Abstract
Aims
To evaluate the tolerability of single oral SDZ RAD doses in stable renal transplant recipients and the pharmacokinetics of ascending SDZ RAD doses when coadministered with steady-state cyclosporin A microemulsion (Neoral).
Methods
This randomized, double-blind, placebo-controlled, sequential study involved 54 patients in six treatment groups; a different SDZ RAD dose (0.25, 0.75, 2.5, 7.5, 15, 25 mg) was assessed in each group. Patients received a single oral dose of SDZ RAD (n = 6) or placebo (n = 3) with their usual Neoral dose. SDZ RAD and cyclosporin A pharmacokinetic parameters were determined.
Results
All SDZ RAD doses were well tolerated, with no discontinuations due to adverse events, serious adverse events, or deaths. Similar proportions of patients receiving SDZ RAD and placebo had at least one adverse event (44% and 50%, respectively). Mean changes in laboratory variables (baseline to endpoint) showed no clinically meaningful differences between SDZ RAD and placebo groups. SDZ RAD was absorbed rapidly and showed dose-proportional pharmacokinetics (dose: 2.5–25 mg), based on systemic exposure. Multiple postabsorptive phases in the pharmacokinetic profile indicate tissue distribution. The elimination half-life ranged from 24 to 35 h across the five highest dose groups. Pharmacokinetics were similar in men and women. Co-administration of escalating single oral SDZ RAD doses did not affect steady-state cyclosporin A pharmacokinetics.
Conclusions
SDZ RAD was well tolerated; safety profiles of SDZ RAD and placebo were similar. SDZ RAD pharmacokinetics were dose-proportional across the range 2.5–25 mg in conjunction with cyclosporin A-based therapy, according to systemic exposure. Cyclosporin A pharmacokinetics were not affected by coadministration of single oral doses of 0.25–25 mg SDZ RAD.
Keywords: cyclosporin A, immunosuppressant, pharmacokinetics, safety, SDZ RAD, transplantation
Introduction
The immunosuppressive properties of rapamycin have been known for more than 15 years [1, 2], but the clinical development of the drug has been hampered by its limited oral bioavailability. A novel immunosuppressant, SDZ RAD, has recently been developed. SDZ RAD is a derivative of rapamycin but differs structurally by having a 2-hydroxyethyl chain at position 40. This modification allowed the development of a solid dosage formulation that is more convenient to administer than rapamycin, which must be prepared from a refrigerated stock solution just before use. SDZ RAD has a mechanism of action similar to that of rapamycin: inhibition of growth factor-driven proliferation of T cells and fibroblasts. SDZ RAD prevents graft rejection in rat models of allotransplantation (kidney, heart) [3]. SDZ RAD and cyclosporin A show synergism in immunosuppression both in vitro and in vivo [4].
The aim of the present study was to evaluate the safety and tolerability of single doses of SDZ RAD (0.25–25 mg) in stable renal transplant recipients and thereby to determine whether further large-scale clinical studies are justified. Other objectives were to determine the pharmacokinetics of ascending single oral doses of SDZ RAD during steady-state dosing with the microemulsion formulation of cyclosporin A (Neoral) and to assess the effect of single-dose SDZ RAD on the steady-state pharmacokinetic profile of cyclosporin A.
This study was presented in part at the American Society of Transplant Physicians’ Sixteenth Annual Meeting, 10–14 May, 1997, Chicago, Illinois.
Methods
Study design
This was a phase-I, multicentre, randomized, double-blind, placebo-controlled, ascending-dose study of the tolerability and pharmacokinetics of SDZ RAD. The study was approved by the local Ethics Committee and patients gave written informed consent to participate in the study. Patients (n = 54) were allocated to six groups. In each group, six patients were randomized to the same single dose of SDZ RAD (0.25, 0.75, 2.5, 7.5, 15, or 25 mg), and three patients randomized to placebo. Patients received study medication under fasting conditions, together with their usual, individually selected Neoral dose. The SDZ RAD doses were evaluated in ascending order, starting with the 0.25 mg dose. Each subsequent dose was not assessed until the safety and tolerability of the previous dose had been evaluated for at least 11 days.
