITS tablets and one 8 mg doxazosin GITS tablet. For both young adult and elderly subjects, and males and females, the pharmacokinetics of doxazosin GITS once daily for 7 days were comparable. Doxazosin GITS was well tolerated in the subjects studied, including young and elderly males and females.
Conclusions
The GITS formulation of doxazosin enhances the pharmacokinetic profile compared with doxazosin standard, allowing more gradual absorption of doxazosin, and a reduced plasma doxazosin peak-to-trough concentration ratio. Thus, doxazosin GITS therapy can be initiated at a therapeutic dose of 4 mg with reduced haemodynamic side-effects.
Keywords: benign prostatic hyperplasia, controlled-release, doxazosin GITS, doxazosin, gastrointestinal therapeutic system, hypertension, pharmacodynamics, pharmacokinetics
Introduction
Doxazosin mesylate, a quinazoline derivative, is established, effective, and well-tolerated for the treatment of hypertension and benign prostatic hyperplasia (BPH) [1–4]. In hypertensive patients, doxazosin lowers blood pressure (BP) by selectively blocking postjunctional α1-adrenergic receptors and antagonizing stimulant activity of noradrenaline, thereby decreasing systemic vascular resistance. In a 5 year study of once-daily treatment with doxazosin or atenolol in patients with mild to moderate hypertension, doxazosin produced a significantly greater beneficial effect on coronary heart disease risk and serum lipid parameters while demonstrating similar antihypertensive efficacy and safety compared with atenolol [2]. Among patients with BPH, doxazosin effectively blocks α1-adrenoceptors present in high density in the prostate and bladder neck, inhibiting sympathetic stimulation of prostatic smooth muscle, thereby reducing prostatic tone and relieving obstruction; this results in improved urinary outflow and symptom relief. In men with BPH, a significant increase in maximal urinary flow rate and improvement in symptoms are observed after both short-and long-term treatment with doxazosin [3]. Both doxazosin plasma concentration and maximal urinary flow rate have been demonstrated to increase linearly with increasing doses, in the range of 2 mg to 8 mg daily, where the clinical response to doxazosin appears to plateau [4].
The pharmacokinetics of doxazosin are linear within a dose range of 1–16 mg [5–7]. After oral administration of the standard immediate-release tablet formulation, doxazosin is rapidly and well absorbed. With this formulation, maximum plasma drug concentrations (Cmax) are attained approximately 2–3 h after dosing. Plasma elimination of doxazosin is biphasic, with a terminal elimination half-life (t1/2) of about 22 h. Oral bioavailability of doxazosin is approximately 65%, reflecting first-pass metabolism. The t1/2 and oral clearance are similar in young and elderly adults [8] and no significant alterations in pharmacokinetics have been observed in individuals with renal impairment vs normal renal function [9]. For patients with hepatic impairment, the Cmax and t1/2 are not significantly different, but the oral clearance has been shown to be 30% lower compared with healthy subjects [10]. Administration with a high-fat meal does not significantly alter the pharmacokinetics of doxazosin [11]; and pharmacokinetics, efficacy, and safety are similar with morning or evening dosing [12, 13].
With the standard formulation, doxazosin therapy is initiated at 1 mg day−1, and the dose is doubled every 7–14 days to a maximum recommended dose of 16 mg day−1 for hypertension or 8 mg day−1 for BPH until BP or urinary flow rate or BPH symptoms are controlled [1]. This regimen can require up to four titration steps to achieve therapeutically effective doses, in a manner likely to avoid first-dose side-effects. The controlled-release gastrointestinal therapeutic system (GITS) [14] formulation of doxazosin has been developed to more precisely control plasma drug levels, thereby enhancing the pharmacokinetic profile and affording a simplified dosing schedule. Doxazosin GITS is designed to permit a higher initial daily dose (4 mg day−1) than doxazosin standard, while avoiding significant first-dose side-effects. Thus, therapeutically effective levels can be reached more rapidly without excessive plasma levels, and more uniform plasma concentrations can also be provided with minimal peak-to-trough fluctuation.
Presented here are the results of in vitro studies assessing performance of the GITS tablet with varying levels of pH and stirring rates, and results of four in vivo single-dose and/or multiple-dose studies designed to characterize the pharmacokinetic profile of doxazosin GITS in healthy young adult and elderly males and females. The single-dose study was designed to assess the basic pharmacokinetic profile of doxazosin GITS and also determine the degree of food effects after a high-fat meal. The objective of the multiple-dose study was to assess the pharmacokinetic characteristics at steady-state. The dose strength bioequivalence study was designed to assess comparative bioavailability and pharmacokinetics of the 4 mg and 8 mg dose strengths. Finally, the objective of the age and gender study was to determine relative bioavailability and pharmacokinetics of the GITS formulation at steady-state for young and elderly individuals and for males and females.
