Abstract
Aims
To determine the systemic dose–response relationships with oral prednisolone and inhaled fluticasone propionate administered in a putative 11:1 mg equivalent basis, in terms of effects on adrenal, bone and haematological markers.
Methods
Twelve asthmatic patients mean (s.e.) age, 28.8 [3.3] years, FEV1 94.7 [3.6]% predicted, FEF25–75 65.5 [6.1]% predicted were studied in a double-blind, double dummy randomised crossover design comparing placebo, inhaled fluticasone propionate via volumatic spacer given twice a day (ex actuator dose 0.44 mg day−1, 0.88 mg day−1, 1.76 mg day−1) and oral prednisolone given once daily (5 mg day−1, 10 mg day−1, 20 mg day−1). All treatments were for 4 days at each dose level with a 7-day washout at crossover. Measurements were made at 08.00 h after the last dose of each dose level for plasma cortisol, serum osteocalcin and blood eosinophil count.
Results
There were significant dose-related effects for suppression of all three endpoints with both prednisolone and fluticasone propionate. Parallel slope analysis revealed a calculated dose ratio for relative potency of 8.5:1 mg (95% CI 5.7–11.2) comparing Pred with FP for morning cortisol. The magnitude of suppression with FP was less for osteocalcin and eosinophils than for cortisol.
Conclusions
Systemic tissues exhibit different dose–response relationships for the effects of inhaled and oral corticosteroids with suppression of cortisol being greater than osteocalcin or eosinophils. For cortisol suppression we observed an 8.5:1 mg relative potency ratio comparing prednisolone with fluticasone propionate. Patients taking high dose inhaled fluticasone propionate should therefore be screened for evidence of impaired adrenal reserve.
Keywords: adrenal suppression, asthma, bone markers, corticosteroids, eosinophils, fluticasone propionate, prednisolone
Introduction
Inhaled corticosteroids have now gained widespread acceptance as first line anti-inflammatory therapy for the treatment of asthma both in Europe and the USA, as reflected in current international management guidelines [1]. It is recognized, however, that inhaled as well as oral corticosteroids are associated with dose-related systemic adverse effects [2]. Glucocorticoid receptors are ubiquitous in bodily tissues and systemic adverse effects can be assessed by a variety of tissue specific markers [3].
Adrenal suppression and suppression of blood eosinophil count are well recognized to be sensitive and reproducible markers of systemic bioactivity with corticosteroids [4]. An important long-term side-effect of corticosteroids is that of altered bone metabolism and the associated fracture risk from osteoporosis. The dominant effect of corticosteroids on bone turnover is a reduction in osteoblast activity and therefore imbalance of the bone multicellular units. Osteocalcin is a protein produced by osteoblasts and as a result is a specific and sensitive surrogate marker of their activity.
Prednisolone is the most widely used oral corticosteroid and is often used as a reference standard in terms of adverse and beneficial effects of anti-inflammatory medication. Dose–response studies have been performed comparing systemic adverse effects of inhaled fluticasone propionate with other inhaled corticosteroids [5–7], but there are no published dose–response data comparing systemic effects of oral prednisolone and inhaled fluticasone propionate. We felt that it was important therefore to perform a dose–response study in asthmatic patients to assess the relative potency for prednisolone and fluticasone propionate in terms of bone, eosinophil and adrenal markers.
In a previous dose–response study the relative nominal milligram potency ratio for oral prednisolone and inhaled budesonide (via spacer) was calculated to be approximately 5:1 for suppression of 08.00 h plasma cortisol [8]. Given that the glucocorticoid potency of fluticasone propionate is twice that of budesonide [9, 10], we therefore chose a putative milligram equivalence ratio of 11:1 for comparing oral prednisolone vs inhaled fluticasone propionate in terms of their likely suppression of 08.00 h cortisol. The doses of prednisolone were given as 5 mg day−1, 10 mg day−1 and 20 mg day−1 and the doses of fluticasone propionate were approximated accordingly as 0.4 mg day−1, 0.88 mg day−1 and 1.76 mg day−1, with both drugs being given at steady state.