Participants
Men and women, aged 18–65 years, were included in the study if they were recipients of a primary cadaveric renal transplant, had undergone transplantation at least 6 months before the start of the study, and were considered to be clinically stable at the start of the study. Their serum creatinine concentration had to be less than 207 μmol l−1, with a creatinine clearance of at least 40 ml min−1 estimated on the basis of the Cockcroft-Gault formula [5]. Whole blood trough cyclosporin A concentrations had to be between 80 and 200 ng ml−1. Patients had to be receiving twice-daily Neoral at a dose that had been stable for at least 3 weeks before screening, combined with prednisone at a dose of up to 15 mg day−1, for at least 3 months.
Exclusion criteria included the following: graft rejection or continued tapering of corticosteroids from previous rejection therapy within 2 months before screening; use of other investigational immunosuppressants within 4 months or other investigational drugs within 4 weeks before screening; hypersensitivity to drugs of the same class as SDZ RAD or to components of the SDZ RAD formulation; liver, heart, or autonomic dysfunction; illness defined as significant by the investigator within 2 weeks before the study; and the use of any drug known to potentiate cyclosporin A nephrotoxicity or to interfere with cyclosporin A pharmacokinetics within 2 weeks before the study (with the exception of calcium antagonists if the dose regimen had been stable for at least 8 weeks before the start of the study). Azathioprine had to have been discontinued at least 4 weeks before the baseline assessment.
Tolerability
Adverse events were reported spontaneously by the patient or discovered from general questioning by the investigator, or after physical examination at any time, as required, up to 4 weeks after receiving SDZ RAD. The severity of the adverse events (mild, moderate, or severe), their relationship to study medication, and the occurrence of death, nonfatal serious adverse events, or adverse events resulting in the discontinuation of medication were recorded.
Patients underwent a general physical examination with ophthalmic assessment, echo- and electrocardiography, vital-signs assessment (blood pressure, pulse, body temperature), haematology, prothrombin time/partial thromboplastin time, blood biochemistry (including creatinine clearance), endocrinology, urinalysis, and markers of inflammation (fibrinogen, C-reactive protein, γ-globulin, and α1–, α2–, and β–proteins). The first laboratory assessment was made between 3 and 90 days before the start of the study (screening); patients returned for subsequent laboratory assessments up to 2 days before drug administration (baseline), on the day of drug administration (day 1), daily until day 7, and then on days 9 and 11.
Pharmacokinetic assessments
Whole blood samples (3.5 ml) were collected by means of a catheter inserted into a forearm vein. Samples for the determination of cyclosporin A concentrations were taken over one dosing interval (just before and up to 12 h after drug administration) one day before administration of SDZ RAD or placebo (day −1). After concomitant intake of Neoral with SDZ RAD or placebo, concentrations of cyclosporin A were again determined over one complete dosing interval (day 1) and, in addition, just before each morning dose of Neoral until 11 days after SDZ RAD or placebo intake. Samples for the determination of SDZ RAD concentrations were collected just before and up to 192 h after drug administration. Samples were immediately stored below −20° C pending analysis.
Cyclosporin A concentrations in whole blood were measured using a commercially available radioimmunoassay (Cyclo-Trac, INCSTAR Corp., Stillwater, Minnesota, USA). The limit of quantification (LOQ) was 15 ng ml−1. Precision and accuracy were 5.7–17.7% and −1.7 to +3.5%, respectively, at concentrations of quality control samples between 15 and 2540 ng ml−1. SDZ RAD concentrations in whole blood were quantified by means of a high-performance liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry method [6]. The LOQ was 0.75 ng ml−1. For the three quality control samples (0.75, 10, and 125 ng ml−1) precision and accuracy ranged between 9 and 11% and −12 to −7%, respectively.
Pharmacokinetic parameters were determined for both SDZ RAD and cyclosporin Ausing noncompartmental methods [7].
For cyclosporin A ratios of tmaxss, Cmaxss, Cminss, and AUCτsswith and without coadministration of SDZ RAD or placebo were also calculated.
Statistical analysis
Because of the small number of patients in the study and within each group, data from the 18 patients receiving placebo were pooled for analysis. Data from the 36 patients receiving SDZ RAD (n = 6 per group) were analysed by dose level and also as a pooled SDZ RAD group (n = 36). Patients failing to provide data at any visit were excluded from the analysis for that visit and data were not carried forward to subsequent time points. For each patient, the endpoint was taken as the last observation after baseline.