Methods
In vitro studies
Two sets of experiments were conducted to assess the performance of the GITS tablet in conditions simulating the gastrointestinal (GI) environment. Because oral extended-release formulations encounter varying pH levels along the GI tract, the release-rate profiles for doxazosin GITS were determined in artificial gastric fluid (pH =1.2), artificial intestinal fluid (pH =7.5), and water. The release from doxazosin GITS systems was measured using the USP Apparatus 2 at 37° C, and with a paddle speed of 75 rev min−1.
Dissolution characteristics of many oral extended-release formulations are frequently susceptible to and altered by agitation and motility along the GI tract. The doxazosin GITS tablets are coated with a semipermeable membrane that does not dissolve; this results in a stable shape that does not erode or disintegrate. Thus, the drug-release mechanism of the system is expected to be independent of external agitation. The effect of agitation on the dissolution characteristics of doxazosin GITS 4 mg and 8 mg tablets in artificial gastric fluid was studied at paddle (stirring) speeds of 50, 75, and 100 rev min−1.
Clinical pharmacology studies
Participants
All participants were healthy, as determined from a detailed medical history and complete physical examination including vital signs, complete blood count, blood chemistries, urinalysis, urine drug screen, and electrocardiogram. Written informed consent was obtained from each participant prior to enrolment, and the studies were conducted with appropriate ethical guidelines.
Study designs
Two single-dose studies and two multiple-dose studies were conducted to characterize the pharmacokinetic profile of doxazosin GITS in healthy volunteers. Two studies included comparisons with doxazosin standard. All studies were conducted in compliance with the Declaration of Helsinki (1964) and the Hong Kong (1989) Revision.
Single-dose/food-effect study
This open-label, randomized, three-way crossover study in 24 young, healthy male volunteers (aged 18–40 years) assessed the comparative bioavailability of single 8 mg doses of doxazosin GITS under fasting and fed conditions and a single doxazosin standard 2 mg dose under fasting condition. Each subject was randomly assigned to one of three treatment sequences, with a 7 day washout period between treatments. Under fasting condition, each dose was given after an overnight 10 h fast; subjects continued to fast for 4 more hours postdose. Under fed condition, each dose was given within 15 min of finishing a high-fat breakfast consisting of two slices of buttered toast, two eggs fried in butter, two slices of bacon, 4 oz hash-brown potatoes, and 8 oz whole milk. Blood samples were drawn immediately before dosing; hourly up to 4 h postdose; every 2 h thereafter up to 16 h postdose; and at 24 h postdose, then once daily up to 96 h postdose. Plasma was immediately separated from the blood samples and frozen until assayed.
Multiple-dose crossover study
This open, randomized, multiple-dose, two-way crossover study assessed the pharmacokinetic characteristics of multiple doses of doxazosin GITS 4 mg and 8 mg and doxazosin standard 4 mg and 8 mg in 35 healthy male subjects (aged 18–60 years). Each subject was randomized to one of the following initial treatment arms: placebo for 7 days, doxazosin GITS 4 mg for 7 days, then 8 mg for 7 days; or doxazosin standard 1 mg for 2 days, 2 mg for 5 days, 4 mg for 7 days, and 8 mg for 7 days. After a 7 day washout period, subjects crossed over to the alternate regimen. Blood samples were drawn for each treatment arm predose on days 1, 13, 14, 20, and 21; as well as 1, 2, 3, 4, 6, 8, 10, 12, 18, and 24 h postdose on days 14 and 21, and 48, 72, and 96 h after the last dose on day 21. Plasma was immediately separated from the blood samples and frozen until assayed.
Dose strength bioequivalence study
An open, randomized, single-dose, two-way crossover study was performed in 24 healthy male subjects (aged 18–40 years) to determine the relative bioavailability and pharmacokinetics of two 4 mg doxazosin GITS tablets vs one 8 mg doxazosin GITS tablet. There was a 7 day washout period between treatments. Blood samples were obtained predose, and 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 24, 48, 72, and 96 h postdose.
Age and gender study
An open, multiple-dose parallel-group study was conducted to determine the steady-state relative bioavailability and pharmacokinetics of doxazosin GITS 4 mg in 41 healthy young (18–40 years of age) and elderly (≥65 years of age) males and females. Each subject received one 4-mg doxazosin GITS tablet for 7 consecutive days. Blood samples were collected predose and 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 24 h postdose on days 1 and 7; predose on days 5 and 6; and 48, 72, and 96 h after the last dose on day 7.
Dosage forms
Two strengths of doxazosin GITS tablets, 4 mg and 8 mg (Cardura XL™, Pfizer Inc, New York, NY), were used in the bioavailability and pharmacokinetic studies. Four commercially available doxazosin standard tablet strengths, 1 mg, 2 mg, 4 mg, and 8 mg (Cardura™), Pfizer Inc, New York, NY, were included in the studies.
