Abstract
Aims
The role of magnesium (Mg) intake in the prevention and treatment of diseases is greatly debated. Mg biodistribution after chronic Mg supplementation was investigated, using state-of-the-art technology to detect changes in free ionized Mg, both at extra- and intracellular levels.
Methods
Thirty young healthy male volunteers participated in a randomised, placebo (P)-controlled, double-blind trial. The treated group (MgS) took 12 mmol magnesium lactate daily for 1 month. Subjects underwent in vivo31P-NMR spectroscopy and complete clinical and biological examinations, on the first and last day of the trial. Total Mg was measured in plasma, red blood cells and 24 h urine ([Mg]U). Plasma ionized Mg was measured by ion-selective electrodes. Intracellular free Mg concentrations of skeletal muscle and brain tissues were determined noninvasively by in vivo31P-NMR at 3T. NMR data were automatically processed with the dedicated software MAGAN.
Results
Only [Mg]U changed significantly after treatment (in mmol/24 h, for P, from 4.2±1.4 before to 4.1±1.3 after and, for MgS, from 3.9±1.1 before to 5.1±1.1 after, t =2.15, P =0.04). The two groups did not differ, either before or after the trial, in any other parameter, whether clinical, biological or in relation with the Mg status.
Conclusions
Chronic oral administration of Mg tablets to young healthy male volunteers at usual pharmaceutical doses does not alter Mg biodistribution. This study shows that an adequate and very complete noninvasive methodology is now available and compatible with the organization of clinical protocols which aim at a thorough evaluation of Mg biodistribution.
Keywords: brain, dietary supplements, erythrocytes, ion-selective electrode, magnesium, nuclear magnetic resonance, plasma, skeletal muscle
Introduction
Only a fraction of the Western population has a Mg intake that satisfies the recommended dietary allowance (RDA) of Mg, which is 0.19 mmol kg−1 day−1 [1]. Whether or not increasing magnesium intake might have a role in the prevention and treatment of diseases is a matter of debate.
Changes in Mg stores in response to chronic Mg supplementation have been particularly difficult to demonstrate. In normal subjects and even in patients with a supposed Mg deficit, very conflicting results have been reported on the effect of a Mg supplementation on Mg concentrations in serum [2], blood cells [3–5] or muscle [6].
A crucial impediment has been that, in most studies, total plasma and/or erythrocyte Mg concentration were taken as the only or main evaluation criterion [2, 3, 5]. Yet, blood Mg only represents approximately 1% of Mg body-content and it is now well established that correlations between Mg contents of various tissues are at the best weak, and most often, nonexistent.
Thus, blood mononuclear cell Mg was found not to correlate with serum or red blood cell Mg [7], whilst a significant correlation was noted with concentrations of Mg in muscle biopsies of normal subjects [8]. Total Mg content of lymphocytes was related to total skeletal muscle Mg concentration only in some subclasses of subjects [9]. In normal human subjects, a weak inverse correlation was recently reported between serum total Mg concentration and skeletal muscle ionized intracellular Mg determined by 31P-NMR [10]. However, no such correlation was detected in earlier and similar works on smaller population samples [11, 12]. Plasma and erythrocyte Mg were reported to change independently during Mg oral supplementation [2, 3].
In pathology, and particularly in the heart, studies of Mg status, have shown little or no correlation between blood Mg, and Mg content of tissues [13–16]. This shows how unsound it may be to draw conclusions concerning Mg stores, from blood data only.
Mg in tissues and body fluids is present in different forms (bound to proteins, bound to small organic molecules and free ionized). This must also be given some consideration, as one of these forms might be specifically altered by certain conditions or diseases, whilst total Mg is not. For instance, both serum ionized Mg and erythrocyte Mg were significantly reduced in type II diabetic patients compared with nondiabetic controls whereas total serum Mg did not differ between the two groups [17]. Ionized but not total serum Mg appeared to be decreased early after stroke [18] and after head injuries [19]. Since ionized Mg is the physiologically active fraction, it is inappropriate to make inferences on the biological properties of Mg solely from its total concentration.
As a consequence, any comprehensive analysis of the Mg status of individuals or population subclasses calls for a multisystemic investigation. Ideally, one ought to determine for each organ of interest, total Mg stores as well as Mg repartition into its various forms, at both intra- and extracellular levels. Obviously, particular attention should be given to the free ionized fraction.