For the tolerability analysis, the number of patients experiencing an adverse event was recorded and summarized by treatment group. The incidence rates of all adverse events were summarized by body system, severity, and treatment group. Changes in vital signs, laboratory data, electrocardiography, and physical examination data were summarized by treatment group, and any clinically significant abnormalities were recorded.
For pharmacokinetic analyses, the dose proportionality of Cmax and AUC for SDZ RAD was assessed using linear regression on non-normalized data and one-factor analysis of variance (anova) on logarithmically transformed dose-normalized data with least-squares comparisons between pairs of cohorts. The Kruskal–Wallis test (the nonparametric equivalent of anova) was performed on dose-normalized data. The relationships of the dose-normalized Cmax and AUC with body weight were also explored. For cyclosporin A, a two-factor anova with dose, time, and the interaction term (time · dose) as sources of variation including estimate statements was determined to assess the dose level of SDZ RAD at which a pharmacokinetic interaction with cyclosporin A occurred. Potential changes in morning predose cyclosporin A concentrations (Cminss) were investigated over time (11 days) after coadministration of single oral doses of SDZ RAD (0.25–25 mg) or placebo, using Hotelling’s T2 test (SAS 6.08, SAS Institute Inc., Cary, North Carolina, USA).
Results
All 54 patients completed the study, although 16 patients in the SDZ RAD groups (44%) and 10 patients in the placebo group (56%) violated an entry criterion (mainly serum creatinine level ≥207 μmol l−1, use of azathioprine within 4 weeks of baseline, use of Neoral for <3 months before study, or <150 000 platelets/mm3). However, these were judged, on a case-by-case basis, to be minor deviations that did not necessitate exclusion from the trial.
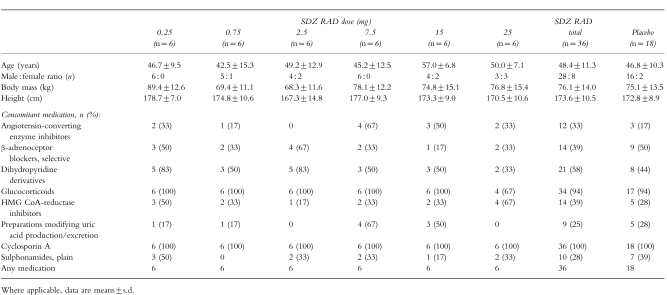
Baseline patient characteristics and concomitant medications are shown in Table 1. Age, weight, and height in the SDZ RAD and placebo groups were similar; the absolute number/proportion of women in the SDZ RAD group was higher than in the placebo group (8/22%vs 2/11%, respectively). Differences at baseline between the SDZ RAD and placebo groups were noted for the incidences of hyperparathyroidism (4/11%vs 5/28%), gastrointestinal disorders (7/19%vs 10/56%), hyperlipidaemia (10/28%vs 2/11%), hyperuricaemia (9/25%vs 8/44%), polycythaemia (7/19%vs 2/11%), cataract (5/14%vs 1/6%). Most of the patients in the SDZ RAD and placebo groups (32/89% and 17/94%, respectively) had hypertension at baseline. This was reflected by the relatively high proportions of patients receiving antihypertensive medication concomitantly with study medication.
Table 1.
Baseline patient characteristics and concomitant medications.
Tolerability
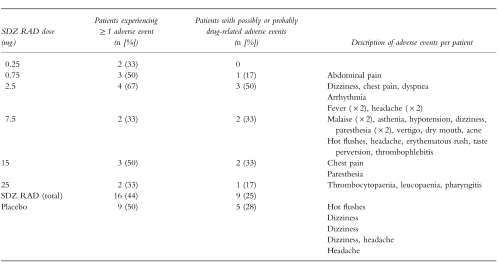
The overall incidence of adverse events is shown in Table 2. No deaths, serious adverse events, or events that led to discontinuation of study medication were reported. No adverse event was considered to be definitely related to the study medication. The most frequently reported adverse events were headache in the SDZ RAD group (11% of patients) and dizziness in the placebo group (22%). Most adverse events (26/43 in the SDZ RAD group and 8/13 in the placebo group) were classified as mild; the remainder were classified as moderate. Adverse events in the SDZ RAD group were more diverse than those in the placebo group; a total of 36 adverse events occurred in the 36 patients in the SDZ RAD group (i.e. mean: 1 event per patient), with at least one adverse event in each of the 16 body systems considered. This compared with a total of 13 adverse events occurring in the 18 patients in the placebo group (mean: 0.7 events per patient), involving six body systems (general cardiovascular, nervous system, gastrointestinal system, musculoskeletal system, skin and appendages, and general body as a whole).
