Abstract
Aims
To define the pharmacokinetic profile of efavirenz (EFV) in HIV-1 infected patients, when administered alone or with nelfinavir (NFV).
Methods
Eleven HIV-positive patients, in steady-state treatment with EFV and 11 patients in steady-state treatment with EFV+NFV, were evaluated. Blood samples for pharmacokinetic analysis were obtained during a dosage interval. Plasma concentrations of EFV were determined by h.p.l.c.
Results
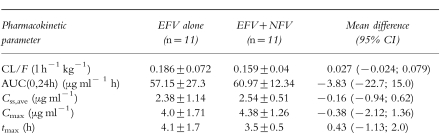
No significant difference was found between the principal pharmacokinetic parameters of EFV when administered alone or in combination with NFV (mean AUC: 57.1–7727.3 vs 60.9±12.3 μg ml−1 h; mean CL/F: 0.18±0.072 vs 0.16±0.04 l h−1 kg−1; mean Cmax: 4.0±1.7 vs 4.3±1.2 μg ml−1, and mean tmax: 4.1±1.7 vs 3.5±0.5 h) Mean trough plasma concentrations (C0) of EFV were 1.64±0.93 μg ml−1, with and without NFV. A good correlation was found between C0 and AUC(0,24h) (r = 0.96; P < 0.01).
Conclusions
Despite the common metabolic pathway, there was no significant influence of NFV on the pharmacokinetics of EFV. EFV exhibits a relatively low interindividual variability and a dosing regimen of 600 mg day−1 assures plasma concentrations that are adequate for inhibition of viral replication.
Keywords: efavirenz, HIV-1 infection, nelfinavir, pharmacokinetic interaction
Introduction
Efavirenz (EFV, DMP 266) is a new, highly potent non-nucleoside reverse transcriptase inhibitor (NNRTI), dosed once daily, recently approved for reduction of HIV-1 RNA load[1]. EFV has a sustained antiretroviral efficacy when used in combination with either a protease inhibitor (PI) or/and with nucleoside reverse transcriptase inhibitors (NRTIs)[2, 3]. Treatment with EFV plus nelfinavir (NFV), the most recently commercialized PI, produces a significant decrease in plasma viral load and an increase in CD4+cell count in both antiretroviral therapy-naive and NRTI-experienced patients[4, 5].
Both EFV and NFV are biotransformed in the liver, mainly by CYP 3A4 isoenzyme, thus, potential pharmacokinetic interactions should be considered when the two drugs are administered concomitantly[6].
The objective of this study was to investigate the pharmacokinetics of EFV after multiple oral doses, when administered alone and in combination with NFV to HIV-1 infected patients.
Methods
Patients
HIV-1 infected patients treated with a combination therapy containing EFV (group 1) or EFV+NFV (group 2), were eligible for this study. EFV (600 mg) was given daily at bedtime. NFV (750 mg) was dosed every 8 h.
Other medications included in group 1 therapy were didanosine (ddI), stavudine (d4T) and hydroxyurea (HU). Group 2 received d4T as additional medication.
The multiple-dose treatment study required a period of at least 4 weeks of EFV or EFV+NFV treatment.
Patients included in this study were heavily pretreated and had failed protease inhibitor containing regimens. As result, both therapies were considered as rescue treatment.
All enrolling patients at baseline met the subsequent criteria: transaminases values less than 2.5 times the upper limit of normal value (40 mU ml−1), creatinine <1.2 mg dl−1(106 μmol l−1).
Before entering the pharmacokinetic study, the medical histories of the patients were recorded. A physical examination and haematological, biochemical and virological tests were performed. Concurrent use of other different medications was notified at the beginning of therapy. Approval for the study was obtained from the local Ethics Committee and each patient provided written informed consent.
Collection of blood samples
In accordance with each patient’s availability, serial blood samples (3 ml) for EFV pharmacokinetic evaluation were obtained during a dosing interval at the following times: 0 (trough level, prior to drug administration), 1, 2, 3, 4, 5, 6, 8, 12, 16 and 24 h post dose. Plasma was separated at 2500 g, inactivated in a bath at 56° C for 45 min and then frozen at −20° C until analysis.
Pharmacokinetic analysis
Plasma samples were analysed for EFV concentrations by h.p.l.c. with u.v. detection[7]. The assay had a limit of detection of 0.01 μg ml−1. Quality control samples at different concentrations of EFV, analysed with each analytical run, had coefficients of variation for precision and accuracy of <10% for all the concentrations examined.