In the present study, Mg biodistribution after chronic Mg supplementation has been revisited. The quantity of Mg given in the treatment was chosen to represent approximately the RDA of a 70 kg adult. We have taken advantage of recent advances in technology to tackle the issue of changes in free ionized Mg, in blood and also intracellularly and noninvasively, in selected organs. Skeletal muscle was chosen because it represents 40% of body mass and it constitutes, second to bones, the largest Mg store in the body. Brain Mg was also investigated. Indeed, many of the functional symptoms that Mg has been claimed to improve or suppress, such as chronic fatigue syndrome, anxiety, insomnia, post-menstrual syndrome [20, 21], are disorders with largely predominant neurological components. We chose to administer Mg in a similar way to that when it is prescribed to treat these functional symptoms and to determine whether it could alter neuronal free intracellular Mg content.
Plasma or serum ionized Mg can conveniently be assayed using ion selective electrodes. Unfortunately, this technique cannot be used to measure intracellular free ionized Mg noninvasively in tissues and organs. Fluorescent dyes, which are common and practical tools for isolated and cultured cells, exhibit major toxicity problems, which preclude their use in vivo. The only currently available method that allows noninvasive determination of free intracellular Mg in living tissues and organs is phosphorus nuclear magnetic resonance spectroscopy (31P-NMR). This has not been routinely exploited, presumably because of high examination costs and the low availability of wide-bore high-field NMR spectrometers.
This study was designed to investigate specifically the effects of Mg administered in pharmaceutical tablets to young adult volunteers at the dose and for the duration usually recommended in medical practice. The consequences on Mg biodistribution were evaluated by tracking changes in total plasma, erythrocyte and urine content, in free ionized serum concentration and in free ionized intracellular concentrations of brain and skeletal muscle.
Methods
Study population
The study included 30 healthy young male volunteers, who had given informed written consent. The volunteers were in good health, were within 10% of ideal body weight and were free from individual history of diabetes, dyslipidaemia, spasmophilia, hypertension and cardiovascular events. Clinical and biological examinations were performed before inclusion into the protocol. The protocol was conducted in accordance with the Declaration of Helsinki and French regulations governing biomedical research and was approved by the Pitié-Salpêtrière ethics committee (C.C.P.P.R.B.)
Magnesium supplementation
The protocol was carried out as a double-blind trial, during which volunteers were given 3 tablets, twice daily, of either magnesium supplement (n =15, group MgS), or placebo (n =15, group P), for a period of 28–35 days. Magnesium tablets contained 470 mg of magnesium lactate (48 mg magnesium), amounting to a 12 mmol daily intake of Mg, and 5 mg pyridoxine. This corresponds precisely to a scheme proposed by pharmaceutical industry in France for oral Mg supplementation. Placebo and treatment tablets had strictly identical external appearance. Throughout the treatment, volunteers phoned an answering machine mornings and evenings once they had taken their tablets and messages were recorded and controlled. Observance of the treatment was further verified by counting residual tablets which volunteers returned on the last day of the trial. Subjects took no other medication during this period, and agreed to restrict their consumption of cigarettes (<5 per day) and alcohol (<600 kcal per day). Investigators were only informed of magnesium or placebo tablet content upon completion of the study.
Magnesium status evaluation
Total magnesium concentrations were determined by standard laboratory colourimetric assay using the methylthymol blue method, in plasma ([Mg]P), lysed erythrocytes ([Mg]RBC) and 24-h urine ([Mg]U). Ionized magnesium ([Mg2+]P) was measured in plasma by ion selective electrodes on a NOVA Mercury 8 blood analysis automat (Stat Profile Ultra, Nova, Waltham). Calibration of the automat was performed weekly. Intracellular free magnesium concentrations of skeletal muscle ([Mg2+]Musc), and brain tissues ([Mg2+]Br), were determined by in vivo31P-NMR spectroscopy. These measurements were performed, along with complete clinical and biological examinations on the first and last days of the trial, following an overnight fast.