Table 2.
Adverse events.
Mean values for systolic and diastolic blood pressure, pulse rate, body mass, and body temperature were similar in all treatment groups throughout the study. Echocardiograms (taken only at baseline) revealed abnormalities in 28% of patients in the SDZ RAD groups and in 39% of those in the placebo group (data not shown). No clinically significant differences in luteinizing hormone, follicle-stimulating hormone, or testosterone concentrations were found between the SDZ RAD and placebo groups.
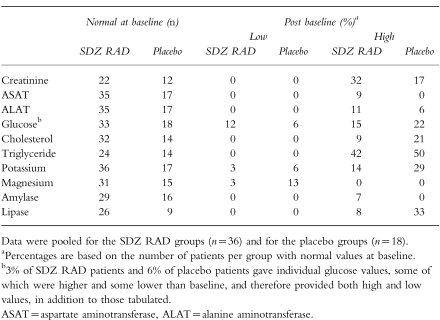
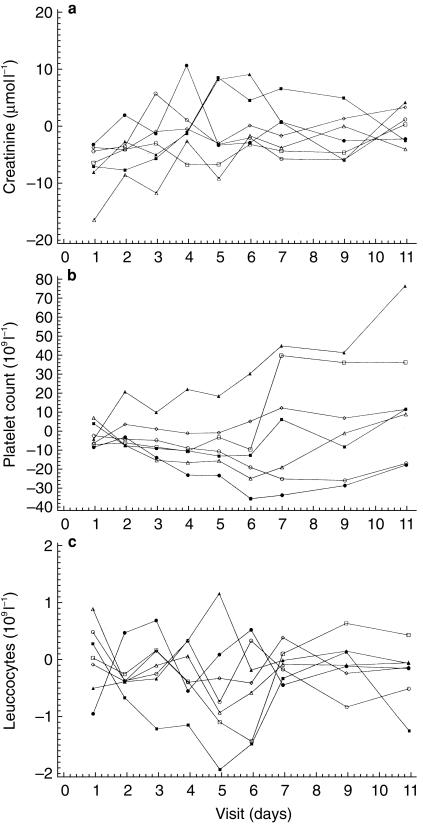
The incidence of clinically significant haematologic and biochemical abnormalities (high or low values compared with baseline) was generally similar for the SDZ RAD and placebo groups (Table 3). However, a trend towards lower platelet counts was seen in patients receiving SDZ RAD doses of 15 or 25 mg. This was most apparent in the 15 mg SDZ RAD treatment group, with the lowest mean counts on days 6 and 7 after dosing and recovery by day 11 after dosing (Figure 1b). The largest individual decrease in platelets occurred in a 52-year-old woman who had received a 25 mg SDZ RAD dose and who had a platelet count of 133×109l−1 on day 9, compared with the lower limit of the expanded normal range of 149×109l−1. This patient also had a decreased leucocyte count (2.1×109 l−1) on day 9. Leucocyte counts also differed generally between SDZ RAD and placebo groups: 17% of patients receiving SDZ RAD who had normal leucocyte counts at baseline shifted to a low postbaseline count, compared with none on placebo (Figure 1c). One mild case of leucopaenia and one mild case of leucocytosis were also reported in patients receiving SDZ RAD. None of these haematologic abnormalities had clinical sequelae.
Table 3.
Patients experiencing a shift in biochemical variables from normal baseline to abnormal postbaseline values.
Figure 1.
Mean change from baseline over time for creatinine (a), platelet count (b), and leucocytes (c); n = 6 for SDZ RAD doses (▪ 0.25 mg, □ 0.75 mg, ▴ 2.5 mg, ▵ 7.5 mg, • 15 mg, ○ 25 mg) and n = 18 for placebo (◊).