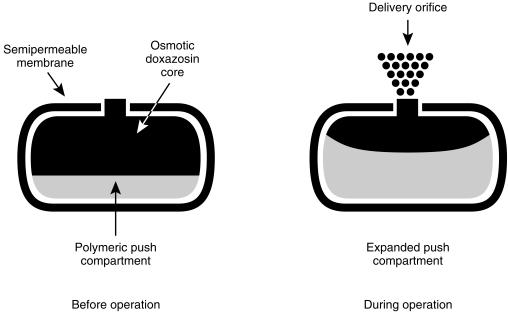
The GITS tablet (Figure 1) employs a membrane-controlled, osmotically powered push-pull process to release doxazosin in the GI lumen at an approximately steady (apparent zero-order) rate for 12–16 h, such that relatively constant plasma concentrations of drug are maintained throughout the 24 h dosing interval. The tablet includes a pull layer containing the drug and osmotic excipients and a push layer containing only osmotic excipients, bonded together by tablet compression to form a single tablet-shaped core. This core is coated by a hard cellulosic membrane that is permeable to water but impermeable to ions, the drug, and osmotic excipients. A precisely dimensioned orifice is drilled through the membrane by a high-speed laser on the drug-layer side of the tablet core to conform to predefined limits.
Figure 1.
Doxazosin in the gastrointestinal therapeutic system (GITS), a push-pull osmotically activated, controlled-release formulation. After ingestion, as the tablet passes through the gastrointestinal tract, water is absorbed into both compartments at a constant rate. Doxazosin is drawn into suspension in the top compartment. Osmotically active excipients in the nondrug layer begin to expand at a precisely controlled rate, pushing against the drug layer such that the doxazosin suspension is expelled through the laser-drilled orifice at a controlled rate.
When the system is placed in an aqueous environment, water enters it because an osmotic pressure gradient is established across the membrane by the attraction of the osmotic excipients in the two tablet layers. Water is drawn into the tablet at a constant rate dictated by the properties of the outer membrane. A suspension of the micronized drug is formed in situ in the drug layer, and the polymeric osmotic excipients in the other layer begin to expand. As the push layer expands, the doxazosin suspension is released through the delivery orifice, a laser-drilled hole on the drug-layer side large enough to prevent osmotic pressure buildup within the system, but small enough to prevent uncontrolled leaching of the drug. The volume of the system remains essentially constant throughout this process; thus, the drug delivery rate equals the rate at which water permeates the membrane. Doxazosin is delivered continuously in this manner as the GITS tablet moves along the GI tract.
Analytical methods
Plasma samples were analysed using a validated high-performance liquid chromatography (h.p.l.c.) assay with fluorescence detection [15]. Doxazosin was extracted from plasma sample after addition of internal standard prazosin and 50% ammonium hydroxide into ethyl acetate. Separation, subsequent detection, and quantification of doxazosin were achieved on a C8 column of an isocratic, reverse-phase h.p.l.c. assay system coupled to a fluorescence detector set at an excitation wavelength of 248 nm and an emission filter at 370 nm. Peak height ratios of doxazosin to the internal standard were computed for all study samples, calibration standards, and quality-control samples. Quality-control samples of 0.8, 4, and 16 ng ml−1 were prepared, stored, and analysed along with the study samples to verify analyte stability, assay accuracy, and precision.
The assay was linear over the calibration standard concentration range for doxazosin of 0.2–20 ng ml−1. The limit of quantification was determined to be 0.2 ng ml−1. The slopes of the standard curves were consistent among all runs with correlation coefficients >0.994. The variability of the calibration standards from 0.2 to 20 ng ml−1 ranged from 1.9% to 7.5%. The interday and within-day precision for the quality-control samples was >93%. The mean quality-control samples deviated <4% from the nominal concentrations. Thus, the assay was linear, reproducible, precise, and specific; and demonstrated that doxazosin was stable in human plasma during 2 months of storage at −20° C.
Pharmacokinetic methods
The area under the plasma concentration-time curve (AUC) for doxazosin from time zero to the last measurable sampling time point (t) or end of dosing interval of 24 h (τ), was calculated using the trapezoidal rule. Total AUC was determined as the sum of AUC (t) plus the area extrapolated to infinity by division of the last measurable plasma concentration by the apparent elimination rate constant λz for the treatment. The Cmax and the time of maximum concentration (tmax) were determined directly from concentration-time data for each subject. The t1/2 was calculated as 0.693/λz, where λz was determined from the slope of the terminal log-linear phase of the plasma concentration-time curve by linear regression. The peak-trough fluctuation index (FI) was calculated at steady state from (Cmax–Cmin)/Cave, where Cmin =minimum concentration at the end of the dosing interval and Cave =AUC (τ)/24. The accumulation index (AI) was determined by dividing AUC (τ) of day 7 to AUC (τ) of day 1.