NMR spectroscopy
Spectra were acquired using a 3 Tesla, 72-cm free bore whole-body magnet (Oxford Instruments, Oxford, UK) interfaced to a Bruker Medspec 30/100 console (Karlsruhe, Germany). Subjects lay supine in the magnet for both brain and muscle measurements. For the calf muscle, a double tuned 1H-31P surface coil (φ=5 cm) was positioned facing the gastrocnemius. It was used both for optimization of field homogeneity (shimming) (1H water line width at half height less than 30Hz) and collection of 31P data (accumulation of 900 scans with an interpulse delay of 1 s, collected over a 4 kHz bandwidth, with 2 k complex data points). For brain measurements, the head of the subject was centred in a 1H pseudo-Helmholtz coil (φ=20 cm). This coil was used to acquire the water signal to correct for magnet inhomogeneity by localized shimming of a 12×12×12 cm3 volume at the back of the head (stimulated echo sequence, TE=35 ms, TM=25 ms, water line width at half height less than 10Hz). The 31P signal was acquired using an ellipse-shaped surface coil (6×8 cm) facing the occipital lobe, by accumulating 440 scans with an interpulse delay of 2 s, over a 4 kHz bandwidth, with 2 k complex data points. A flexible mat containing a circuit placed between the coil and the head, was used to deliver surface spoiling gradients for 350 μs prior to acquisition in order to eliminate signal originating from scalp and bone [22].
NMR examinations lasted altogether approximately 25 min for calf and 40 min for head. The subjects were taken out of the magnet and allowed to stretch between both sessions. Shimming was performed manually (so as to obtain the best possible resolution for 31P data) and lasted up to 10 min. This is critical for the precision of measurement of free intracellular magnesium concentrations, because of the low responsiveness of differential δ ATP to Mg concentration changes in the range of usual biological values.
31P-NMR data processing
This low responsiveness is certainly one of the more problematic limitations of the technique. In order to remove variability, and hence uncertainty, introduced by observer intervention, a robust automatic processing of the 31P-NMR signal is highly desirable. An original method has recently been proposed. This is fully automated and dedicated to the determination of free intracellular Mg from 31P-NMR data. The software MAGAN (CRIS, Belgium) which runs on a SUN workstation, was used for processing the data of this study. The novelty is the use of a Wigner distribution to analyse the NMR signals. In this time-frequency domain representation of signals, cross-terms originate between resonances which oscillate at a frequency equal to the difference in chemical shift between resonances [23]. This is advantageous in determining , the observed difference between chemical shifts of the ATP α- and β-phosphate groups.
MAGAN first proceeds by an overall identification of resonances in the 31P signal by noniterative analysis in the time domain (TLS or HSVD). Then, is determined precisely by analysis of the cross-terms between the α and β ATP resonances generated by the discrete pseudo-Wigner distribution. The concentration of free intracellular Mg [Mg2+] is obtained by solving the following quadratic equation, which takes into account significant exchange processes [24]:
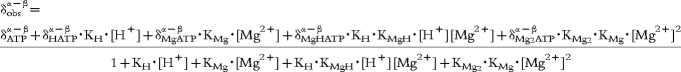 |
[1] |
In this equation, δX represents chemical shift of pure compound X, KX is the formation constant of XATP complexes and the proton concentration [H+] is calculated from the measured difference between δ of creatine and inorganic phosphate (), and KH the formation constant of H2PO4:
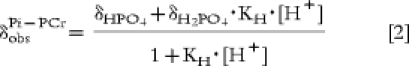 |
[2] |
This totally automated approach has been compared in terms of accuracy, reproducibility and sensitivity to variations of , to manual 31P spectroscopy data analysis by experimented observers (peak-picking of Fourier-transformed spectra after exponential filtering to a line broadening of 1Hz) [25]. Unlike other more classical automated processing schemes, either in the time or frequency domains, which were previously experimented, the results obtained by MAGAN were equivalent to those obtained from manual processing, in all three respects.
Preliminary experiments for reproducibility
To address the reproducibility of the methodology and its ability to detect small changes in intracellular Mg concentration, preliminary experiments were conducted. Muscle free ionized Mg content was determined on two separate occasions in the right median gastrocnemius of healthy volunteers. Inter-study variability was found to be remarkably low, 1.3% for the ATP, resulting in an interstudy variability for calculated Mg of only 4.1%, in the usual ranges of concentrations and pHs [26]. For the number of subjects enrolled in the present study, this means that 31P-NMR should be able to pick up changes in free intracellular Mg of the order of 3.5%, i.e. less than 20 μm. The sensitivity of the automated procedure MAGAN towards small variations of ATP was also assessed. The ATP was modified acutely by pH variations during brief ischaemic episodes. In this test, MAGAN performed slightly better than manual spectrum analysis [25].
