Abstract
Aims
In diabetics, acarbose causes a reduction of blood glucose and triglyceride levels. The aim of this study was to assess the effect of this drug in non diabetic subjects with hypertriglyceridaemia.
Methods
Thirty non diabetic patients with hypertriglyceridaemia type IIb or IV (24 males, six females; mean age 51.1 ±10.2 years) were studied. They were stratified into two groups depending on their basal triglyceride concentration (group A: triglyceride values ≤4.5 mmol l−1; group B triglyceride values > 4.5 mmol l− 1). Treatment consisted of 4 week courses of diet plus acarbose (50 mg twice daily) alternating with 4 weeks of diet alone for a total period of 16 weeks.
Results
Mean triglyceride values decreased significantly during the first and third cycles of therapy, i.e. diet plus acarbose treatment cycles in both patient groups. Group A also had significant reductions in total cholesterol and HDL cholesterol concentrations after completion of the acarbose treatment. Reduction of triglyceride levels was observed after both acarbose courses in patients affected by hypertriglyceridaemia type IIb. A marked reduction of triglyceride concentrations was achieved by patients affected by hypertriglyceridaemia type IV after the second acarbose course only.
Conclusions
Diet alone did not reduce triglyceride concentrations to normal values in our patients. The data suggest that acarbose is a useful adjunct to dietary control in non-diabetic patients affected by severe hypertriglyceridaemia.
Keywords: acarbose, hypertriglyceridaemia, hypolipaemic drugs
Introduction
Acarbose is a pseudotetrasaccharide of microbial origin which competitively, selectively and reversibly inhibits alpha glucosidase activity in the brush border membrane of the small intestine. This enzyme is essential for the degradation of starch, dextrins, maltose and sucrose to absorbable monosaccharides [1, 2]. The effects of acarbose on carbohydrate metabolism are well documented in patients affected by non insulin dependent diabetes mellitus (NIDDM). This drug prevents abnormally high rises in postprandial blood glucose concentrations, reduces hyperinsulinaemia and may reduce insulin resistance [3, 4]. The possibility that hypertriglyceridaemia, which is closely linked to carbohydrate and insulin metabolism [5], could also be modified by acarbose has been explored in normal and genetically obese rats [6, 7]. Administration of acarbose to NIDDM patients and healthy volunteers reduces serum triglyceride values, but has little or no effect on total cholesterol levels [4, 8–10].
We have assessed the efficacy of acarbose in the treatment of non diabetic subjects, with hypertriglyceridaemia type IIb or IV, classified according to the WHO classification [11].
Methods
Patients’ characteristics
Thirty non-diabetic patients (24 males, six females; mean age 51.1±10.2 years) without a family history of diabetes mellitus and with hypertriglyceridaemia type IIb or IV, classified according to the WHO classification [11] were studied. None of the patients had responded with a reduction in triglyceride concentrations to dietary control alone.
The specific inclusion criteria were:
plasma triglyceride concentrations > 2.85 mmol l−1, cholesterol concentrations 4.13–7.75 mmol l−1, age <70 years.
The exclusion criteria were:
-
unstable or Prinzmetal’s angina, previous myocardial infarction, drug addiction, limited cognitive function, impaired liver function, increased creatinphosphokinase serum levels, hypothyroidism, kidney disease, diabetes mellitus, gout and alcohol consumption. Also patients taking the following drugs were excluded: oestrogens, progesterones, corticosteroids, diuretics, β-adrenoceptor blockers, cimetidine, retinoids, allopurinol and benzodiazepines, other lipid lowering therapy.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research review committee. Informed consent was obtained from each patient.
Protocol
The study had a 6-week run in period during which treatment consisted only of a low fat (max 20% total calories) diet (1600 kCal day−1) with less than 300 mg day−1 intake of cholesterol. The diet was made up of 55% carbohydrates, 20% proteins and 25% fats (9% saturated fatty acids, 9% monounsaturated and 7% polyunsaturated fatty acids).
The 16 week study protocol was divided into 4 week courses as follows: diet plus acarbose therapy (4 weeks), alternating with dietary control alone (4 weeks). Thus in the first and third 4 week periods diet plus acarbose therapy were administered, while dietary control alone was followed in the second and fourth 4 week periods. Acarbose dosage consisted of 50 mg twice daily.
Fasting serum concentrations of total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides were determined at the start of the study and after each treatment cycle. Glucose values were determined after a 12 h fast and 2 h postprandially at the start of the study. Fasting glycaemia was also assessed after each treatment course.
