Abstract
Aims
Voluntary adverse drug reaction (ADR) reporting schemes have operated since the early sixties in many Western countries. It is generally recognized, however, that only a small proportion of ADRs is actually reported. The current survey was conducted to assess attitudes towards reporting of ADRs, and to study which types of ADRs are reported.
Methods
A questionnaire seeking reasons for nonreporting was sent to a random sample of 10% of medical practitioners in The Netherlands in October 1997. After 6 weeks, a reminder was sent to those who had not responded.
Results
One thousand four hundred and forty-two (73%) questionnaires were returned, of which 94% were complete. The percentage of GPs (51%) which had ever reported an ADR to the national reporting centre was significantly higher than the percentage of specialists (35%), who reported more often to the pharmaceutical industry (34%vs 48%). 86% of GPs, 72% of surgical specialists and 81% of medical specialists had ever diagnosed an ADR, which they had not reported. Uncertainty as to whether the reaction was caused by a drug (72%), the ADR being trivial (75%) or too well known (93%) were the most important reasons for not reporting. 18% were not aware of the need to report ADRs, 22% did not know how to report ADRs, 38% did not have enough time, 36% thought that reporting was too bureaucratic and only 26% of Dutch physicians knew which ADRs to report. A serious ADR, an unlabelled ADR, an ADR to a new drug, history of reporting of one or more ADRs, and specialty were all independently associated with reporting of 16 hypothetical ADRs. Surgical and medical specialists tended to report less often than GPs.
Conclusions
There is a considerable degree of underreporting, which might partly be explained by lack of knowledge and misconceptions about spontaneous reporting of adverse drug reactions.
Keywords: adverse drug reaction, spontaneous reporting
Introduction
Voluntary adverse drug reaction (ADR) reporting schemes have operated since the early sixties in many Western countries. These surveillance systems enable physicians and pharmacists to report suspected ADRs and thus act as a tool to identify new ADRs and risk factors predisposing to recognized ADRs.
It is acknowledged that only a small proportion of ADRs are actually reported to national reporting centres and pharmaceutical companies [1]. For example, it has been demonstrated that only 4–7% of episodes of drug-associated anaphylaxis in The Netherlands were reported to the national reporting centre [2, 3].
Insight into reasons for underreporting should enable national reporting centres to take appropriate measures to increase reporting rates. An earlier attitudinal survey of ADR reporting in The Netherlands showed that the lack of availability of report forms and shortage of time were important reasons for nonreporting [4]. Another survey conducted among general practitioners (GPs) revealed that unfamiliarity with the Dutch national reporting centre was the most important reason for not reporting an ADR [5]. In continuation with previous studies on attitudes towards reporting of ADRs [6, 7], the European Pharmacovigilance Research Group (EPRG) initiated this study to further assess attitudes towards reporting of ADRs, and to study the types of ADRs that are reported.
Methods
Setting
The Inspectorate for Health Care maintains a database with the addresses of all health care workers in The Netherlands. From this database all general practitioners (GPs), surgical specialists (surgeons, ear, nose and throat surgeons, eye surgeons, urologists, gynaecologists), and medical specialists (anaesthetists, cardiologists, dermatologists, internists, paediatricians, pulmonary physicians, gastroenterologists, neurologists, psychiatrists, rheumatologists) were selected. All other physicians and all physicians aged 65 years or over were removed from the database. A random sample of approximately 10% was selected from the remaining physicians. Small changes in the order and phrasing of the questions were made after a pilot phase. In October 1997, the final questionnaire was mailed to the random sample and in November 1997 a reminder was sent to those who had not responded.
The questionnaire included questions regarding reasons for nonreporting. The attitudes of physicians regarding adverse drug reaction reporting were assessed with 16 hypothetical ADRs for which the physicians were asked to decide whether they would report these to their national ADR monitoring centre (options: ‘yes’, ‘no’ or ‘not sure’). The 16 hypothetical ADRs concerned different combinations of three options: serious vs nonserious ADR, ADR to new vs established drugs, and an ADR described in the product information vs a newly discovered ADR (labelled vs unlabelled ADR).
