Abstract
Aims
To examine the influence of cisapride on the pharmacokinetics of ethanol and the impact of gastric emptying monitored by the paracetamol absorption test.
Methods
Ten healthy male volunteers took part in a cross-over design experiment. They drank a moderate dose of ethanol 0.30 g kg−1 body weight exactly 1 h after eating breakfast either without any prior drug treatment or after taking cisapride (10 mg three times daily) for 4 consecutive days. In a separate study, the same dose of ethanol was ingested on an empty stomach (overnight fast). Paracetamol (1.5 g) was administered before consumption of ethanol to monitor gastric emptying. Venous blood was obtained at 5–10 min intervals for determination of ethanol by headspace gas chromatography and paracetamol was analysed in serum by high performance liquid chromatography (h.p.l.c.).
Results
The maximum blood-ethanol concentration (Cmax) increased from 3.8±1.7 to 5.6±2.3 mmol l−1 (±s.d.) after treatment with cisapride (95% confidence interval CI on mean difference 0.28–3.28 mmol l−1). The area under the blood-ethanol curve (AUC) increased from 6.3±3.5 to 7.9±2.6 mmol l−1 h after cisapride (95% CI −0.74–3.9 mmol l−1 h). The mean blood ethanol curves in the cisapride and no-drug sessions converged at ≈2 h after the start of drinking. Both Cmax and AUC were highest when the ethanol was ingested on an empty stomach (Cmax 9.5±1.7 mmol l−1 and AUC 14.6±1.9 mmol l−1 h), compared with drinking 1 h after a meal and regardless of pretreatment with cisapride.
Conclusions
A small but statistically significant increase in Cmax occurred after treatment with cisapride owing to faster gastric emptying rate as shown by the paracetamol absorption test. However, the rate of absorption of ethanol, as reflected in Cmax and AUC, was greatest after drinking the alcohol on an empty stomach. The cisapride–ethanol interaction probably lacks any clinical or forensic significance.
Keywords: bioavailability, blood ethanol, cisapride, first-pass metabolism
Introduction
Cisapride is widely used in the treatment of various gastrointestinal disorders especially gastroesophageal reflux disease (for review see [1]). This prokinetic drug promotes gastric emptying of solid and liquid meals in healthy subjects and in those suffering from various gastrointestinal disorders [2, 3]. Cisapride stimulates gastrointestinal motor activity by indirectly facilitating cholinergic transmission in the myenteric plexus [4].
We were interested to know whether cisapride influenced the absorption kinetics of ethanol because gastric emptying is an important determinant of the peak blood-alcohol concentration reached after drinking [5]. In forensic investigations, factors influencing a person’s Cmax such as any concomitant drug treatment are often discussed and debated in litigation [6]. The impact of cisapride on the absorption kinetics of ethanol has not been studied under conditions when this medication is normally used, that is, just before meals [7].
In this study, healthy volunteers were pretreated with cisapride for 4 consecutive days before they ingested ethanol 0.30 g kg−1 exactly 1 h after a meal. The pharmacokinetic profiles of ethanol were compared with a no-drug control treatment and in a separate study, the same dose of ethanol was consumed after an overnight fast. In each of the drinking experiments, gastric emptying was monitored by the paracetamol absorption test.
Methods
Subjects
Ten healthy male volunteers all nonsmokers and not taking any medication participated in this study. Their ages ranged from 21 to 28 years (mean 26.0 years) and their body weights ranged from 65 to 97 kg (mean 81.9 kg). The subjects were in good health as shown by clinical examination and laboratory tests and urea-breath tests were negative, thus confirming the absence of Helicobacter pylori. The test protocol was approved by the ethics committee of the Faculty of Health Sciences in Linköping, and all volunteers gave their informed consent in writing.
Procedure
At about 08.00 h after an overnight (10 h) fast, the dose of ethanol (0.30 g kg−1) was ingested either on an empty stomach, 1 h after a meal without any prior drug treatment or 1 h after a meal following treatment with 10 mg cisapride (Prepulsid, Janssen-Cilag, Belgium) three times daily before meals for 4 consecutive days. The last tablet of cisapride was taken 15 min before eating a standardized breakfast. The breakfast consisted of 300 ml nonsweetened coffee, 400 ml low-fat milk, one boiled egg, 100 g white bread, 8 g low-fat margarine, 40 g ham, 40 g low-fat hard cheese and 20 g caviar paste (3188 kJ energy) and was eaten in 15 min. The alcoholic drink was prepared from ethanol solvent (96% v/v), which was diluted with pure orange juice to give a 20% v/v cocktail. The control (fasting) phase of the study started with the subjects taking 1.5 g paracetamol (Alvedon tablets (0.5 g), Astra, Sweden) dissolved in 150 ml water before the dose of ethanol was consumed. In the phases of the study when alcohol was given after breakfast, the paracetamol tablets were taken 1 h before the dose of ethanol, which was consumed within 5 min.