Methods
Patients
Twelve (six female) stable mild to moderate, nonsmoking, asthmatic patients, mean age (s.e. mean): 28.8 [3.3] years, were recruited into the study. They had a mean forced expiratory volume in one second (FEV1): 94.7 [3.6]% predicted, and mid-expiratory flow (FEF25–75): 65.5 [6.1]% predicted which reflects the calibre of the smaller airways. All patients were receiving less than or equal to 800 μg day−1 of inhaled corticosteroid. (Median dose: 300 μg day−1, range: 100–800 μg day−1). Eight patients were taking beclomethasone dipropionate (2 patients on 100 μg day−1, three patients on 200 μg day−1, two patients on 400 μg day−1 and one patient on 500 μg day−1); and four patients were taking budesonide (one patient on 200 μg day−1, three patients on 800 μg day−1). No patient had received oral corticosteroids within the previous 6 months or were taking medications known to alter steroid disposition (e.g. anticonvulsants, etc.). All subjects had normal full blood count and biochemical profile (including urea and electrolytes, liver function tests, and bone markers) and normal urinalysis. Approval for the study was obtained from the Tayside medical ethics committee and all subjects gave written informed consent. We were unable to evaluate ACTH stimulation response in our study because it is contraindicated in the UK data sheet (‘Synacthen’, Novartis, UK) for use in asthmatic or atopic subjects because of reports of potentially fatal anaphylactic reactions.
Study design
A double-blind, double-dummy placebo controlled randomised crossover design was used. Subjects attended for initial screening where FEV1and FEF25–75were measured using a Vitalograph Compact spirometer (Vitalograph Ltd Buckingham, UK) and were eligible for inclusion if their FEV1 was greater than 70% predicted. Spirometry was also measured at each subsequent visit, although efficacy was not an end point due to the short duration of treatment. Patients were randomised to receive either oral prednisolone 5 mg per tablet (Biorex Laboratories Ltd, Enfield, UK), or inhaled fluticasone propionate 0.11 mg per actuation as dose ex actuator (nominal dose of 0.125 mg) (as Flovent metered dose inhaler, Glaxo-Wellcome Inc, USA) via a 750 m1 Volumatic spacer (Allen and Hanburys, UK).
Each drug sequence was given over a total of 12 days with six patients receiving fluticasone propionate first in sequence and the other six patients receiving prednisolone first in sequence. Fluticasone propionate was given via a volumatic spacer in twice daily divided doses at 08.00 h and 22.00 h whereas prednisolone was given orally once daily at 08.00 h. The doses were given sequentially as follows each for 4 days; prednisolone: one tablet once daily, two tablets once daily, four tablets once daily (i.e. 5 mg day−1, 10 mg day−1 and 20 mg day−1, respectively); fluticasone propionate: two puffs twice daily, four puffs twice daily, eight puffs twice daily (dose ex actuator: 0.44 mg day−1, 0.88 mg day−1 and 1.76 mg day−1, respectively). Patients received placebo tablets whilst taking inhaled fluticasone propionate, and inhaled placebo (MDI plus Volumatic spacer) when taking oral prednisolone, using the corresponding number of puffs or tablets in order to make the trial double-dummy. Prior to each 12 day drug sequence (i.e. either fluticasone propionate or prednisolone) patients received both one placebo tablet per day and two puffs bid of placebo MDI (via Volumatic spacer) for 4 days. The patients’ usual inhaled corticosteroid therapy was discontinued during the placebo and treatment periods. There was also a 7-day washout between each of the 12 day treatment sequences where patients received their usual maintenance inhaled corticosteroid therapy. Each inhaler was discharged twice prior to use and patients used the spacer according to the manufacturers instructions breathing from residual volume to total lung capacity. Patients were instructed to use single puffs without delay, with each dose being followed by mouth-rinsing. Prior to the study, each individual spacer was initially prewashed in detergent, left to dry and then coated with 20 puffs.
The inhalers and tablets were masked and sealed in envelopes by a pharmacist along with instruction sheets at the beginning of the trial in order to make it investigator blind. Prior to the study and at each visit subjects were given detailed tuition, by a third party, in how to use their inhaler with the Volumatic spacer device. Each subject received a written instruction sheet to follow while taking their inhaler at home and a simple tick chart was used as an aide to compliance.