Statistical methods
The pharmacokinetic parameters of AUC, Cmax, tmax, and t1/2 for all treatments within each study were compared using an analysis of variance (anova) method as appropriate for the study design. The model used in the anova tested for the effects of treatment, treatment sequence, subject-within-treatment sequence, and interaction between treatment and sequence for all crossover studies. Steady-state was assessed in the multiple-dose crossover study by comparing 24 h values following 5, 6, and 7 daily doses, respectively, at each dose level for each formulation using anova with terms for subject and day. The analysis was performed using the PROC GLM procedure in SAS version 6.06 (SAS Institute, Cary, NC). Statistical tests were done at a nominal significance level of 0.05. Least-square means of each parameter for each treatment were used in the statistical analysis. Demographic parameters and pharmacokinetic parameters are expressed as mean±s.d. and mean plasma concentration curves with standard error. For the bioequivalence analysis, the geometric mean and confidence limits for Cmax and AUC were taken to estimate the geometric mean ratio between treatment effects and the 90% confidence interval (CI) of the ratio.
Safety assessments
Standard haematologic and plasma biochemistry laboratory tests and urinalysis were performed and an electrocardiogram obtained at screening and at the end of each study. All reported or observed adverse experiences were recorded regardless of their severity or relationship to treatment. Sitting BP and pulse rates were measured at screening, at baseline and at prespecified time intervals appropriate for the individual studies. Where these times coincided with pharmacokinetic blood sampling, haemodynamic measurements were performed before blood sample collection. In the single-dose studies, sitting BP and pulse rates were measured predose and 1, 2, 4, 6, 12, and 24 h postdose. In the multiple-dose crossover study, these measures were obtained predose and 4 h postdose on the first day of each treatment phase; predose and 1, 2, 4, 6, 12, and 24 h postdose on days 14 and 21 of each treatment phase; and prior to discharge. In the age/gender study, sitting BP and pulse rates were measured predose each day of treatment; at 1, 2, 4, 6, 8, and 12 h postdose on days 1 and 7; and 24 h postdose on day 7.
Results
In vitro studies
The doxazosin GITS tablet performed consistently well in vitro in environments designed to simulate varying conditions of pH and motility encountered in the GI tract. The GITS tablet was fully operational and afforded similar release rates at pHs of 1.2 (artificial gastric fluid), 7.5 (artificial intestinal fluid), and in water. Likewise, the release profile of doxazosin GITS was not affected by external agitation at paddle speeds of 50, 75, and 100 rev min−1.
Clinical pharmacology studies
Single-dose/food-effect study
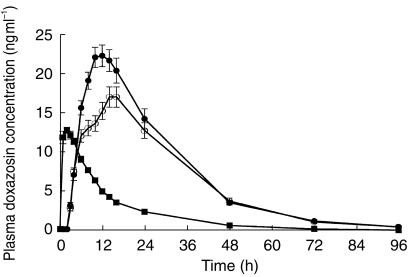
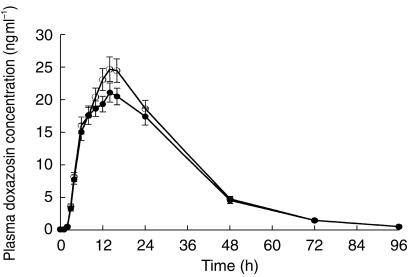
All 24 subjects (mean age, 27±6 years) enrolled in the study completed each treatment arm. The mean observed doxazosin plasma concentration-time profiles after administration of single doses of doxazosin GITS 8 mg under fed and fasting conditions and doxazosin standard 2 mg under fasting conditions are shown in Figure 2. Plasma levels of doxazosin increased more rapidly with the standard formulation than with GITS, achieving maximum levels 2–3 h after dosing with doxazosin standard compared with 14–16 h after dosing with doxazosin GITS. The GITS formulation also produced a 2 h absorption lag time and a more gradual decline in plasma levels over the course of 24 h postdose, but a terminal half-life comparable with that of doxazosin standard.
Figure 2.
Mean (±s.e.mean) plasma doxazosin concentrations following single oral doses of doxazosin standard 2 mg, fasted (▪); doxazosin GITS 8 mg, fasted (○); and doxazosin GITS 8 mg, fed (•); in 24 healthy volunteers.