Blood sampling and analysis of ethanol and paracetamol
Venous blood was obtained through an indwelling catheter which was flushed with a few drops of heparin-saline solution to prevent coagulation between obtaining consecutive specimens. The specimens for ethanol analysis were drawn into 5 ml Vacutainer tubes containing NaF (20 mg) and heparin (143 units) at 0, 10, 20, 30, 40, 50, 60, 90, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210, 220, 230 and 240 min after start of drinking. The concentration of ethanol was determined by headspace gas chromatography according to a well established method used in forensic toxicology [8]. An ethanol concentration of 0.2 mmol l−1 was easily measurable and the precision, expressed as the coefficient of variation, of a single determination was 1% at a mean blood-ethanol concentration of 4.3 mmol l−1.
Vacutainer tubes (5 ml) without any preservatives were filled with blood at 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 140, 150, and 170 min after ingestion of paracetamol and the concentrations were determined in serum by h.p.l.c. as described in detail elsewhere [9].
Pharmacokinetic analysis
Blood-ethanol and serum-paracetamol profiles were plotted for each subject and the peak concentration (Cmax) and the time of reaching the peak concentration (tmax) were noted. The areas under the blood-ethanol curves were calculated from the beginning of ethanol administration and up to 240 min postdrinking by the linear trapezoidal method. The AUC for paracetamol was measured up to 170 min after administration. Treatment effects for blood-ethanol and serum paracetamol data were evaluated based on intraindividual differences and 95% confidence limits on the mean difference were calculated.
Results
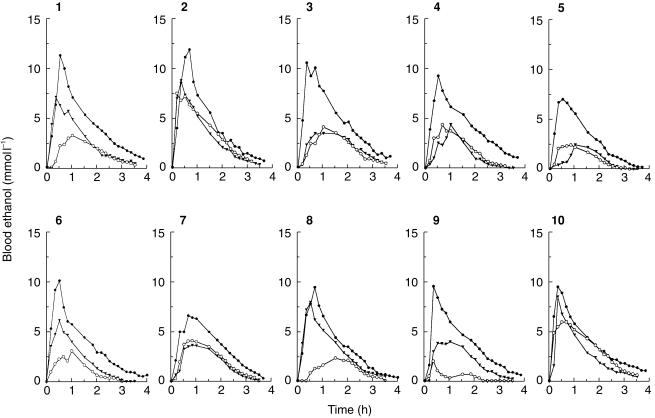
Figure 1 shows blood-ethanol profiles for the 10 subjects in the three drinking phases of the study. Although there were large interindividual variations in the shapes of these blood alcohol concentration (BAC) profiles, it was obvious that drinking on an empty stomach resulted in higher Cmaxand AUC compared with 1 h after eating a meal. This held despite pretreatment with cisapride. The concentration-time profiles of ethanol showed a rising phase corresponding to the absorption of ethanol before a peak BAC was reached and then a postabsorptive pseudolinear phase followed by a curvilinear disappearance phase until alcohol was no longer measurable.
Figure 1.
Individual blood-ethanol profiles for 10 healthy men after they drank 0.3 g kg −1 on an empty stomach (•), 1 h after a eating breakfast either without cisapride (○) or after administration of 10 mg cisapride three times daily 15 min before meals for 4 days (▾).