Measurements
The subjects attended the laboratory, after both placebo periods and after each dose level of both treatments, at 07.30 h 9.5 h after taking the eighth dose (at 22.00 h) of inhaled medication or placebo and 23.5 h after taking the fourth dose of oral medication, i.e. every 4 days in both limbs of the study. A cannula was inserted into an antecubital fossa vein to permit blood sampling, and subjects then rested supine for 30 min. After the rest period, blood samples were taken for measurement of plasma cortisol, serum osteocalcin and blood eosinophils at 08.00 h.
Assays
All assays were performed in duplicate in a blinded fashion by a separate technician. Plasma cortisol was measured using a commercial radio-immunoassay (r.i.a.) kit which has 11% cross reactivity for prednisolone but no cross reactivity with fluticasone propionate or its metabolites (Incstar Ltd, Wokingham, Berkshire). The coefficient of variability (CV) for analytical imprecision for within the assay was 4.3% and between the assay was 7.2%. The lower limit of the normal reference range for 08.00 h plasma cortisol, in our laboratory, is 150 nmol l−1 (5.4 μg dl−1). Plasma osteocalcin was measured using a r.i.a. kit (Incstar Ltd, Wokingham, Berkshire). The within assay CV was 5.9%. The eosinophil count was measured using a SE-9000 Haematology analyser (Sysmex UK Ltd, Bucks, UK).
Statistical analysis
The study was designed with sample size of 12 with 80% power (beta error = 0.2) to detect a 20% difference in 08.00 h cortisol (the primary end point) between treatments with the alpha error set at 0.05 (two-tailed) All data were analysed using a ‘Statgraphics’ software package (STSC Software Group, Rockville, Maryland, USA). Osteocalcin was analysed geometrically in order to normalize its distribution.
Regression analysis was applied to investigate whether for either drug, fluticasone or prednisolone, there was a significant dose–response relationship, as percentage suppression for each of the three end points. For a given end point parallel slope analysis was then applied to both drugs together. In the presence of a significant fit for the common parallel slope with both drugs, a dose ratio was calculated for relative potency on a milligram equivalent basis. This was only possible for effects on cortisol.
In addition, all active treatments and both placebos were compared by an overall multifactorial analysis of variance (manova) using treatment, dose, subject and period as factors, followed by Bonferroni’s multiple range testing to obviate multiple pair-wise comparisons. The Bonferroni’s multiple range test was set with 95% confidence intervals and hence any significant differences are reported at the P < 0.05 level. For each dose level (low, medium and high) 95% CI were calculated for the comparative response ratio between the two drugs. The number of individual values of 08.00 h plasma cortisol with an abnormal low level (<150 nmol l−1 or <5.4 μg dl−1) were analysed using the Chi-square test.
Results
There were no significant differences between the FEV1(s.e. mean) values as percentage predicted comparing placebo with low (L) medium (M) or high (H) doses of each drug: placebo 89.4 (5.8), prednisolone L:91.5 (5.2), M:92.0 (5.4), H:90.1 (4.7); fluticasone propionate L:91.3 (4.2)., M:96.2 (4.9). H:94. 1 (4.4); or FEF25–75 values (as percentage predicted): placebo: 65.3 (8.5), prednisolone L:62.2 (5.7), M:64.4 (7.2). H:61.4 (6.5); fluticasone propionate L:56.3 (6.7), M:69.2 (8.2), H:65.6 (5.4).
There were no significant carryover effects between the first and second placebos in sequence using any of the systemic parameters measured: 08.00 h plasma cortisol 415.2 vs 395.5 nmol l−1 eosinophils 0.33 vs 0.30×109 l−1, or osteocalcin 1.0 vs 1.2 nmol l−1. There were also no significant differences between the placebos prior to each treatment sequence (prednisolone vs fluticasone propionate): 08.00 h plasma cortisol 420 vs 390 nmol l−1, eosinophils 0.27 vs 0.36×109 l−1, or osteocalcin 1.12 vs 1.0 nmol l−1.