Statistical analyses
Comparisons were made with the Chi-square test for discrete variables and with the t-test for continuous variables using SPSS for Windows 7.5 with rejection of the null hypothesis at a value <0.05. In all analyses, the answers to questions left blank were treated as missing.
Conditional logistic regression analysis on the 16 hypothetical questions was performed in SAS, with matching on respondent, to assess the effect on reporting of an ADR to a new drug, of a serious ADR, or of an unlabelled ADR. For this analysis, the outcome was dichotomized: ‘yes’vs other (‘no’ and ‘not sure’) and the effects of reporting determinants were expressed as odds ratios with 95% confidence limits.
The influence of the history of reporting one or more ADRs, gender, age, type of specialization and time since specialization was assessed with a multiple linear regression model in which the total number of confirmative answers (‘yes’) to the 16 hypothetical questions formed the outcome. In the model, age (30–40, 41–50, >50 years), time since specialization (0–10, 11–20, >20), type of specialization (GP, surgical specialist, medical specialist), gender, and history of reporting were entered as categorical values.
Results
A total of 1984 questionnaires were sent. 1442 (72.7%) questionnaires were returned, of which 1357 (94.1%) were complete. The remaining 85 questionnaires were returned blank. All analyses are therefore based on the 1357 completed questionnaires. Of the responders, 1053 (77.6%) were male. The median age was 46 years, and the median time since specialization was 14 years. Responders did not differ significantly from nonresponders with regard to gender, age and time since specialization. Ninety-five percent of medical practitioners who returned the questionnaire were currently engaged in medical practice including the prescription of medicines.
Ninety-eight percentage of GPs, surgical and medical specialists had ever diagnosed an ADR in one of their patients. 85.6% of GPs, 71.9% of surgical specialists and 81.1% of medical specialists had ever diagnosed an ADR, which they had not reported to the national reporting centres or pharmaceutical industry. The percentage of GPs that ever reported an ADR to the national reporting centre was significantly greater than the percentage of specialists. In contrast, medical specialists reported more often to the pharmaceutical industry (Table 1).
Table 1.
Number (%) of medical practitioners who ever reported an ADR to the national reporting centre (NRC) or to the pharmaceutical industry (PI).
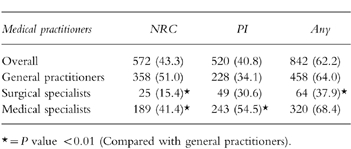
Table 2 lists various possible reasons for not reporting a suspected ADR. As was expected, uncertainty as to whether the reaction was caused by a drug, the ADR being trivial, or too well known, were the three most important reasons for not reporting. Unfamiliarity with the national reporting scheme was also a reason for nonreporting. This was further demonstrated by the observation that only 25.7% of GPs, 23.2% of surgical specialists and 26.2% of medical specialists were familiar with the criteria specifying which ADRs to report. 31.7% of Dutch physicians would not report an ADR if another physician had prescribed the medicine, 27.2% would not report an ADR if the patient had purchased the drug over the counter.
Table 2.
Reasons not to report an ADR.