Pharmacokinetic and statistical analysis
EFV concentration-time data were analysed using the population pharmacokinetic program P-Pharm (Version 3, Simed, Creteil, France). P-Pharm uses a Maximum a-Posteriori Probability (MAP) Bayesian fitting procedure to combine prior knowledge and individual available information to estimate individual parameters, and computes approximate statistical tests to evaluate the distribution properties of the differences between the expected and the observed data. The pharmacokinetic parameters of EFV were determined from a two-compartment open model. This model produced the smallest log likelihood, Akaike information criterion, and the best fit of the data.
The following pharmacokinetic descriptors were obtained: Cmax (maximum concentration), tmax(time to Cmax), Css,ave (average plasma drug concentration during the dosing interval at steady-state), AUC(0,24h) (area under the plasma concentration-time curve during the dosing interval τ), C0 (trough concentration) and CL/F (the total body clearance/fraction of the dose absorbed assumed as equal to 1). AUC was calculated by DoseF/CL and Css,ave by AUC/τ. C0, Cmax and tmax were noted directly.
Differences in CL/F, AUC, Css,ave, Cmax and tmax were compared using analysis of variance. A P value <0.05 was considered statistically significant.
Results
Twenty-two patients were equally distributed in the two treatment groups and there was no statistical difference between treatment groups with respect to age (39±16 vs 37±13 years), weight (73±14 vs 64±9 kg), SGOT (49±23 vs 41±23 mU ml−1), SGTP (49±26 vs 49±31 mU ml−1), creatinine (0.9±0.1 vs 0.9± 0.2 mg dl−1), CD4 + cell count (115±135 vs 221± 227 cell μl−1) and HIV RNA (3.7±2.3 vs 4.4± 1.4 Log10).
The pharmacokinetic evaluation was performed within 4–20 weeks after initiation of treatment (mean: 10.7 weeks). A total of 155 measurements were obtained from 22 patients enrolled in the study (mean: 7 time points for each patient).
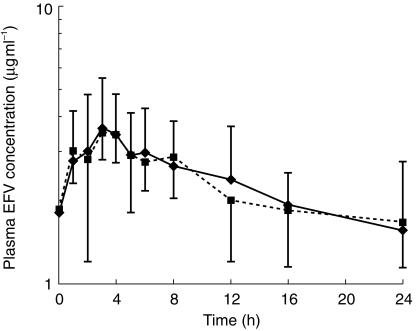
Mean plasma concentration-time curves for EFV in the two groups of patients are shown in Figure 1.
Figure 1.
Mean plasma concentration-time profiles for EFV (+ or − s.d.) in the two groups of patients. (♦ EFV alone, ▪ EFV+NFV).
The principal pharmacokinetic parameters for EFV in the presence and absence of NFV are reported in Table 1. There values were not statistically significant.
Table 1.
Pharmacokinetic parameters (mean±s.d.) of EFV in the two groups of patients (see text).
We were able to obtain EFV trough plasma concentrations (C0) from 15 out of 22 patients, with a mean value of 1.64±0.93 μg ml−1. By linear regression analysis, trough concentrations proved to be predictive for EFV body systemic exposure, as demonstrated by the good correlation found between AUC(0,24h) and C0 values (AUC(0,24h) (μg ml−1 h)=0.038 C0 (μg ml−1) −0.663; r = 0.96, P < 0.01).
Discussion
NFV, like other protease inhibitors (PIs), is extensively metabolized by cytochrome P450 (CYP) isoenzymes (mainly CYP 3A4) and is also an inhibitor of this enzyme system[8–10]. EFV is metabolized by the same pathway. Thus, coadministration of this drug with a PI may result in alteration of the respective plasma concentrations, depending up on the relative inhibitory potency of the agents involved[11]. The coadministration of EFV and NFV, in healthy volunteers, resulted in a small increase of NFV AUC (15–20%), while EFV plasma concentrations were not influenced by NFV[6]. We now confirm in HIV-1 infected patients the absence of any influence of NFV on the disposition of EFV, despite this common metabolic pathway and an inhibitory effect of NFV on cytochrome P450 isoenzymes.
Our data are also in good agreement with the relatively limited data available in HIV-positive patients after multiple oral doses of EFV (600 mg day−1), that reported a mean AUC of 58.1 μg ml−1 h, a mean Cmax of 4.1 μg ml−1 and a mean trough plasma level of 1.8 μg ml−1[12]. Our results obtained in HIV-positive patients, show that a dosing regimen of 600 mg daily−1 assures mean EFV Css,ave of 2.46 (range: 1.26– 5.37) μg ml−1 (n = 22) and mean trough concentrations of 1.64 (range: 0.62–2.5) μg ml−1 (n = 15). Previous studies using once daily dosing with 200–600 mg of EFV reported median trough plasma concentrations of 0.6–1.45 μg ml−1 that would be adequate to inhibit the replication of wild type and many mutant strains of HIV-1[13].