Dose–response relationships
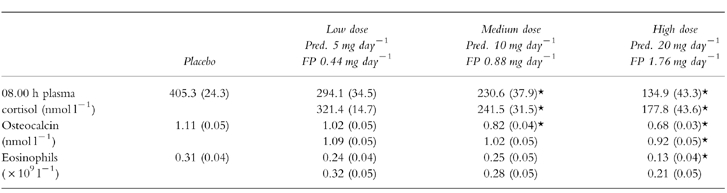
Mean values for each of the three parameters for both fluticasone propionate and prednisolone are shown in Table 1. With fluticasone propionate there was significant suppression at M and H for 08.00 h plasma cortisol, at H dose only for osteocalcin, and at no dose for blood eosinophil count. This shows that the effects of fluticasone propionate are greater on cortisol compared with osteocalcin or eosinophils. For prednisolone, there were significant differences between placebo and medium and high dose for both 08.00 h plasma cortisol and osteocalcin, and with high dose only for eosinophils.
Table 1.
Mean (s.e.mean) for prednisolone (Pred.) and fluticasone propionate (FP) and pooled placebo for: 08.00 h plasma cortisol, osteocalcin and eosinophils at low, medium and high dose levels. Asterix denotes significant difference from placebo.
Regression analysis showed significant dose–response relationships for percentage suppression with each end-point for both drugs: prednisolone (08.00 h plasma cortisol P < 0.005 eosinophils P < 0.05, osteocalcin P < 0.001); fluticasone propionate (08.00 h plasma cortisol P < 0.01, eosinophils P < 0.05, osteocalcin P < 0.05). This showed a dose-ratio for relative potency of 8.5:1 mg (95% CI 5.7–11.2) in terms of milligram equivalence for comparison of prednisolone:fluticasone (Figure 1). It was not possible to calculate a dose-ratio for either eosinophil count or osteocalcin.
Figure 1.
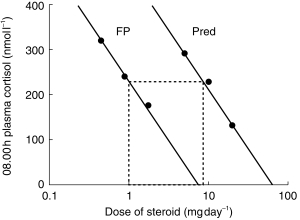
Log dose–response plot for 08.00 h plasma cortisol suppression to show dose-ratios for relative potency. Doses of oral prednisolone (Pred) were 5 mg day−1, 10 mg day−1 and 20 mg day−1. Doses of inhaled fluticasone propionate (FP) were 0.44 mg day−1, 0.88 mg day−1 and 1.76 mg day−1. Parallel fitted slope analysis was used to calculate the equivalent dose of prednisolone causing the same degree of suppression as compared with 1 mg fluticasone. The relative dose ratio for Pred. vs FP was calculated at 8.5:1 mg (95% CI 5.7–11.2).
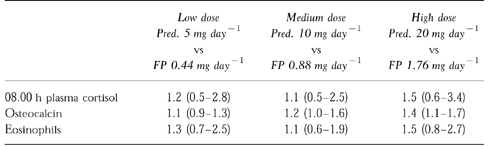
Response ratios showed no significant differences at any dose level for effects on 08.00 h plasma cortisol or eosinophils but a significant difference in osteocalcin at medium and high doses (Table 2).
Table 2.
Response ratios shown as fold difference (95% CI for difference) for prednisolone (Pred.) vs fluticasone propionate (FP) for: 08.00 h plasma cortisol, osteocalcin and eosinophils at low, medium and high doses. Confidence intervals which exclude unity show a significant (P<0.05) difference between the two drugs at a given dose level.
Individual data (Figure 2) showed no significant difference in the numbers of individual results with abnormal low values for 08.00 h cortisol (<150 nmol l−1 or <5.4 μg dl−1) comparing all doses of both drugs: fluticasone propionate (n = 9/36) vs prednisolone (n = 15/36) (P = 0.21).
Figure 2.
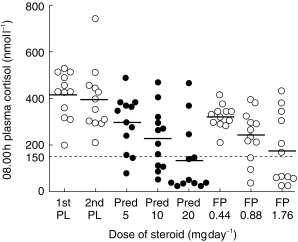
Individual values for 08.00 h plasma cortisol with each treatment. Horizontal bars represent mean values. The interrupted line represents the lower end of the normal reference range at <l50 nmol l−1 (or <5.4 μg dl−1). There was no significant difference between fluticasone and prednisolone in terms of the number of abnormal values: n = 9/36 for fluticasone vsn = 15/36 for prednisolone (P = 0.21).
There was no significant correlation between 08.00 h plasma cortisol and the degree of airway calibre as FEV1% predicted, with either fluticasone propionate or prednisolone at any dose level.