The variability for colourimetric assays of total Mg were 4% for repeated measurements on a given sample, and, respectively, 7, 5 and 30% for different samples of plasma, lysed erythrocytes and 24 h urine from a same subject.
It is worth noting that intracellular free Mg levels determined by NMR in vivo are very similar to Mg concentration assayed by microfluorimetric dye in isolated cells [27].
Statistical analysis
Data were compared before and after treatment and between P and MgS using bilateral Student’s t-tests. The level of significance was taken as 5%.
Results
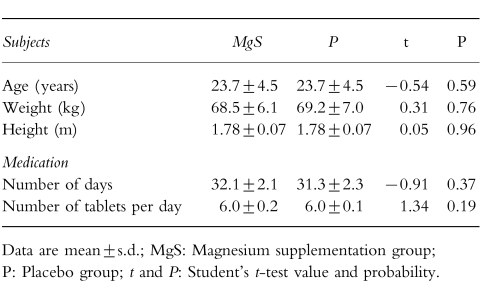
After inclusion into the protocol, the volunteers’ compliance was excellent. Two volunteers had to be excluded from the study because they had stopped treatment more than 12 h before the second NMR session. They were replaced by other recruits who were given reserve treatment tablets with identical codes and content. No incident which could be related to either medication or to NMR occurred during or following the protocol. Duration and quantity of medication were identical for both P and MgS groups (Table 1). Mean subject characteristics (age, weight and height) are also recorded in Table 1 and were not different between P and MgS subjects.
Table 1.
Subject characteristics and treatment observance.
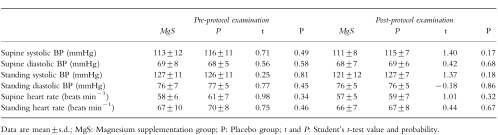
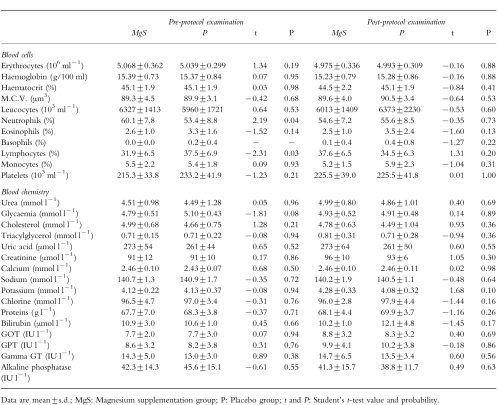
In Table 2, the means of blood-pressure and heart rates are given for the two groups, before and after treatment. They were indistinguishable both before and after the protocol. The complete mean biological analyses for MgS and P groups measured before and after the trial are tabulated (Table 3). None of the parameters differentiate the two groups.
Table 2.
Blood pressure and heart rate data.
Table 3.
Clinical biology data.
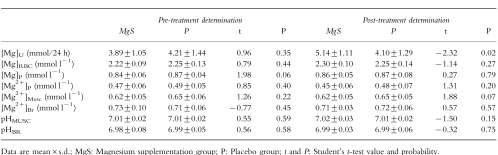
Two aspects were considered in evaluating evolution of Mg status. First, P and MgS groups were compared, as for other biometrical and clinical data, by testing all parameters for differences between the two groups, before and after supplementation. The quantity of Mg determined according to the various modalities as well as intracellular pH in brain (pHBR) and muscle (pHMUSC) measured by 31P-NMR are reported in Table 4 for both groups, before and after medication. At the beginning of the protocol, pHBR, pHMUSC and all of the six parameters used to assess Mg status were equivalent in the two groups. After 1 month of supplementation, the group receiving Mg had a higher excretion of Mg in urine [Mg]U, than the group given placebo (5.14±1.11 and 4.10±1.29 mmol/24 h, respectively, t =−2.32, P =0.02). However, none of the other Mg values ([Mg]P, [Mg]RBC, [Mg2+]P, [Mg2+]Musc and [Mg2+]Br) differed between the group after Mg supplementation compared with the group taking placebo.
Table 4.
Magnesium biodistribution.