EFV interindividual variability was relatively low, with a coefficient of variation of about 40% for the main pharmacokinetic parameters. This reduced variability may in part be due to the improved compliance of a once daily dosing regimen. Moreover, measuring of predose plasma concentration provides a consistently reliable prediction of the full AUC during a dosing interval for each patient, as indicated by the good correlation (r = 0.96) we found between EFV trough concentrations (C0) and the systemic exposure (AUC(0,24h). That is EFV plasma concentration at time zero can predict 96% of drug exposure with a single concentration point. This parameter may be particularly useful for monitoring systemic exposure in HIV-patients with liver impairment, since it has been demonstrated that hepatic dysfunction may lead to EFV plasma concentrations higher than normal[14], or in patients receiving combination therapies with high risk of drug interactions (mainly with inhibitors of CYP450), which could result in drug accumulation to toxic concentrations.
References
- 1.Young SD, Britcher SF, Tran LO, et al. L-743,726 (DMP-266): a novel, highly potent non-nucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995;39(12):2602–2605. doi: 10.1128/aac.39.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddler S, Kahn J, Hicks C, et al. Durable clinical anti-HIV-1 activity (72 weeks) and tolerability for Efavirenz (DMP 266) in combination with Indinavir (IDV) [DMP 266–003, Cohort IV]. 12th World AIDS Conference; June 28–July 3; Geneva. 1998. abstract 12359. [Google Scholar]
- 3.Haas D, Hicks C, Seekins D, et al. A phase II, double-blind, placebo-controlled, dose-ranging study to assess the antiretroviral activity and safety of Efavirenz (DMP-266) in combination with open-label zidovudine (ZDV) with Lamivudine (3TC) at 24 weeks (DMP 266–005). 5th Conference on Retroviruses and Opportunistic Infections; February 1998; Chicago. Abstract 698. [Google Scholar]
- 4.Kagan S, Jemsek J, Martin DG, et al. Initial effectiveness and tolerability of nelfinavir (NFV) in combination with efavirenz (EFV,Sustiva,DMP 266) in antiretroviral therapy naive or nucleoside analogue experienced HIV-1 infected patients: characterisation in a phase II, open-label, multi-center study at >36 weeks (study DMP 266–024). 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 24-27; San Diego. 1998. Abstract 394. [Google Scholar]
- 5.Jemsek J, Kagan S, Martin G, et al. Combination with DMP 266 in antiretroviral therapy naive or nucleoside analogue experienced HIV-infected patients (DMP 266–024). 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 24–27; San Diego. 1998. AbstractI–102. [Google Scholar]
- 6.Fiske WD, Benedek IH, White SJ, et al. Pharmacokinetic interaction between efavirenz (EFV) and nelfinavir mesylate (NFV) in healthy volunteers. 5th Conference on Retroviruses and Opportunistic Infections; February; Chicago. 1998. abstract 349. [Google Scholar]
- 7.Villani P, Pregnolato M, Banfo S, et al. High-performance liquid chromatography method for analyzing the antiretroviral agent Efavirenz (EFV, DMP 266) in human plasma. Ther Drug Monit. 1999;21:346–350. doi: 10.1097/00007691-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors ritonavir, saquinavir, and indinavir. Br J Clin Pharmacol. 1997;44:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kravcik S, Sahai J, Kerr B, et al. Nelfinavir mesylate (NFV) increases saquinavir-soft gel capsule (SQV−SGC) exposure in HIV+ patients. Fourth Conference on Retroviruses and Opportunistic Infections; January; Washington, DC. 1997. abstract 389. [Google Scholar]
- 10.Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with nelfinavir in HIV-infected patients. AIDS. 1997;11:F117–F120. doi: 10.1097/00002030-199715000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Fiske WD, Benedek IH, Joseph JL, et al. Pharmacokinetics of Efavirenz (EFV) and Ritonavir (RTV) after multiple oral doses in healthy volunteers. 12th World AIDS Conference; June 28–July 3; Geneva. 1998. abstract 42269. [Google Scholar]
- 12.DuPont Pharmaceuticals Company. Sustiva™. (efavirenz capsules). Prescribing information 1998 Sep 17.
- 13.Bacheler LT, Anton E, Baker D, et al. Impact of mutation, Plasma protein binding and pharmacokinetics on clinical efficacy of the HIV-1 non nucleoside reverse trancriptase inhibitor, DMP 266. 37th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September; Toronto. 1997. Abstract I–115. [Google Scholar]
- 14.Maserati R, Villani P, Seminari E, et al. High plasma levels of nelfinavir and efavirenz in two HIV-positive patients with hepatic disease. AIDS. 1999;13:12–13. doi: 10.1097/00002030-199905070-00025. [DOI] [PubMed] [Google Scholar]