Relatively high proportions of patients in both the SDZ RAD and placebo groups experienced a shift from normal baseline to abnormal (either high or low) postbaseline values for certain biochemical variables. Serum creatinine increased to abnormal values (≥30% above baseline) in seven patients receiving SDZ RAD (Figure 1a); however, such an increase in serum creatinine is not unusual in renal allograft recipients. Although a higher proportion of patients in the SDZ RAD group (32%) than in the placebo group (17%) shifted to high postbaseline serum creatinine concentrations, the estimated creatinine clearance rate was similar for the SDZ RAD and placebo groups. In both groups, relatively high proportions of patients with normal baseline triglyceride levels had high postbaseline triglyceride levels (42% and 50%, respectively). A lower proportion of patients in the SDZ RAD group than in the placebo group had normal baseline cholesterol and high postbaseline cholesterol (9% and 21%, respectively). Blood concentrations of markers of inflammation (fibrinogen, C-reactive protein, γ-globulin, and α-1–, α-2–, and β–proteins) did not change significantly between baseline and the end of the study and did not differ significantly between SDZ RAD and placebo treatment groups. As in the case of haematologic abnormalities, none of the changes in laboratory variables had clinical sequelae.
Pharmacokinetics
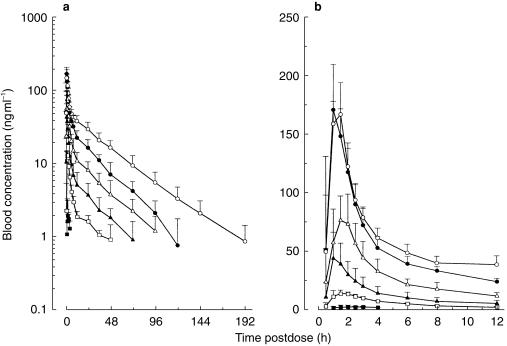
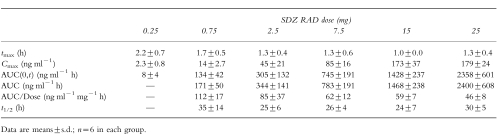
Mean SDZ RAD whole blood concentration–time profiles after single doses of 0.25, 0.75, 2.5, 7.5, 15, and 25 mg are shown in Figure 2. At a dose of 0.25 mg, maximum SDZ RAD concentrations were only just above the assay LOQ, and therefore pharmacokinetic characterization was not possible. The graph indicates reproducible pharmacokinetics within, and similar half-lives among, the different dose levels. The derived pharmacokinetic variables for SDZ RAD are shown in Table 4. SDZ RAD was absorbed rapidly; whole blood concentrations were measurable in most patients 30 min after the dose. Peak concentrations were reached on average 1.0–2.2 h after the dose. The increase in Cmax from the 0.25 mg dose to the 15 mg dose was not proportional to the dose over the entire range (Table 4). Dose-normalized AUC values were not significantly different for doses in the range 2.5–25 mg but were higher at the 0.75 mg dose level (Table 4). Dose-normalized Cmax and AUC for 0.75 and 2.5 mg SDZ RAD tended to increase with low body weight. The elimination half-life was approximately 1 day (25 h) for the 2.5, 7.5, and 15 mg doses and somewhat longer for the 0.75 and 25 mg doses (difference not significant).
Figure 2.
Whole blood concentration–time profiles of SDZ RAD on (a) semilogarithmic and (b) linear scales (initial 12 h postdose time interval) after single oral administration of SDZ RAD. Data are means+s.d.; n = 6 for each dose, ▪ 0.25 mg, □ 0.75 mg, ▴ 2.5 mg, ▵ 7.5 mg, • 15 mg, ○ 25 mg.
Table 4.
Pharmacokinetic parameters of SDZ RAD after single oral administration.