The following parameters were determined only at the start and end of the 16 week protocol: total glycated haemoglobin levels (HbA1); urea and creatinine levels; aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (gamma-GT), alkaline phosphatase (ALP) and creatinphosphokinase (CPK) concentrations; blood cell counts and haemoglobin levels; thyroid hormones values (free-T3, free-T4, TSH) and urine test for glucose.
Total and LDL cholesterol and triglyceride values were determined using enzymatic methods [12–14]. HDL cholesterol was determined after precipitation of lipoproteins containing ApoB by phosphotungsten acid/magnesium chloride [15]. HbA1 was determined chromatographically (546 nm; Glyco Hb Kit—Cat. N.5344—Helena Laboratories—Diagnostic System). Other parameters were assessed using routine laboratory methods.
Each subject’s body weight and height were recorded and body mass index (BMI) calculated (Table 1) after a run-in period and before the start of therapy. Side-effects, heart rate and arterial pressure were recorded at each examination at the end of each month. Statistical analysis on all data extrapolated was performed using anova test followed by Bonferroni’s t-test. A P value < 0.05 was considered statistically significant.
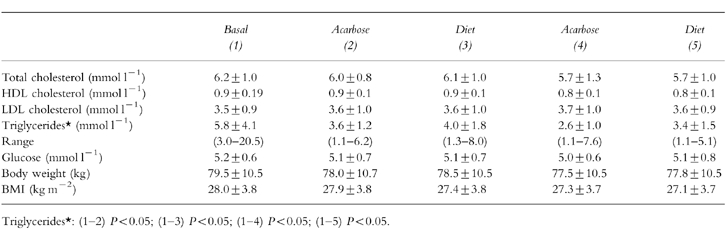
Table 1.
Mean values±s.d. in 30 non-diabetic patients with hypertriglyceridaemia, determined before start of therapy and at end of each of four cycles.
Results
Mild side-effects were observed in six subjects and mainly consisted of gastrointestinal disorders (flatulence, nausea and diarrhoea, etc.). Laboratory tests (glucose, urea and creatinine levels; AST, ALT, gamma-GT, ALP and CPK values; blood cell counts and haemoglobin levels; free-T3, free-T4 and TSH values and HbA1 concentrations were within their normal ranges at the start of the study and none of them showed significant variations at the end of treatment. We observed a slight reduction in mean total cholesterol value from 6.2±1.0 mmol l−1 at the start compared with 5.7±1.0 mmol l−1 at the end of treatment (Table 1). No significant changes were observed in mean HDL cholesterol and LDL cholesterol levels (Table 1).
Mean triglyceride value decreased significantly from 5.8±4.1 mmol l−1 to 3.6±1.2 mmol l−1 (P < 0.05) during the first cycle of the therapy (diet plus acarbose treatment). Triglycerides were again reduced significantly (P < 0.05) at the end of the third cycle (diet plus acarbose therapy) and increased after the final cycle (dietary control alone) (Table 1).
Efficacy of acarbose according to groups A and B
When the population was stratified into two groups based on basal triglyceride concentrations: (group A = triglyceride values ≤4.5 mmol l−1 and group B = triglyceride values >4.5 mmol l−1) (Table 2), group A showed a slight reduction in mean total cholesterol levels after the two cycles of acarbose treatment.
Table 2.
Stratification analysis of plasma triglyceride data (mmol l−1) based on type of hypertriglyceridaemia in 30
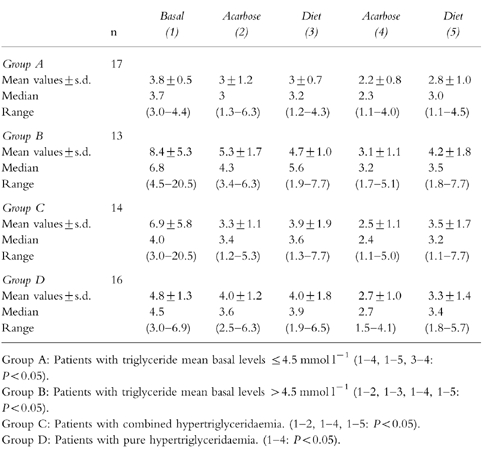
However, both groups showed similar changes in mean triglyceride values during the study. Triglycerides decreased significantly at the end of the first 4 week course using acarbose (P < 0.05 in both groups), and thereafter fell significantly during the second acarbose cycle (P < 0.05 in both groups A and B) (Table 2).
At the end of the treatment group B showed no significant changes in mean total cholesterol, HDL cholesterol and LDL cholesterol concentrations, while group A showed a slight decrease in mean HDL cholesterol level.