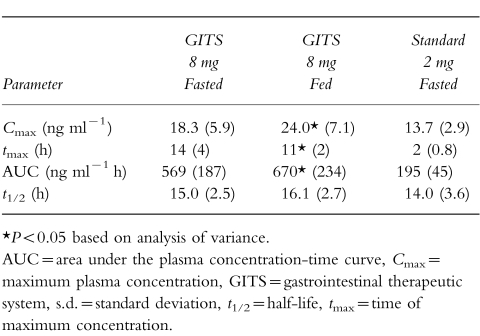
Mean pharmacokinetic data for each regimen are presented in Table 1. The Cmax achieved following a single 8 mg dose of doxazosin GITS was similar to that achieved with the 2 mg doxazosin standard dose (both under fasting conditions). The extent of bioavailability of a single doxazosin GITS tablet was approximately 75% of that of doxazosin standard.
Table 1.
Mean (±s.d.) pharmacokinetic parameters of doxazosin GITS 8 mg vs doxazosin standard 2 mg in the single-dose/food-effect study in 24 healthy males.
Analysis of pharmacokinetic parameters after treatment in the fed vs fasted state demonstrated that doxazosin GITS can be given with or without food, and without dose adjustment. Cmax and AUC were approximately 31% and 18% higher, respectively, after GITS administration under fed vs fasting conditions (P <0.05); however, these differences are not clinically significant. The tmax occurred earlier in the fed condition (11 h) compared with the fasted state (14 h), although this difference was not statistically significant. The Cmax following a single 2 mg doxazosin standard tablet under fasted state was similar to that after an 8 mg GITS tablet under fasted condition. The t1/2 was similar for each regimen.
Multiple-dose crossover study
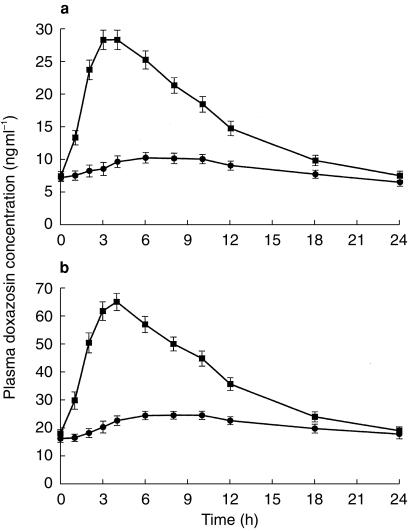
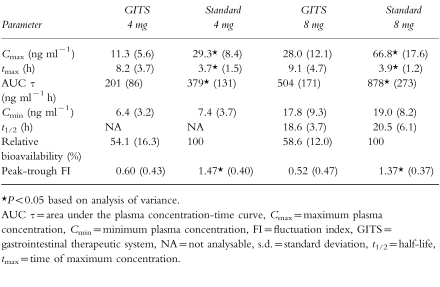
Of the 35 subjects enrolled (mean age, 32±9 years), 31 subjects completed the two treatment phases; four subjects discontinued during the study for reasons unrelated to the study drug. Steady-state was shown to have been attained by day 7 of dosing for each formulation at both the 4 mg and 8 mg doses. The mean plasma doxazosin concentration-time curves at steady state for both formulations are presented in Figure 3a and 3b for the 4 mg and 8 mg doses, respectively. The GITS formulation produced a gradual increase in doxazosin plasma concentrations, to a mean Cmax of 11.3 ng ml−1 with the 4 mg dose attained at 8 h and 28.0 ng ml−1 with the 8 mg dose attained at 9 h. Plasma concentrations increased more rapidly and to higher levels with the doxazosin standard formulation, as evidenced by a mean Cmax of 29.3 ng ml−1 attained at 4 h with 4 mg and 66.8 ng ml−1 attained at 4 h with the 8 mg dose. Plasma drug concentrations declined gradually with both formulations. A terminal t1/2 of 18.6 h was observed with doxazosin GITS and 20.5 h with the standard tablet.
Figure 3.
Mean (±s.e.mean) plasma concentration of doxazosin over 24 h at steady state with 4 mg (a) and 8 mg (b) doses of the GITS (•) and standard formulations (▪) in 31 healthy volunteers.
Mean pharmacokinetic parameters of doxazosin after multiple doses of 4 mg and 8 mg of the GITS and standard formulations are in Table 2. At each respective dose, the GITS formulation produced a lower doxazosin Cmax, a longer tmax and a lower AUC compared with doxazosin standard; while affording a comparable Cmin. Statistically significant differences between formulations were observed for mean Cmax, tmax, AUC (τ), and peak-trough FI, but there were no significant differences in Cmin and t1/2. The Cmax values were lower for doxazosin GITS, being 39% and 42% of those for the standard formulation at the 4 mg and 8 mg GITS doses, respectively. Likewise, the peak-trough FI was significantly lower after GITS (41% and 38% of the standard formulation for the 4 mg and 8 mg doses, respectively [P <0.05]), indicating that steady-state concentrations remain more uniform with the GITS formulation, independent of dose level. Within each formulation, the peak-trough FI values were independent of dose level. The relative bioavailability of doxazosin from the GITS formulation compared with the standard formulation was 54% at the 4 mg dose and 59% at the 8-mg dose. Comparison of data for the 8 mg dose and 4 mg dose indicated that both formulations approached dose proportionality.