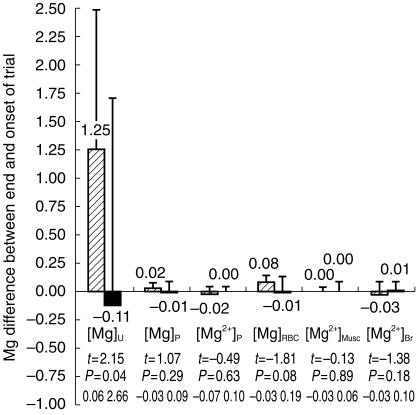
Secondly, the data were analysed by testing the evolution of Mg status parameters, within each group, before and after the trial. Similarly, only [Mg]U, showed any modification for the group receiving a supplement, with an approximate increase of 30% (3.89±1.05 and 5.14±1.11 mmol/24 h before and after MgS, respectively, t =2.15, P =0.04). This is illustrated in Figure 1 which shows variation of Mg concentrations following the trial. Again, all other values used to characterize Mg biodistribution remained unaffected by the treatment. Likewise, intracellular pH of brain and muscle, measured by NMR were also stable.
Figure 1.
Difference measured between end and onset of trial of: total Mg in plasma [Mg]P, lysed erythrocytes [Mg]RBC and 24-h urine [Mg]U, ionized magnesium [Mg2+]P in plasma and intracellular free magnesium in skeletal muscle [Mg2+]Musc and brain [Mg2+]Br. All values are expressed in mmol l−1, except [Mg]U which is in mmol/24 h, and are given as the mean for the groups receiving magnesium supplement (MgS) (hatched bars) or placebo (P) (closed bars). Error bars are s.d.. Statistical significance is given for each measurement by Student’s t-test value, probability P and 95% confidence interval for the difference between placebo and Mg supplemented volunteers.
Thus, although the increase in [Mg]Uis a clear indicator of Mg intake in the MgS group, there was no measurable repercussion of changes in Mg concentration either by routine clinical measurement of total Mg in blood and red blood cells, or by ion selective electrodes in plasma. Finally no variation of the free intracellular ion concentration could be found within brain or peripheral muscle cells using noninvasive 31P-NMR spectroscopy.
Discussion
Chronic Mg administration to normal subjects and to patients without overt Mg depletion has provided conflicting results. For compartments so far accessible to clinical investigation, evidence suggesting that Mg biodistribution can be altered to a biologically significant extent, is scarce (see introduction). A major contribution of the present study was to extend that conclusion to other essentially unexplored biological pools, namely ionized blood and intracellular brain and skeletal muscle Mg. The study is strengthened by the number and diversity of Mg parameters that were confronted.
Regarding muscle, similar results to those obtained in the present work, have been very recently reported for a Mg supplementation to athletes who had a low initial concentration of Mg in serum [28]. Indeed, Mg is often given to sportsmen in the hope of improving their muscular performance and recovery from exercise. In a placebo controlled double-blind trial, the authors found no increase in serum, blood cell or skeletal muscle concentrations of Mg, after 3 weeks of Mg oxide supplementation, whereas Mg renal excretion was increased in subjects receiving medication. Nor did they find any difference in neuromuscular excitability, exercise performance or muscle related symptoms (such as muscular cramps and weakness) between the group receiving Mg and that receiving placebo. These results, although there is no measure of Mg in brain, nor of ionized Mg in plasma and serum, comfort our own findings on supplementation in healthy volunteers.
The conclusion of an absence of response to Mg supplementation observed in the present protocol on a well-characterized subclass, young male Caucasian adults, of normal healthy subjects, cannot be generalized to the whole population. In particular, it cannot be taken to indicate similar results for patients with observed Mg depletion. The many biological effects observed after chronic Mg supplementation, mainly to patients cannot be ignored. Mg supplementation (15.8 mmol Mg per day for a month) was found to improve glycaemic control in noninsulin-dependent diabetic patients [29], in elderly hypertensive subjects [30] and in thiazide-treated hypertensive patients [19, 31].
Mg also seems to possess hypolipaemic properties. Supplementation of Mg produced a significant reduction of plasma cholesterol and LDL-cholesterol and an increase in HDL-cholesterol of type II diabetic patients [32]. Similar findings were reported in healthy Japanese subjects, with concurrent increases in LCAT activity and apolipoprotein A1. In types IV and IIb hyperlipidaemic patients, Mg supplementation induced a significant decrease in plasma triglycerides, but not in cholesterol [33].