Efficacy of acarbose according to WHO IIb or IV classes
The patients were also grouped according to the type of hypertriglyceridaemia: combined hypertriglyceridaemia (Group C = WHO IIb class, n = 14) and pure hypertriglyceridaemia (Group D = WHO IV class, n = 16).
In type IV hypertriglyceridaemia no marked changes in mean total cholesterol, LDL cholesterol and HDL cholesterol concentrations were detected throughout the treatment period, while mean triglyceride values showed a progressive reduction after drug treatment, especially during the second cycle using acarbose (P < 0.05) (Table 2).
Mean triglyceride concentrations reduced significantly during the first and second drug treatment cycles (P < 0.05) in patients with type IIb hypertriglyceridaemias (Table 2), with a slight decrease in mean total cholesterol value.
Discussion
The most commonly used drugs to treat hypertriglyceridaemia are the fibrates and statins. Fibrates act on different stages of lipoprotein metabolism, stimulating lipoprotein-lipase activity and inhibiting liver synthesis of VLDL [16, 17]. The side-effects caused by fibrates sometimes necessitate suspension of treatment and include nausea, vomiting, diarrhoea, hepatomegaly with formation of bile stones, myalgia and increased serum transaminase concentrations [18]. The effect of newer statins (e.g. atorvastatin and cerivastatin) in reducing triglyceride serum levels, is represented by the inhibition of Apo B synthesis, a main fraction of VLDL [19]. Side-effects include enhancement of serum ALT, AST and CPK levels, asthenia and muscular pain [19]. In NIDDM patients, good glucose control is often associated with a reduction in serum triglyceride levels. In patients with hypertriglyceridaemia without diabetes and/or IGT (impaired glucose tolerance), there is evidence of hyperinsulinaemia and insulin resistance [20]. Acarbose may exert its effect on serum triglyceride levels either by a direct action i.e. decreasing chylomicron remnant production through impairment of triglyceride synthesis in the small intestine [21] or through its effects on postprandial serum glucose [22] and insulin levels. It has been suggested that acarbose, both by decreasing hyperglycaemia and hyperinsulinaemia (less pronounced down-regulation of insulin receptors) during the hours following a meal, could improve insulin sensivity [23]. Acarbose acts locally on the intestine and after its administration, only minimal amounts reach the peripheral tissues (only 1–4% of the drug in its unchanged form [24]). In this study when dietary control alone was not capable of reverting triglyceride concentrations to normal, administration of acarbose achieved a significant decrease in mean triglyceride levels in 30 non diabetic subjects with moderate or severe hypertriglyceridaemia.
The action of acarbose is more evident with the second course of treatment, possibly due to better compliance. Acarbose was efficacious both in the moderate and severe forms (group A and group B) and in combined hypertriglyceridaemia. It also resulted in a slight decrease in total cholesterol concentrations, but no significant change in HDL-cholesterol and LDL-cholesterol concentrations. There were no significant changes in BMI with acarbose therapy.
In conclusion acarbose reduced triglyceride levels in non diabetic patients with hypertriglyceridaemias. It did not affect fasting glucose levels or have serious side-effects.
Therapy with acarbose and diet represents another approach to treatment of hypertriglyceridaemia in non diabetics and may have a role in patients who cannot take fibrates or statins because of side-effects.