Discussion
Our results in asthmatic patients showed that there were no significant differences in the degree of cortisol suppression exhibited by oral prednisolone and inhaled fluticasone propionate which were administered on a putative 11:1 mg equivalent basis. When the same data were fitted by parallel slope analysis, we observed a 8.5:1 mg relative potency ratio for oral prednisolone compared with fluticasone propionate. Our results seem to be approximately in line with those of Jennings et al. [8] in healthy volunteers where there was a 5:1 milligram equivalent dose-ratio between oral prednisolone and inhaled budesonide via a spacer, assessed by effects on 08.00 h cortisol. This would also be consistent with a two fold difference in glucocorticoid potency between budesonide and fluticasone as assessed by the McKenzie skin vasoconstrictor assay [9, 10]. In another study with prednisone dependent asthmatics, Toogood et al. [4] reported a dose ratio of 7.6:1 with prednisolone vs budesonide given via a spacer for effects on early morning cortisol. It is probably invalid to compare dose ratios between different groups of subjects because there is a large degree of interindividual variability in hypothalamic-pituitary-adrenal axis response to exogenous corticosteroids. The severity of asthma will also influence the ratio of systemic effects between an oral and inhaled corticosteroid as systemic activity will vary according to lung bioavailability. In this respect patients had mild to moderate asthma although they did have reduced small airway calibre as evidenced by the mean value for FEF25–75 of 60% predicted.
The interindividual variability can be seen by the dispersion of values in Figure 2 and may be related to possible effects of inhaler technique, airway calibre or glucocorticoid receptor responsiveness. We took great care to eliminate possible differences in inhaler technique by using the spacer. We also found no significant correlation between airway calibre and the degree of cortisol suppression induced by inhaled fluticasone propionate. Indeed it was evident that even with oral prednisolone there was considerable variability in suppression, suggesting that factors other than inhaler technique and lung bioavailability are important in determining systemic bioactivity. This is more likely to represent tissue specific differences in glucocorticoid metabolism or possibly individual glucocorticoid receptor responsiveness [3]. It is therefore also important to look at the individual responses to inhaled and oral corticosteroids as well as the mean data. Our results showed no significant difference in the number of individual low values (<150 nmol l−1 or 5.4 μg dl−1) between oral prednisolone and fluticasone propionate when all doses were compared. In this respect, it is known that patients who have an abnormally low value for 08.00 h plasma cortisol and urinary free cortisol excretion whilst receiving inhaled corticosteroid medication, also exhibit a subnormal dynamic response to ACTH stimulation [11–13].
In this study we have analysed markers for the systemic activity of different tissues, namely bone, blood and adrenal cortex. We may not have achieved the steep part of the dose response curve for observed effects on osteocalcin or eosinophils in response to fluticasone propionate. However it could be argued that it would not be clinically relevant to evaluate doses of fluticasone propionate greater than 1.76 mg day−1 as this is the highest recommended dose by the manufacturers. Our findings with fluticasone propionate are in keeping with Jennings et al. [8] and Toogood et al. [4], using budesonide in terms of a greater suppression with cortisol compared with the effects on eosinophils or osteocalcin. None the less, our data are reassuring in that the bone appears to be less sensitive than the adrenal gland to the systemic effects of inhaled fluticasone propionate.
The high degree of first-pass metabolism of the swallowed dose for fluticasone propionate [14], will result in the systemic bioactivity being predominantly determined by the lung bioavailability, as there is no first-pass metabolism in the lungs [15]. It is important therefore to assess whether airway calibre was altered by fluticasone propionate treatment, as this might conceivably result in attenuated lung bioavailability. In this respect we found no differences in effects on either FEV1or FEF25–75between fluticasone and prednisolone, and so altered lung bioavailability is unlikely to explain their relative systemic effects. The efficacy of the corticosteroids was not an end point in this study because the duration of treatment was relatively short and the airways effects, which can take up to 1 month, were not seen. Furthermore, our patients had well controlled mild to moderate asthma, and were therefore probably at the top of the dose–response curve for corticosteroid efficacy [2].