Increasing Mg intake chronically often has a modest effect on the blood pressure of hypertensive patients, with a decrease of a few mmHg in diastolic and systolic arterial pressures [34–37]. Chronic treatment with Mg and potassium has also been shown to decrease cardiac events and total mortality by approximately 50% in patients with suspected acute myocardial infarction [38].
Beneficial effects of Mg supplementation compared with placebo have also been published in a variety of other diseases such as asthma [39], chronic alcoholism [40] and attention deficit hyperactivity disorder of children [41]. Finally, in a large cohort of 400 hundred individuals having taken either a Mg-rich diet or a regular diet for 10 years, mortality and morbidity were reduced by 50% in the high-Mg group as compared with the controls [42]. Although in these clinical observations, trial design was sometimes suboptimal.
It is well established that Mg has calcium channel blocking properties and stimulates the Na/K pump. However, the exact mechanisms of the above effects are still essentially unexplained. One can nonetheless assume that they ought to be mediated through modifications in Mg biodistribution, and mainly ionized intra-and extracellular Mg. These remain undocumented, to a very large extent.
This work shows that Mg supplementation in Mg- adequate subjects fails to alter the various Mg pools (transient changes were not tracked and cannot be ruled out). Perhaps more importantly, it demonstrates that an adequate and very complete non invasive methodology is now available and compatible with the organization of clinical protocols. This should make it possible to investigate the effects, if they exist, of therapeutic intervention on Mg biodistribution in patients with Mg deficits. It avoids the use of invasive and destructive methods such as muscle biopsies [43].
References
- 1.Elin RJ. Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol. 1994;102:616–622. doi: 10.1093/ajcp/102.5.616. [DOI] [PubMed] [Google Scholar]
- 2.Becchi MA, Borella P, Michelini N. Effects of oral magnesium supplementation on plasma and erythrocytes levels of the cation during maximal effort. Med Dello Sport. 1990;43:231–239. [Google Scholar]
- 3.Borella P, Ambrosini G, Concari M, Bargellini A. Is magnesium content in erythrocytes suitable for evaluating cation retention after oral physiological supplementation in marginally magnesium-deficient subjects? Magnes Res. 1993;6:149–153. [PubMed] [Google Scholar]
- 4.Desbiens NA, Marx JJJ, Haas RG, Reinhart RA. Can the magnesium content of mononuclear blood cells be altered by oral magnesium supplementation? Clin Biochem. 1992;25:289–292. doi: 10.1016/0009-9120(92)80035-f. [DOI] [PubMed] [Google Scholar]
- 5.Howard JM, Davies S, Hunnisett A. Red cell magnesium and glutathione peroxidase in infertile women—effects of oral supplementation with magnesium and selenium. Magnes Res. 1994;7:49–57. [PubMed] [Google Scholar]
- 6.Terblanche S, Noakes TD, Dennis SC, Marais D, Eckert M. Failure of magnesium supplementation to influence marathon running performance or recovery in magnesium-replete subjects. Int J Sport Nutr. 1992;2:154–164. doi: 10.1123/ijsn.2.2.154. [DOI] [PubMed] [Google Scholar]
- 7.Elin RJ. Status of the determination of magnesium in mononuclear blood cells in humans. Magnesium. 1988;7:300–305. [PubMed] [Google Scholar]
- 8.Sjogren A, Floren CH, Nilsson A. Magnesium and potassium status in healthy subjects as assessed by analysis of magnesium and potassium in skeletal muscle biopsies and magnesium in mononuclear cells. Magnesium. 1987;6:91–99. [PubMed] [Google Scholar]
- 9.Dyckner T, Wester PO. Skeletal muscle magnesium and potassium determinations: correlation with lymphocyte contents of magnesium and potassium. J Am Coll Nutr. 1985;4:619–625. doi: 10.1080/07315724.1985.10720104. [DOI] [PubMed] [Google Scholar]