References
- 1.Puls W, Keup U, Krause HP, Thomas G, Hoffmeister F. Glucosidase inhibition. A new approach to the treatment of diabetes, obesity and hyperlipoproteinaemia. Naturwissenschaften. 1977;64:563–537. doi: 10.1007/BF00483562. [DOI] [PubMed] [Google Scholar]
- 2.Muller L, Puls W. Pharmacology of alpha-glucosidase inhibitors. In: Caspary WF, editor. Structure and function of the small intestine. Amsterdam: Excepta Medica; 1988. pp. 281–300. [Google Scholar]
- 3.Tuomilehto J. Acarbose monotherapy in the treatment of non-insulin-dependent diabetes mellitus-a review. In: Creutzfeldt w., editor. Acarbose for the Treatment of Diabetes Mellitus. Berlin: 2nd International Symposium on Acarbose; 1987. pp. 12–14. Berlin, Springer Verlag 1988: 104-116, November. [Google Scholar]
- 4.Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs. 1988;35:214–243. doi: 10.2165/00003495-198835030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Nestel PJ, Carroll KF, Havenstein N. Plasma triglyceride response to carbohydrates, fats and caloric intake. Metabolism. 1970;19:1–18. doi: 10.1016/0026-0495(70)90112-5. [DOI] [PubMed] [Google Scholar]
- 6.Krause HP, Keup U, Thomas G, Puls W. Reduction of carbohydrate-induced hypertriglyceridaemia in (fa fa) ‘Zucker’ rats by the α-glucosidase inhibitor acarbose (BAY g5421) Metabolism. 1982;31:710–714. doi: 10.1016/0026-0495(82)90202-5. [DOI] [PubMed] [Google Scholar]
- 7.Zavaroni I, Reaven GM. Inhibition of carbohydrate-induced hypertriglyceridaemia by a disaccharidase inhibitor. Metabolism. 1981;30:417–420. doi: 10.1016/0026-0495(81)90125-6. [DOI] [PubMed] [Google Scholar]
- 8.Leonhardt W, Hamfeld M, Fischer S, Schulze J, Spengler M. Beneficial effects on serum lipids in non-insulin dependent diabetics by acarbose treatment. Drug Res. 1991;41:435–438. [PubMed] [Google Scholar]
- 9.Hillebrand I, Boehme K, Frank G. The effects of the α-glucosidase inhibitor BAY g5421 (acarbose) on postprandial blood glucose, serum insulin, and triglyceride levels: Dose-time-response relatioship in man. Res Exp Med (Berl) 1979;175:87–94. doi: 10.1007/BF01851237. [DOI] [PubMed] [Google Scholar]
- 10.Homma Y, Irie N, Yano Y, Nakaya N, Goto Y. Changes in plasma lipoprotein levels during medication with a glucosidase-hydrolase inhibitor (acarbose) Tokai J Exp Med. 1982;7:393–396. [PubMed] [Google Scholar]
- 11.Beaumont JL, Carlson LA, Cooper GR, Fejfar Z, Fredrickson DS, Strasser T. Classification of hyperlipidemias and hyperlipoproteinaemias. Bull Wld Hlth Org. 1970;43:891–915. [PMC free article] [PubMed] [Google Scholar]
- 12.Roschlau P, Bernt E, Gruber W. Enzymatische Bestimmung des Gesamtcholesterins in Serum. Chem Klin Biochem. 1974;12:403–407. [PubMed] [Google Scholar]
- 13.Wahlefeld AW. Triglycerides determination after enzymatic hydrolysis: In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 4. New york: Academic Press; 1974. pp. 1831–1835. [Google Scholar]
- 14.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Virella MF, Stone P, Ellis S, Coltwell JA. Cholesterol determination in high density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 16.Kesaniemi YA, Grundy SM. Influence of gemfibrozil and clofibrate on metabolism of cholesterol and plasma triglycerides in man. Jama. 1984;251:2241–2246. doi: 10.1001/jama.1984.03340410049031. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman Y, Oschry Y, Eisenberg S. Abnormal regulation of LDL receptor activity and abnormal cellular metabolism of hypertriglyceridemic low density lipoprotein: normalization with bezafibrate therapy. Eur J Clin Invest. 1987;17:538–543. doi: 10.1111/j.1365-2362.1987.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 18.Sirtori CR, Franceschini G. Effects of fibrates on serum lipids and atherosclerosis. Pharmacol Ther. 1988;37:167–191. doi: 10.1016/0163-7258(88)90024-1. [DOI] [PubMed] [Google Scholar]
- 19.Bakker-Arkema RG, Davidson MH, Goldstein RJ, et al. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA. 1996;275:128–133. [PubMed] [Google Scholar]
- 20.Zavaroni I, Bonora E, Pagliara M, et al. Risk factors for coronary artery disease in healthy people with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:202–206. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995;18:303–311. [PubMed] [Google Scholar]
- 22.Malaguarnera M, Giugno I, Panebianco MP, Pistone G. Beneficial effects of acarbose on familiar hypertriglyceridemias. Clin Pharmacol Ther. 1998;36:441–445. [PubMed] [Google Scholar]
- 23.Baron A, Neumann C. on behalt of the PROTECT Study Group. PROTECT interim results: a large multicenter study of patients with type II diabetes. Clin Ther. 1997;19:282–295. doi: 10.1016/s0149-2918(97)80116-6. [DOI] [PubMed] [Google Scholar]
- 24.Ahr HJ, Boberg M, Krause HP, et al. Pharmacokinetics of acarbose. Part I. Absorbtion, concentration in plasma, metabolism and excretion after single administration of 14C-acarbose to rats, dogs and man. Arzneimittelforschung/Drug Res. 1989;39:1254–1260. [PubMed] [Google Scholar]