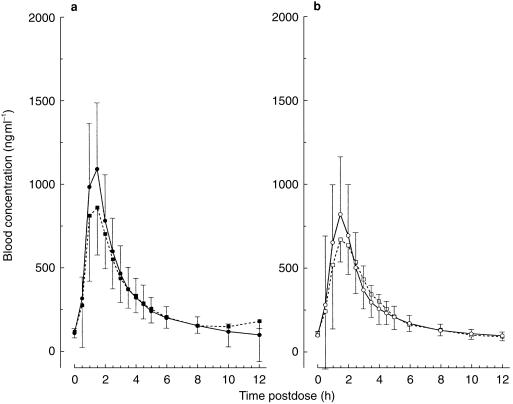
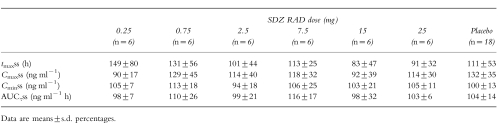
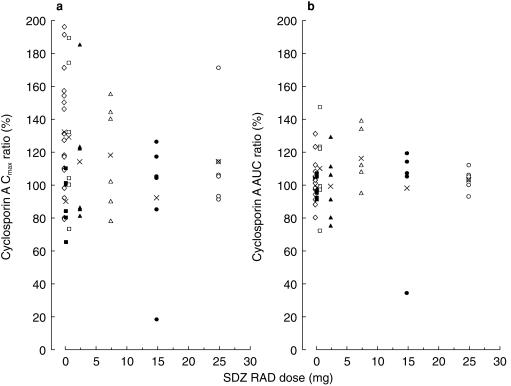
Morning predose cyclosporin A whole blood concentrations were similar across the six dose groups and were well within the target range of 80–200 ng ml−1 (Table 5). Table 5 also provides the respective daily Neoral doses. Similar doses of Neoral were given as capsule and solution, with means of 224±62 mg (n = 42) and 235±70 mg (n = 12), respectively. Overlapping morning trough-normalized (untransformed) whole blood concentration–time profiles of cyclosporin A after administration of Neoral alone or with a single oral dose of 25 mg SDZ RAD or placebo are illustrated in Figure 3. The means of the individual ratios of tmaxss, Cmaxss, Cminss, and AUCτss for cyclosporin A (coadministration of Neoral with SDZ RAD or placebo on day 1/Neoral alone on day −1) are presented in Table 6. Individual results relative to the means of the ratios for Cmaxss and AUCτss are shown in Figure 4. No significant difference in these pharmacokinetic parameters was seen among the different groups, indicating no effect of single oral doses of 0.25–25 mg SDZ RAD on the steady-state pharmacokinetics of cyclosporin A.
Table 5.
Baseline predose cyclosporin A whole blood concentrations and daily Neoral dose.
Figure 3.
Morning trough-normalized whole blood cyclosporin A concentration–time profiles after administration of Neoral alone (▪, □) or coadministered with a single dose of (a) placebo (•) or (b) 25 mg SDZ RAD (○). Data are means + or − s.d.; n = 18 for placebo; n = 6 for SDZ RAD.
Table 6.
Ratios (in percentage) of cyclosporin A steady-state pharmacokinetic variables with and without (baseline) coadministration of SDZ RAD.
Figure 4.
Effect of single oral doses of SDZ RAD on steady-state cyclosporin A ratios for Cmax and AUC (coadministration on day 1/Neoral alone on day −1); n = 6 for each dose, ▪ 0.25 mg, □ 0.75 mg, ▴ 2.5 mg, ▵ 7.5 mg, • 15 mg, ○ 25 mg, ◊ placebo, X mean.
Discussion
In this small group of stable primary renal transplant recipients, evaluations of adverse events, laboratory investigations, vital signs, and physical examinations indicated that single doses of SDZ RAD in the range 0.25–25 mg were well tolerated. SDZ RAD also showed a favourable pharmacokinetic profile and had no effect on steady-state cyclosporin A pharmacokinetics when coadministered with the microemulsion formulation of cyclosporin A, Neoral.
No overall difference was seen between the adverse event profiles of SDZ RAD and placebo. However, some haematologic differences were noted between patients receiving SDZ RAD and those receiving placebo. In particular, there was evidence of reduced platelet counts at doses of 15 or 25 mg and a general trend towards lowered leucocyte counts in patients receiving SDZ RAD. The maximum decrease in platelets was observed around day 6–7. This may be of clinical significance, given that rapamycin is known to reduce both platelet and leucocyte counts [8], larger studies must be performed before definitive conclusions can be drawn regarding the haematologic effects of SDZ RAD. In preclinical safety studies platelet counts were generally decreased at 1.5 mg kg−1 SDZ RAD and above with no changes in monkeys. A relatively high proportion of patients with normal baseline triglyceride levels had high postbaseline levels, regardless of whether they subsequently received SDZ RAD or placebo, but a lower proportion of patients receiving SDZ RAD than receiving placebo had high postbaseline cholesterol levels. This contrasts with increased cholesterol levels in patients receiving rapamycin [8]. SDZ RAD did not have any adverse effect on renal function (determined by estimated serum creatinine clearance rates) or blood pressure, a feature that it shares with rapamycin [8]. These favourable tolerability results contrast with those of a recent placebo-controlled study of rapamycin, in which more adverse events occurred in patients receiving single doses of the active agent than in those receiving placebo [9].