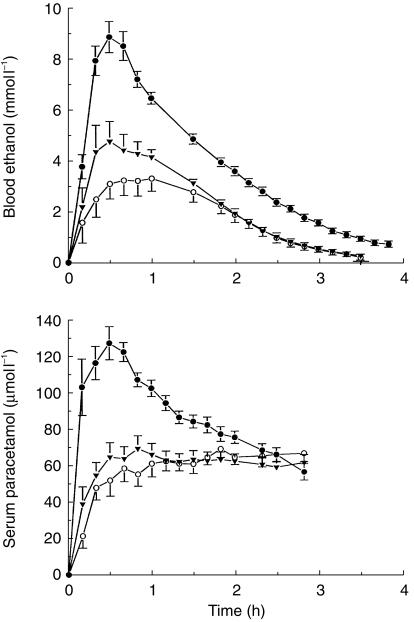
Figure 2 (upper plot) shows the average BAC profiles. The mean±s.d. Cmaxwas 9.5±1.7 mmol l−1 when ethanol was taken on an empty stomach compared with Cmax of 3.8±1.7 mmol l−1when the same dose of alcohol was consumed 1 h after food (95% CI on mean difference 4.39–6.99 mmol l−1) The corresponding AUCs were 14.6±1.9 mmol l−1 h and 6.3±3.5 mmol l−1 h (95% CI 6.1–10.6 mmol l−1 h). Pretreatment with cisapride resulted in a higher Cmax 5.6±2.3 mmol l−1 compared with no-drug treatment 3.8±1.7 mmol l−1 (95% C.I. 0.28–3.28 mmol l−1) and AUC was also higher 7.9±2.6 mmol l−1 h compared with the no-drug control session 6.3±3.5 mmol l−1 h (95% CI −0.74– 3.9 mmol l−1 h).
Figure 2.
(upper plot) Mean blood-ethanol profiles (±s.e. mean) in 10 healthy men after oral administration of ethanol 0.3 g kg−1 body weight on an empty stomach (•), 1 h after breakfast either without any prior drug treatment (○) or after 10 mg cisapride was given three times daily 15 min before meals for 4 days (▾). The lower plot shows the serum paracetamol curves (mean ±s.e. mean) after a dose of 1.5 g was taken mixed with water before drinking the dose of alcohol.
The mean curves for cisapride and no-drug treatment differed only during the first 2 h after drinking, that is, during the absorption phase of ethanol kinetics. Thereafter the curves overlapped until all the alcohol was cleared from the bloodstream. By contrast, after drinking on an empty stomach the BAC profile remained above the cisapride and no-drug curves for the 4 h sampling period.
Figure 2 (lower plot) shows the mean serum-paracetamol profiles for the three drinking experiments. In the fasting state, Cmax (137.7±26.7 μmol l−1) and AUC (244.8±30.9 μmol l−1 h) were significantly greater than Cmax (85.4±15.5 μmol l−1) and AUC (179.9± 35.6 μmol l−1 h) for the cisapride plus food condition; the mean difference in Cmax was 52.3 μmol l−1 (95% CI 33.0–71.5 μmol l−1) and for AUC 64.8 μmol l−1 h (95% CI 47.5–82.2 μmol l−1 h). No significant difference in Cmax was noted for alcohol plus food with and without cisapride; mean difference 6.3 μmol l−1 (95% CI −4.4–17.1 μmol l−1). However, AUCs under these conditions did differ significantly; mean difference 18.7 μmol l−1 h (95% CI 4.1–33.4 μmol l−1 h). The relative position of the serum-paracetamol curves in terms of AUC and Cmax agreed well with the BAC curves, namely fasting > cisapride+food > no-drug+food.
Discussion
Previous studies have shown that drugs that promote gastric emptying (metoclopramide [10], erythromycin [11] and ranitidine [12]) increase the bioavailability of ethanol whereas anticholinergic agents [10], low-dose aspirin [13] and smoking [14] delay gastric emptying resulting in a lower peak BAC and AUC. However, the rate of absorption of ethanol cannot be used as an indicator of gastric emptying because part of the dose becomes absorbed already from the stomach [15]. Accordingly, we used the serum paracetamol profiles (Figure 2, lower plot) to judge the impact of cisapride on stomach emptying and rate of absorption of alcohol because paracetamol is not absorbed from the stomach at all [16].
After treatment with cisapride a 43% increase in blood-ethanol Cmax was noted compared with the no-drug control phase of the study. This relatively large percentage change (43%) should be considered in relation to the modest mean difference in BAC observed experimentally (1.8 mmol l−1). The Cmax for blood-ethanol was higher by 143% when the dose of alcohol was consumed on an empty stomach compared with 1 h after eating a meal. The mean increase in Cmax was 5.7 mmol l−1.
Dziekan et al. [7] recently reported that treatment with cisapride increased Cmax when subjects drank alcohol (0.50 g kg−1) on an empty stomach but not when taken together with food. However, the study design involved drinking and eating at the same time and not drinking 1 h after finishing the meal as in our study. According to Dziekan et al. the Cmaxfor plasma-ethanol was increased by 2.3 mmol l−1or 14% of the control level when red wine was consumed in three portions over 25 min. We extend this finding and report here a modest but statistically significant increase in peak BAC after pretreatment with cisapride even when the dose of alcohol was ingested 1 h after a meal.