- 10.Ryschon TW, Rosenstein DL, Rubinow DR, Niemela JE, Elin RJ, Balaban RS. Relationship between skeletal muscle intracellular ionized magnesium and measurements of blood magnesium. J Lab Clin Med. 1996;127:207–213. doi: 10.1016/s0022-2143(96)90080-3. [DOI] [PubMed] [Google Scholar]
- 11.Carlier PG, Wary C, Jehenson P, Bloch G. Magnesium content in plasma and erythrocytes is uncorrelated with brain and muscle free intracellular concentrations as determined in vivo by 31P-NMR spectroscopy. In: Halpern MJ, Durlach J, editors. Current Research in Magnesium. London: John Libbey; 1996. pp. 25–26. [Google Scholar]
- 12.Rosenstein DL, Ryschon TW, Niemela JE, Elin RJ, Balaban RS, Rubinow DR. Skeletal muscle intracellular ionized magnesium measured by 31P-NMR spectroscopy across the menstrual cycle. J Am Coll Nutr. 1995;14:486–490. doi: 10.1080/07315724.1995.10718540. [DOI] [PubMed] [Google Scholar]
- 13.Moller JB, Klaaborg KE, Alstrup P, Arendrup H, Klitgard NA, Pedersen KE. Magnesium content of the human heart. Scand J Thorac Cardiovasc Surg. 1991;25:155–158. doi: 10.3109/14017439109098102. [DOI] [PubMed] [Google Scholar]
- 14.Ralston MA, Murnane MR, Kelley RE, Altschuld RA, Unverferth DV, Leier CV. Magnesium content of serum, circulating mononuclear cells, skeletal muscle, and myocardium in congestive heart failure. Circulation. 1989;80:573–580. doi: 10.1161/01.cir.80.3.573. [DOI] [PubMed] [Google Scholar]
- 15.Reinhart RA, Marx JJJ, Broste SK, Haas RG. Myocardial magnesium: relation to laboratory and clinical variables in patients undergoing cardiac surgery. J Am Coll Cardiol. 1991;17:651–656. doi: 10.1016/s0735-1097(10)80179-2. [DOI] [PubMed] [Google Scholar]
- 16.Camara EJ, Cruz TR, Nassri JM, Rodrigues LE. Muscle magnesium content and cardiac arrhythmias during treatment of congestive heart failure due to chronic chagasic cardiomyopathy. Braz J Med Biol Res. 1986;19:49–58. [PubMed] [Google Scholar]
- 17.Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non- insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:767–770. doi: 10.1007/BF00401149. [DOI] [PubMed] [Google Scholar]
- 18.Altura BT, Memon ZI, Zhang A, et al. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular muscle cells. Neurosci Lett. 1997;230:37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- 19.Memon ZI, Altura BT, Benjamin JL, Cracco RQ, Altura BM. Predictive value of serum ionized but not total magnesium levels in head injuries. Scand J Clin Lab Invest. 1995;55:671–677. doi: 10.3109/00365519509075397. [DOI] [PubMed] [Google Scholar]
- 20.Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298–301. doi: 10.1111/j.1526-4610.1991.hed3105298.x. [DOI] [PubMed] [Google Scholar]
- 21.Durlach J, Bac P, Durlach V, Bara M, Guiet-Bara A. Neurotic, neuromuscular and autonomic nervous form of magnesium imbalance. Magnes Res. 1997;10:169–195. [PubMed] [Google Scholar]
- 22.Chen W, Ackerman JJ. Localized 13C-[1H] NMR. of rat liver in vivo using surface-spoiling gradients. NMR Biomed. 1989;2:267–273. doi: 10.1002/nbm.1940020515. [DOI] [PubMed] [Google Scholar]
- 23.Leclerc JH. The pseudo-Wigner distribution of double and triple resonances. J Magn Reson. 1992;100:171–182. [Google Scholar]
- 24.Halvorson HR, Vande Linde AM, Helpern JA, Welch KM. Assessment of magnesium concentrations by 31P NMR in vivo. NMR Biomed. 1992;5:53–58. doi: 10.1002/nbm.1940050202. [DOI] [PubMed] [Google Scholar]
- 25.Leclerc JHJ, Wary C, Carlier PG. Determination of intracellular magnesium concentration by P-31 nuclear magnetic resonance spectroscopy-a new quantification technique based on the Wigner distribution. In: Smetana R, editor. Advances in Magnesium Research. 1. Magnesium in Cardiology. London: John Libbey; 1997. pp. 577–585. [Google Scholar]
- 26.Carlier PG, Bidault N, Wary C, Jehenson P, Leroy-Willig A, Bloch G. Workshop on Magnetic Resonance Imaging and Spectroscopy of Muscle. Liverpool: 1994. A reproducibility study of magnesium free intracellular concentration in human skeletal muscle by P31 NMR spectroscopy; p. P25. [Google Scholar]