The pharmacokinetics and dose-proportionality of SDZ RAD were investigated after a single administration of 0.25–25 mg doses. SDZ RAD was rapidly absorbed at all dose levels, with Cmax reached within 1–2 h after administration. The dose-normalized AUC indicated no deviation from dose-proportional pharmacokinetics within the dose range 2.5–25 mg. The multiphasic postabsorptive pharmacokinetic profile showed that SDZ RAD was distributed into tissues. The elimination half-life in stable renal transplant patients was similar to that in monkeys [unpublished data] and twofold shorter than in rats [unpublished data]. Co-administration of single oral doses of 0.25–25 mg SDZ RAD did not affect the steady-state pharmacokinetics of cyclosporin A given as Neoral. SDZ RAD doses of at least 0.75 mg are recommended, from a pharmacokinetic viewpoint, in the conduct of future multiple-dose safety, tolerability, and pharmacokinetic studies.
The single-dose pharmacokinetics of SDZ RAD obtained in this entry-into-humans study can be compared with those previously reported for rapamycin by Brattström and colleagues [9]. The correlation between AUC and dose was much stronger for SDZ RAD in the present study than for rapamycin. The clinical relevance of this observation merits confirmation with multiple administration of the two drugs. The elimination half-life of SDZ RAD was approximately twofold shorter than that of rapamycin, with means of 28 h and 60 h, respectively. Consequently, under multiple dosing conditions, the accumulation potential of SDZ RAD is expected to be smaller than that of rapamycin. In neither our nor the rapamycin study, significant differences in the predose morning concentrations and AUC of cyclosporin A were seen due to coadministration of SDZ RAD or rapamycin. Again, multiple administration of SDZ RAD is required to explore fully the effect on the steady-state pharmacokinetics of cyclosporin A.
In conclusion, the first human data obtained on the tolerability and pharmacokinetics of SDZ RAD in stable renal transplantation make it a candidate for further investigation as a potential addition to immunosuppressive regimens based on cyclosporin A.
References
- 1.Chen HF, Wu JP, Xu DS, Luo HY, Daloze PM. Reversal of ongoing heart, kidney and pancreas allograft rejection and suppression of accelerated heart allograft rejection in the rat by rapamycin. Transplantation. 1993;56:661–666. doi: 10.1097/00007890-199309000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Yatscoff RW, Wang P, Chan K, Hicks D, Zimmerman J. Rapamycin distribution pharmacokinetics and therapeutic range investigations. Ther Drug Monit. 1995;17:666–671. doi: 10.1097/00007691-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Schuurman H-J, Cottens S, Fuchs S, et al. SDZ RAD, a new rapamycin derivative. Synergism with cyclosporine. Transplantation. 1997;64:32–35. doi: 10.1097/00007890-199707150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative. Pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–34. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Jean C, Laplanche R. Basel: Novartis Pharma AG; 1997. Method of determination of SDZ RAD in blood by liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry (HPLC/APCI/MS). Method description and preliminary validation. [Google Scholar]
- 7.Gibaldi M, Perrier F. Pharmacokinetics. Drugs and the Pharmaceutical Sciences. Vol. 15. New York and Basel: Marcel Dekker Inc; 1982. [Google Scholar]
- 8.Murgia MG, Jordan S, Kahan BD. The side effect profile of Sirolimus: a phase I study in quiescent cyclosporine-treated renal transplant patients. Kidney Int. 1996;47:209–216. doi: 10.1038/ki.1996.28. [DOI] [PubMed] [Google Scholar]
- 9.Brattström C, Säwe J, Tydén G, et al. Kinetics and dynamics of single oral doses of Sirolimus in sixteen renal transplant recipients. Ther Drug Monit. 1997;19:397–406. doi: 10.1097/00007691-199708000-00007. [DOI] [PubMed] [Google Scholar]