It is also worth noting that the dosing schedules of the two drugs may have influenced the diurnal profile for adrenocortical activity, in that prednisolone was given once daily in the morning and fluticasone given in the morning and evening, in considering the effects of fluticasone propionate it has been shown that suppression of a spot 08.00 h plasma cortisol sample closely mirrors the effects on an integrated 24 h plasma cortisol profile [6, 16–18]. This is perhaps not surprising in that the maximal degree of diurnal HPA-axis suppression coincides with peak levels as measured at 08.00 h. The peak to trough variability for fluticasone propionate is much less than other steroids [19], reflecting the long elimination half life of 14.4 h [20], and the time of dosing is therefore not as important with respect to cortisol suppression. Indeed, it has been shown that significant adrenal suppression occurs with fluticasone when administered with a 24 h dosing interval [21].
We specifically chose to use enteric coated prednisolone as this is the most commonly prescribed formulation in our own unit. In a study evaluating the pharmacokinetic profile of enteric coated prednisolone, there was a lag in absorption such that there was an appreciable concentration remaining at 24 h after dosing [22]. Furthermore, the corresponding 24 h plasma cortisol profile showed that the lowest value coincided with the time point at 24 h after dosing. Thus, the suppressive effects of fluticasone propionate and enteric coated prednisolone are likely to be comparable on 08.00 h and 24 h cortisol measurements.
What might be the clinical relevance of our results? The finding of an 8.5:1 mg dose ratio for adrenal suppression suggests that fluticasone exhibits both topical and systemic glucocorticoid activity. This may explain why high dose inhaled fluticasone (2 mg day−1) has been shown, to have a prednisolone sparing effect [23] and it may be possible to wean patients off prednisolone as a consequence of substituting the systemic effect of prednisolone with the systemic effect of fluticasone. This is illustrated that by the case reports of cushingoid features and adrenal suppression with inhaled fluticasone propionate in both adults and children [24, 25] as well as the recent report of adrenal insufficiency in a patient who changed her inhaled corticosteroid treatment from 1000 μg day−1 of fluticasone propionate to 800 μg day−1 budesonide [26].
Acknowledgments
This study was supported by a University of Dundee department grant and received no support from the pharmaceutical industry.
References
- 1.British Thoracic Society. The British Guidelines on asthma management: 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21. [Google Scholar]
- 2.Lipworth BJ. Airway and systemic effects of inhaled corticosteroids in asthma: dose response relationship. Pulm Pharmacol. 1996;9:19–27. doi: 10.1006/pulp.1996.0002. [DOI] [PubMed] [Google Scholar]
- 3.Lipworth BJ, Seckl JL. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997;52:476–482. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johannsson SA. Bioequivalent doses of budesonide and prednisone in moderate and severe asthma. J Allergy Clin Immunol. 1989;84:668–700. doi: 10.1016/0091-6749(89)90297-2. [DOI] [PubMed] [Google Scholar]
- 5.Clark DJ, Lipworth BJ. Adrenal suppression with chronic dosing of fluticasone propionate compared with budesonide in adult asthmatic patients. Thorax. 1997;52:55–58. doi: 10.1136/thx.52.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boorsma M, Andersson N, Larsson P, Ullman A. Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J. 1996;9:1427–1432. doi: 10.1183/09031936.96.09071427. [DOI] [PubMed] [Google Scholar]
- 7.Wilson AM, McFarlane LC, Lipworth BJ. Dose response effect for adrenal suppression with repeated twice daily inhaled fluticasone priopionate and triamcinolone acetonide in adult asthmatics. Am J Respir Crit Care Med. 1997;156:1274–1277. doi: 10.1164/ajrccm.156.4.97-03029. [DOI] [PubMed] [Google Scholar]