The higher Cmaxand AUC observed after pretreatment with cisapride can be explained by a more rapid emptying of the stomach and thereby a swifter absorption of ethanol, as suggested by the paracetamol concentration-time profiles [16]. The mean BAC curve after cisapride treatment coincided with the no-drug control BAC curve after about 2 h postdrinking. This suggests that the primary influence of the drug treatment was on the absorption kinetics of ethanol and not on the rate of clearance because the time required to reach zero BAC was about the same for the two test conditions (see Figure 2). The differences observed between the mean blood-ethanol curves under fasting and food conditions is somewhat more difficult to explain because the concentration-time profiles do not overlap at all. The bioavailability of ethanol as reflected in AUC is evidently diminished when alcohol is consumed 1 h after a meal presumably because a presystemic metabolism of part of the dose occurs. This might be explained by the nonlinear saturation kinetics of ethanol so that with rapid absorption, e.g. after drinking on an empty stomach, the metabolising enzymes in the liver are saturated with substrate more quickly and for a longer time leading to a higher AUC [17]. When the absorption of ethanol is slow and more prolonged, e.g. after eating a meal before drinking, the peripheral BAC remains low and there is more scope for hepatic first pass metabolism (FPM) to occur [18].
The difference between fasting and fed BAC profiles could also be explained if part of the dose of ethanol was retained in the stomach for a prolonged period of time, such as, by binding to constituents of the meal and therefore not reaching the portal vein and liver for several hours [18–20]. If at this time peripheral BAC is below the Vmaxof hepatic ADH the part of the dose of alcohol bound to food particles in the stomach can be cleared from the blood during its first passage through the liver as a consequence of Michaelis-Menten elimination kinetics [18]. Among other possibilities, the ethanol metabolising enzymes in the fed state might be inherently more effective compared with after food-deprivation for 10 h [21]. Hepatic blood-flow is also enhanced after eating a meal and this might help to facilitate a more rapid clearance of ethanol. Although ethanol is not normally considered a flow limited high extraction drug, the blood flow to the liver might be an important determinant of AUC when small amounts of alcohol are ingested and especially when the absorption rate is slow and highly variable as occurs when drinking after a meal [22].
After drinking on an empty stomach the dose of ethanol was absorbed so fast in some subjects that the peak BAC was higher than expected for the dose administered (0.3 g kg−1) and the volume of distribution of ethanol (0.7 l kg−1). The overshooting BAC saturates the hepatic ADH enzymes and this prevents any significant FPM under these conditions [19].
In conclusion, our study demonstrates that taking the drug cisapride causes a small increase in both Cmax and AUC when ethanol (0.30 g kg−1) was ingested 1 h after eating a meal. The mechanism appears to be an accelerated gastric emptying and more rapid saturation of metabolising enzymes and thus a diminished first-pass metabolism. The increases in peak BAC and AUC after cisapride treatment were not remarkable and was less than that observed when ethanol was consumed on an empty stomach. This example of a drug–alcohol interaction probably lacks clinical or forensic significance and the ethanol-induced performance decrement will be more pronounced when alcohol is consumed on an empty stomach.
Acknowledgments
We thank Mrs Kia Bunnfors for technical assistance and Björn Carlsson MSc and Björn Norlander BSc for analysis of serum paracetamol. This study was supported by the Swedish National Board of Forensic Medicine and the Forensic Science Centre of Linköping University.