- 27.Li HY, Quamme GA. Caffeine decreases intracellular free Mg2+ in isolated adult rat ventricular myocytes. Biochim Biophys Acta. 1997;1355:61–68. doi: 10.1016/s0167-4889(96)00117-6. [DOI] [PubMed] [Google Scholar]
- 28.Weller E, Bachert P, Meinck HM, Friedmann B, Bartsch P, Mairbaurl H. Lack of effect of oral Mg-supplementation on Mg in serum, blood cells, and calf muscle. Med Sci Sports Exerc. 1998;30:1584–1591. doi: 10.1097/00005768-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab. 1994;78:1510–1514. doi: 10.1210/jcem.78.6.8200955. [DOI] [PubMed] [Google Scholar]
- 30.Paolisso G, Di Maro G, Cozzolino D, et al. Chronic magnesium administration enhances oxidative glucose metabolism in thiazide treated hypertensive patients. Am J Hypertens. 1992;5:681–686. doi: 10.1093/ajh/5.10.681. [DOI] [PubMed] [Google Scholar]
- 31.Paolisso G, Sgambato S, Gambardella A, et al. Daily magnesium supplements improve glucose handling in elderly subjects. Am J Clin Nutr. 1992;55:1161–1167. doi: 10.1093/ajcn/55.6.1161. [DOI] [PubMed] [Google Scholar]
- 32.Corica F, Allegra A, Di Benedetto A, et al. Effects of oral magnesium supplementation on plasma lipid concentrations in patients with non-insulin-dependent diabetes mellitus. Magnes Res. 1994;7:43–47. [PubMed] [Google Scholar]
- 33.Kisters K, Spieker C, Tepel M, Zidek W. New data about the effects of oral physiological magnesium supplementation on several cardiovascular risk factors (lipids and blood pressure) Magnes Res. 1993;6:355–360. [PubMed] [Google Scholar]
- 34.Dyckner T, Wester PO. Effect of magnesium on blood pressure. Br Med J (Clin Res. eds) 1983;286:1847–1849. doi: 10.1136/bmj.286.6381.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motoyama T, Sano H, Fukuzaki H. Oral magnesium supplementation in patients with essential hypertension. Hypertension. 1989;13:227–232. doi: 10.1161/01.hyp.13.3.227. [DOI] [PubMed] [Google Scholar]
- 36.Widman L, Wester PO, Stegmayr BK, Wirell M. The dose-dependent reduction in blood pressure through administration of magnesium. A double blind placebo controlled cross-over study. Am J Hypertens. 1993;6:41–45. doi: 10.1093/ajh/6.1.41. [DOI] [PubMed] [Google Scholar]
- 37.Witteman JC, Grobbee DE, Derkx FH, Bouillon R, de Bruijn AM, Hofman A. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr. 1994;60:129–135. doi: 10.1093/ajcn/60.1.129. [DOI] [PubMed] [Google Scholar]
- 38.Singh RB, Singh NK, Niaz MA, Sharma JP. Effect of treatment with magnesium and potassium on mortality and reinfarction rate of patients with suspected acute myocardial infarction. Int J Clin Pharmacol Ther. 1996;34:219–225. [PubMed] [Google Scholar]
- 39.Hill J, Micklewright A, Lewis S, Britton J. Investigation of the effect of short-term change in dietary magnesium intake in asthma. Eur Respir J. 1997;10:2225–2229. doi: 10.1183/09031936.97.10102225. [DOI] [PubMed] [Google Scholar]
- 40.Gullestad L, Dolva LO, Soyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16:986–990. doi: 10.1111/j.1530-0277.1992.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 41.Starobrat-Hermelin B, Kozielec T. The effects of magnesium physiological supplementation on hyperactivity in children with attention deficit hyperactivity disorder (ADHD). Positive response to magnesium oral loading test. Magnes Res. 1997;10:149–156. [PubMed] [Google Scholar]
- 42.Singh RB. Effect of dietary magnesium supplementation in the prevention of coronary heart disease and sudden cardiac death. Magnes Trace Elem. 1990;9:143–151. [PubMed] [Google Scholar]
- 43.Dorup I, Skajaa K, Thybo NK. Oral magnesium supplementation restores the concentrations of magnesium, potassium and sodium-potassium pumps in skeletal muscle of patients receiving diuretic treatment. J Intern Med. 1993;233:117–123. doi: 10.1111/j.1365-2796.1993.tb00663.x. [DOI] [PubMed] [Google Scholar]