Table 2.
Comparative mean (±s.d.) pharmacokinetic parameters of 4 mg and 8 mg doxazosin GITS and 4 mg and 8 mg doxazosin standard at steady state in a multiple-dose study in 31 healthy males.
Dose strength bioequivalence study
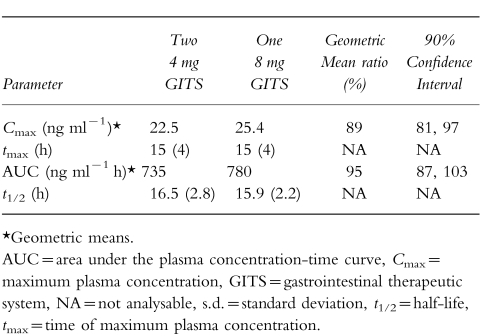
Of the 24 subjects enrolled (mean age 24±4 years), 23 completed the study; 1 subject withdrew his consent 3 days after dosing in the first study phase. Two 4 mg doxazosin GITS tablets were found to produce a mean plasma concentration-time curve (Figure 4) and pharmacokinetic profile (Table 3) similar to that of one 8 mg doxazosin GITS tablet. There were no statistically significant differences between treatments for any of the pharmacokinetic parameters analysed. Following oral administration of either two 4 mg doxazosin GITS or one 8 mg GITS tablet, drug absorption appeared to begin 2 h after dosing; plasma doxazosin concentrations gradually increased until mean maximum concentrations were achieved 15 h postdose.
Figure 4.
Mean (±s.e.mean) plasma concentrations of doxazosin following ingestion of two 4 mg GITS tablets (•) vs one 8 mg GITS tablet (○) in 23 healthy volunteers.
Table 3.
Mean (±s.d.) pharmacokinetic parameters of two 4 mg doxazosin GITS tablets compared with one 8 mg doxazosin GITS tablet in 21 healthy males.
The geometric mean ratios and 90% CI of the ratios for comparisons between the two 4 mg (test) and one 8 mg (reference) dose strengths were 88% (78%, 99%) for Cmax and 92% (80%, 107%) for AUC. There were two subjects who were considered to be pharmacokinetic outliers: one subject who received the two 4 mg tablets and the other who received one 8 mg tablet had AUC ratios of 2.4 and 0.21, respectively. The geometric mean ratios and 90% CI of the ratios excluding the two outliers were 89% (81%, 97%) for Cmax and 95% (87%, 103%) for AUC. These data indicate that the one 8 mg and two 4 mg dose strengths of doxazosin GITS tablets are bioequivalent.
Age and gender study
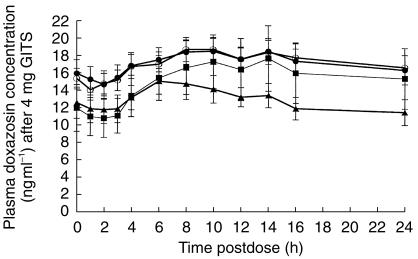
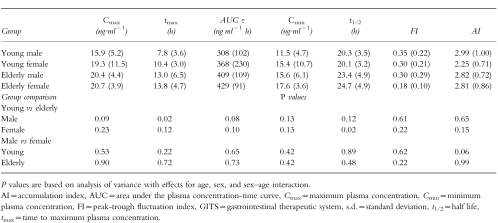
The 41 healthy subjects enrolling in the study included 11 young males (mean age 28±5 years), 10 young females (mean age 28±6 years), 10 elderly males (mean age 71±6 years), and 10 elderly females (mean age 68±3 years); 40 subjects completed the study and 1 young male withdrew due to an adverse event following the first dose. The steady-state plasma doxazosin concentration-time profiles from 0 to 24 h on day 7 (Figure 5) and up to 96 h after the last dose on day 7 were similar for both age and gender groups. Mean pharmacokinetic parameters on day 7 are presented in Table 4. At steady state, the fluctuation index of the GITS formulation was small, ranging from 0.18 for the elderly females to 0.35 for the young males. The degree of accumulation of doxazosin was similar for both age and gender groups and ranged from 2.25 for the young females to 2.99 for young males. The differences observed between the age and gender group comparisons in tmax and t1/2 values were within the intersubject variations; statistical analysis of the Cmax, AUC, Cmin, FI, and AI data showed that none of the age or gender differences was statistically significant.
Figure 5.
Mean (±s.e.mean) plasma concentrations of doxazosin from 0 to 24 h postdose at steady state (day 7) of doxazosin GITS 4 mg in 40 elderly and young male and female volunteers. (• elderly males, ○ elderly females, ▴ young males, ▪ young females).