References
- 1.Wiseman LR, Faulds D. Cisapride. An updated review of its pharmacology and therapeutic efficacy as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1994;47:116–152. doi: 10.2165/00003495-199447010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Madsen JL. Effects of cisapride on gastrointestinal transit in healthy humans. Dig Dis Sci. 1990;35:1500–1504. doi: 10.1007/BF01540568. [DOI] [PubMed] [Google Scholar]
- 3.Maddern GJ, Jamieson GG, Myers JC, Collins PJ. Effect of cisapride on delayed gastric emptying in gastro-oesophageal reflux disease. Gut. 1991;32:470–474. doi: 10.1136/gut.32.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCallum RW, Prakash C, Campoli-Richards DM, Goa KL, Cisapride Cisapride. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1988;36:652–681. doi: 10.2165/00003495-198836060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jones AW, Jönsson KÅ. Food-induced lowering of blood-ethanol profiles and increased rate of elimination immediately after a meal. J Forensic Sci. 1994;39:1084–1093. [PubMed] [Google Scholar]
- 6.Jones AW, Jönsson KÅ, Neri A. Peak blood-alcohol concentration and the time of its occurrence after rapid drinking on an empty stomach. J Forensic Sci. 1991;36:376–385. [PubMed] [Google Scholar]
- 7.Dziekan G, Contesse J, Werth B, Schwarzer G, Reinhart WH. Cisapride increases peak plasma and saliva ethanol levels under fasting conditions. J Int Med. 1997;242:479–482. doi: 10.1111/j.1365-2796.1997.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones AW, Schuberth J. Computer-aided headspace gas chromatography applied to blood alcohol analysis: importance of online process control. J Forensic Sci. 1989;34:1116–1127. [PubMed] [Google Scholar]
- 9.McDowall RD, Doyle E, Murkitt GS, Picot VS. Sample preparation for the HPLC analysis of drugs in biological fluids. J Pharm Biomed Anal. 1989;7:1087–1096. doi: 10.1016/0731-7085(89)80047-0. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons DO, Lant AF. Effects of intravenous and oral propantheline and metoclopramide on ethanol absorption. Clin Pharmacol Ther. 1975;17:578–584. doi: 10.1002/cpt1975175578. [DOI] [PubMed] [Google Scholar]
- 11.Edelbroek MAL, Horowitz M, Wishart JM, Akkermans LMA. Effects on erythromycin on gastric emptying, alcohol absorption and small intestinal transit in normal subjects. J Nucl Med. 1993;34:582–588. [PubMed] [Google Scholar]
- 12.Amir I, Anwar N, Baraona E, Lieber CS. Ranitidine increases the bioavailability of imbibed alcohol by accelerating gastric emptying. Life Sci. 1996;58:511–518. doi: 10.1016/0024-3205(95)02316-x. [DOI] [PubMed] [Google Scholar]
- 13.Kechagias S, Jönsson KÅ, Norlander B, Carlsson B, Jones AW. Low-dose aspirin decreases blood alcohol concentrations by delaying gastric emptying. Eur J Clin Pharmacol. 1997;53:241–246. doi: 10.1007/s002280050369. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RD, Horowitz M, Maddox AF, Wishart JM, Shearman DJC. Cigarette smoking and rate of gastric emptying: effect on alcohol absorption. Br Med J. 1991;302:20–23. doi: 10.1136/bmj.302.6767.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berggren SM, Goldberg L. The absorption of ethyl alcohol from the gastrointestinal tract as a diffusion process. Acta Physiol Scand. 1940;1:246–270. [Google Scholar]
- 16.Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47:415–421. doi: 10.1111/j.1476-5381.1973.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitt MD, Li R, DeMaster EG, Elson M, Furne J, Levitt DG. Use of measurements of ethanol absorption from stomach and intestine to assess human ethanol metabolism. Am J Physiol. 1997;273:G951–G957. doi: 10.1152/ajpgi.1997.273.4.G951. [DOI] [PubMed] [Google Scholar]
- 18.Jones AW, Jönsson KÅ, Kechagias S. Effect of high-fat, high-protein, and high-carbohydrate meals on the pharmacokinetics of a small dose of ethanol. Br J Clin Pharmacol. 1997;44:521–526. doi: 10.1046/j.1365-2125.1997.t01-1-00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortot A, Jobin G, Ducrot F, Aymes C, Giraudeaux V, Modigliani R. Gastric emptying and gastrointestinal absorption of alcohol ingested with a meal. Dig Dis Sci. 1986;31:343–348. doi: 10.1007/BF01311667. [DOI] [PubMed] [Google Scholar]
- 20.Schultz J, Weiner H, Westcott J. Retardation of ethanol absorption by food in the stomach. J Stud Alc. 1980;41:861–870. doi: 10.15288/jsa.1980.41.861. [DOI] [PubMed] [Google Scholar]
- 21.Lemeng L, Bosron WF, Li T-K. Quantitative correlation of ethanol elimination rates in-vivo with liver alcohol dehydrogenase activities in fed, fasted, and food restricted rats. Biochem Pharmacol. 1979;28:1547–1551. doi: 10.1016/0006-2952(79)90471-4. [DOI] [PubMed] [Google Scholar]
- 22.Svensson CK, Edwards DJ, Maurietto PM, et al. Effect of food on hepatic blood flow: Implications in the ‘food effect’ phenomenon. Clin Pharmacol Ther. 1983;34:316–323. doi: 10.1038/clpt.1983.174. [DOI] [PubMed] [Google Scholar]