- 8.Jennings BH, Anderson KE, Johannsson SA. Assessment of systemic effects of inhaled glucocorticoids: comparison of the effects of inhaled budesonide and oral prednisolone on adrenal function and markers of bone turnover. Eur J Clin Pharmacol. 1989;40:77–82. doi: 10.1007/BF00315143. [DOI] [PubMed] [Google Scholar]
- 9.Phillips GH. Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respir Med. 1990;84(Suppl A):19–23. doi: 10.1016/s0954-6111(08)80003-0. [DOI] [PubMed] [Google Scholar]
- 10.English AF, Neate MS, Quint DJ, Sareen M. Biological activities of some corticosteroids used in asthma. Am J Respir Crit Care Med. 1994;149(Suppl):A212. [Google Scholar]
- 11.Brown PH, Blundell G, Greening AP, Crompton GK. Screening for hypothalamo-pituitary-adrenal axis suppression in asthmatics taking high doses of inhaled corticosteroid. Respir Med. 1991;85:511–516. doi: 10.1016/s0954-6111(06)80269-6. [DOI] [PubMed] [Google Scholar]
- 12.Broide J, Soferman R, Kivity S, Golander A, Dickstein G, Spirer Z, Weisman Y. Low dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab. 1995;80:1243–1246. doi: 10.1210/jcem.80.4.7714095. [DOI] [PubMed] [Google Scholar]
- 13.Grebe SKG, Feek CM, Durham JA, Kjakovic M, Cooke R. Inhaled beclomethasone dipropionate suppresses the hypothalamic pituitary adrenal axis in a dose dependent manner. Clin Endocrinol. 1997;47:297–304. doi: 10.1046/j.1365-2265.1997.2391059.x. [DOI] [PubMed] [Google Scholar]
- 14.Harding SM. The human pharmacology of fluticasone propionate. Respir Med. 1990;84(Suppl A):25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grahnen A, Jansson B, Brunden RM, et al. A dose response study comparing suppression of plasma cortisol induced by fluticasone propionate from diskhaler and budesonide from turbuhaler. Eur J Clin Pharmacol. 1997;52:261–267. doi: 10.1007/s002280050287. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly R, Williams KM, Baker B, Badcock CA, Day RO, Seal PJ. Effects of budesonide and fluticasone on 24 hour plasma cortisol: a dose response study. Am J Respir Crit Care Med. 1997;156:1746–1751. doi: 10.1164/ajrccm.156.6.9703003. [DOI] [PubMed] [Google Scholar]
- 18.Wilson AM, Lipworth BJ. 24 hour profiles of adrenocortical activity in asthmatic patients receiving inhaled and intranasal corticosteroids. Thorax. 1999;54:20–27. doi: 10.1136/thx.54.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meibholm B, Hochhaus G, Rahatagi S, et al. Dependency of cortisol suppression on the administration time of inhaled corticosteroids. J Clin Pharmacol. 1997;37:704–710. doi: 10.1002/j.1552-4604.1997.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 20.Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AM, Clark DJ, Devlin M, McFarlane LC, Lipworth BJ. Adrenocortical activity with repeated administration of once daily inhaled fluticasone propionate and budesonide in asthamtic adults. Eur J Clin Pharmacol. 1998;53:317–320. doi: 10.1007/s002280050385. [DOI] [PubMed] [Google Scholar]
- 22.Adair CG, McCallion O, McFarlane LC. A pharmacokinetic and pharmacodynamic comparison of plain and enteric coated prednisolone tablets. Br J Clin Pharmacol. 1992;33:495–499. doi: 10.1111/j.1365-2125.1992.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noonan M, Chervinsky P, Busse WW, et al. Fluticasone propionate reduces oral prednisolone use while it improves asthma control and quality of life. Am J Respir Crit Care Med. 1995;152:1467–1473. doi: 10.1164/ajrccm.152.5.7582278. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman B, Gold M, Wherrett D, Hanna AK. Adrenal suppression in two patients with asthma treated with low doses of inhaled steroid fluticasone propionate. J Allergy Clin Immunol. 1998;101:425–426. doi: 10.1016/S0091-6749(98)70260-X. [DOI] [PubMed] [Google Scholar]
- 25.Duplantier JE, Nelson RP, Morelli AR, Good RA, Kornfeld SJ. Hypothalamic-pituitary-adrenal axis suppression associated with the use of inhaled fluticasone propionate. J Allergy Clin Immunol. 1998;102:699–700. doi: 10.1016/s0091-6749(98)70292-1. [DOI] [PubMed] [Google Scholar]
- 26.Todd GRG, Wright D, Ryan M. Acute adrenal insufficiency in a patient with asthma after changing from fluticasone propionate to budesonide. J Allergy Clin Immunol. 1999;103:956–957. doi: 10.1016/s0091-6749(99)70447-1. [DOI] [PubMed] [Google Scholar]