Table 4.
Mean (±s.d.) pharmacokinetic parameters of doxazosin GITS according to age and gender at steady state (study day 7) during a multiple-dose regimen in 40 elderly or young healthy males and females.
Safety
A total of 123 subjects received doxazosin GITS and 56 subjects received doxazosin standard. The extent of exposure to treatment ranged from a minimum of a single day (one dose) to a maximum of 7 consecutive days per dose, with a maximum treatment period of 21 days. No serious adverse events occurred with doxazosin GITS or doxazosin standard during any of the clinical pharmacology studies. Those treatment-emergent adverse events observed were mild, resolved without intervention, and occurred at similar incidence with doxazosin GITS (56.9%) and doxazosin standard (57.1%), with a similar number of adverse events reported per subject. Only two subjects withdrew from treatment: one subject developed a viral illness after 2 weeks of treatment with doxazosin GITS 4 mg and one subject experienced an episode of dizziness and syncope approximately 17 min prior to the second dose of doxazosin GITS 4 mg. No subjects in any study had serious adverse experiences or clinically significant changes in BP, heart rate, or body weight. No clinically significant treatment-related changes were observed for standard clinical laboratory measures, vital signs, or electrocardiogram.
There was no obvious difference in the type and incidence of specific adverse events between the two treatments. There did not appear to be any difference in the type or overall incidence of adverse events reported between young and elderly age groups, or between males and females. Overall, doxazosin GITS was well tolerated in young or elderly male or female volunteers.
Discussion
The GITS tablet was designed to provide drug delivery rates not dependent on erosion, disintegration, pH, or motility of the GI tract; but, rather, on osmotic processes of the semipermeable membrane, which is permeable to water but not to ions. Thus, changes in these variables are unlikely to affect the release rate and pharmacokinetics of the GITS formulation. This has been demonstrated to be the case with doxazosin GITS, as evidenced by in vitro dosage form release/dissolution-rate data obtained under bioanalogous conditions and by pharmacokinetic studies in human volunteers.
The study in fed and fasted conditions with doxazosin GITS tablet shows minimal effects of food on the pharmacokinetics of doxazosin. Although the effect of food on a dosage form is expected to be formulation dependent, our findings are consistent with an earlier food-effect study with the doxazosin standard tablet [11].
As expected from the design of the GITS dosage form, the drug absorption profile of doxazosin GITS is more gradual and more extended than that of the doxazosin standard tablet, as illustrated by a significantly longer tmax with the GITS formulation, as well as a much lower Cmax (about a third of that observed with the standard formulation) at the same dosage. However, Cmin is not significantly affected by the new formulation, thus assuring the maintenance of adequate plasma levels over the dosing interval. This absorption profile allows the initial dose of doxazosin GITS to be higher (4 mg), eliminating at least two titration steps required with the doxazosin standard formulation. At the same time, the GITS formulation provides a relatively constant plasma doxazosin concentration-time profile at steady state, and considerably reduced peak-to-trough blood level fluctuations compared with doxazosin standard (by about 60%). Avoidance of excessive fluctuations in plasma drug concentrations with the GITS formulation of doxazosin is expected to reduce the incidence of side-effects associated with the relatively high peak plasma concentrations produced by the standard formulation. The relative bioavailability with the GITS formulation compared with the standard formulation is 54% at the 4 mg dose, and 59% at the 8 mg dose.
As is the case with doxazosin standard tablets, the dose strengths of two 4 mg doxazosin GITS tablets are bioequivalent to one 8 mg doxazosin GITS tablet.
Plasma concentration-time curves are similar for all age and gender groups, with drug absorption appearing to begin after approximately 2 h, increasing to reach near-maximum levels 8 h after the first dose. No statistically or clinically significant age-or gender-related differences in pharmacokinetics have been observed at steady state with doxazosin GITS; therefore, no dosage adjustments are warranted based on age or gender. Similar conclusions have been reported with doxazosin standard. A pooled analysis of well-controlled studies has demonstrated that doxazosin standard is equally well tolerated in young and elderly, hypertensive and normotensive patients with BPH [16].
It should be noted that in the clinical pharmacology studies, doxazosin GITS was sometimes initially administered to subjects at the higher single dose of 8 mg. This dose would not be recommended as the initial dose in clinical practice; the recommended initial dose of doxazosin GITS in clinical practice is 4 mg. All regimens of doxazosin standard began with the lower doses of 1 mg or 2 mg recommended in clinical practice.
In conclusion, the improved absorption profile of doxazosin GITS will likely minimize the risk for unintended adverse hypotensive effects; and the relatively constant plasma doxazosin concentration-time profile at steady state and considerably reduced peak-to-trough blood level fluctuations compared with doxazosin standard will likely result in more uniform control of BP and symptoms of BPH, and enhanced tolerability.
References
- 1.Fulton B, Wagstaff AJ, Sorkin EM. Doxazosin: an update of its clinical pharmacology and therapeutic applications in hypertension and benign prostatic hyperplasia. Drugs. 1995;49:295–320. doi: 10.2165/00003495-199549020-00011. [DOI] [PubMed] [Google Scholar]
- 2.Daae LN, Westlie L. A 5-year comparison of doxazosin and atenolol in patients with mild-to-moderate hypertension: effects on blood pressure, serum lipids, and coronary heart disease risk. Blood Press. 1998;7:39–45. doi: 10.1080/080370598437556. [DOI] [PubMed] [Google Scholar]
- 3.Lepor H, Kaplan SA, Klimberg I, et al. Doxazosin for benign prostatic hyperplasia: long-term efficacy and safety in hypertensive and normotensive patients. The Multicenter Study Group. J Urol. 1997;157:525–530. doi: 10.1016/s0022-5347(01)65193-0. [DOI] [PubMed] [Google Scholar]
- 4.Fawzy A, Vashi V, Chung M, Dias N, Gaffney M. Clinical correlation of maximal urinary flow rate and plasma doxazosin concentrations in the treatment of benign prostatic hyperplasia. Multicenter Study Group. Urology. 1999;53:329–335. doi: 10.1016/s0090-4295(98)00506-8. [DOI] [PubMed] [Google Scholar]
- 5.Elliott HL, Meredith PA, Sumner DJ, McLean K, Reid JL. A pharmacodynamic and pharmacokinetic assessment of a new α-adrenoreceptor antagonist, doxazosin (UK 33274) in normotensive subjects. Br J Clin Pharmacol. 1982;13:699–703. doi: 10.1111/j.1365-2125.1982.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent J, Elliott HL, Meredith PA, Reid JL. Doxazosin, an α1-adrenoceptor antagonist: pharmacokinetics and concentration-effect relationships in man. Br J Clin Pharmacol. 1983;15:719–725. doi: 10.1111/j.1365-2125.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott HL, Meredith PA, Vincent J, Reid JL. Clinical pharmacological studies with doxazosin. Br J Clin Pharmacol. 1986;21:27S–31S. doi: 10.1111/j.1365-2125.1986.tb02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent J, Meredith PA, Elliott HL, Reid JL. The pharmacokinetics of doxazosin in elderly normotensives. Br J Clin Pharmacol. 1986;21:521–524. doi: 10.1111/j.1365-2125.1986.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson RV, Bailey RR, Begg EJ, Cowlishaw MG, Sharman JR. Pharmacokinetics and effect on blood pressure of doxazosin in normal subjects and patients with renal failure. Clin Pharmacol Ther. 1986;40:561–566. doi: 10.1038/clpt.1986.224. [DOI] [PubMed] [Google Scholar]
- 10.Vashi V, Penenberg D, Chung M, Walmsley P. Effect of hepatic impairment on the pharmacokinetics of doxazosin. Abstract. Clin Pharmacol Ther. 1997;61:159. doi: 10.1177/00912700022008711. [DOI] [PubMed] [Google Scholar]
- 11.Conway EL, McNeil JJ, Hurley J, et al. The effects of food on the oral bioavailability of doxazosin in hypertensive subjects. Drug Invest. 1993;6:90–95. [Google Scholar]
- 12.Vashi V, Chung M, Dias N, Phillips K. Effect of time of administration on the pharmacokinetics and tolerance of doxazosin in healthy male volunteers. J Clin Pharmacol. 1996;36:325–331. doi: 10.1002/j.1552-4604.1996.tb04208.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirby RS. Morning versus evening dosing with doxazosin in benign prostatic hyperplasia: pharmacokinetics, efficacy and safety. Int J Clin Pract. 1998;52:75–77. [PubMed] [Google Scholar]
- 14.Chung M, Reitberg DP, Gaffney M, Singleton W. Clinical pharmacokinetics of nifedipine gastrointestinal therapeutic system. Am J Med. 1987;83(Suppl 6B):10–14. doi: 10.1016/0002-9343(87)90630-9. [DOI] [PubMed] [Google Scholar]
- 15.Rubin PC, Brunton J, Meredith P. Determination of the vasodilator UK33274 by high-performance liquid chromatography using fluorescence detection. J Chromatogr. 1980;221:193–195. doi: 10.1016/s0378-4347(00)81025-7. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan SA, D’Alisera PM. Tolerability of alpha-blockade with doxazosin as a therapeutic option for symptomatic benign prostatic hyperplasia in the elderly patient: a pooled analysis of seven double-blind, placebo-controlled studies. J Gerontol Biol Sci Med Sci. 1998;53:M201–M206. doi: 10.1093/gerona/53a.3.m201. [DOI] [PubMed] [Google Scholar]