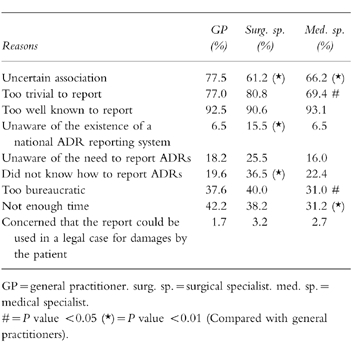
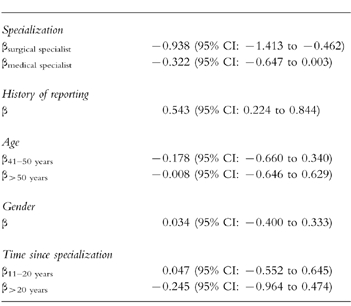
Table 3 shows the assumed reporting rates of the 16 hypothetical ADRs. The table clearly demonstrates that even serious, unlabelled ADRs to new drugs will be substantially under-reported. Conditional logistic regression analysis on the 16 hypothetical ADRs, revealed an odds ratio of reporting of 6.6 (95% CI:6.2–7.1) for a serious ADR, of 2.8 (95% CI: 2.6–2.9) for an unlabelled ADR and 2.4 (95% CI: 2.3–2.6) for an ADR to a new drug. Although the percentage of variability that was explained by regression was small (3%), linear regression on the number, out of 16, of hypothetical ADRs that the respondent would report, showed a significant effect of specialty and history of reporting. Surgical and medical specialists indicated less often than GPs that they would report one of the hypothetical ADRs. Physicians who ever reported an ADR were more inclined to report one of the hypothetical ADRs (Table 4). Age, gender, and time since specialization did not show a significant effect. Entering age and time since specialization as continuous variables did not change the results. Out of the 16 hypothetical ADRs, the national reporting centre would favour reporting of 11 of these. In fact, however, the median number that GPs would report was 5, for surgical specialists it was 3 and for medical specialists it was 4.
Table 3.
Which hypothetical ADRs would you report?
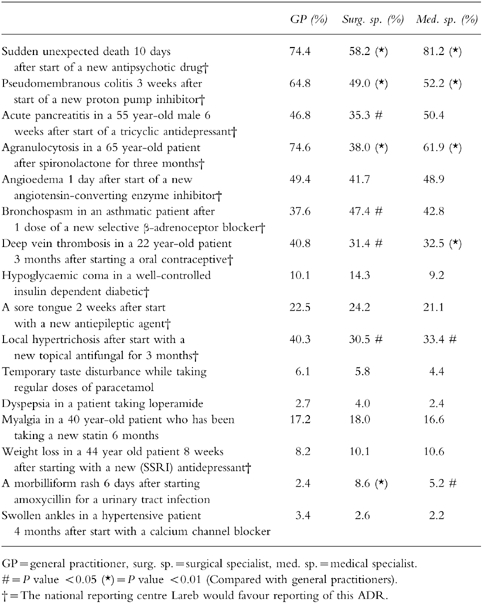
Table 4.
Linear regression coefficients for the number, out of 16, of hypothetical ADRs that the respondent would report.
Discussion
This survey was conducted to assess attitudes of medical practitioners towards the reporting of ADRs. In addition to previous studies, reporting behaviour was studied by analysing reporting percentages of 16 hypothetical ADRs. In 1995 a nationwide regionalized reporting scheme was introduced in The Netherlands. The reporting scheme is coordinated by the national reporting centre Lareb, which advocates the reporting of serious ADRs, unlabelled ADRs, and ADRs to new drugs by professional health care workers.
Seriousness of the ADR, a previously unknown ADR, an ADR to a new drug, type of specialization, history of reporting and certainty as to whether the reaction was caused by a drug were important determinants of reporting. In accordance with earlier studies, a higher percentage of GPs than specialists had ever reported an ADR to the national reporting centres [4, 6]. This phenomenon was further examined by seeking responses to 16 hypothetical ADRs. Corrected for age, gender, time since specialization and a history of reporting an ADR, GPs would report more of these hypothetical ADRs than medical specialists, who in their turn would report more than surgical specialists. Keeping in mind that hypothetical ADRs were used, actual reporting of ADRs might deviate from this pattern. However, analysis of actual ADR reports to the national reporting centre shows that reports from GPs are relatively overrepresented. Medical specialists reported more often to the pharmaceutical industry, possibly because medical specialists are more often involved in clinical trials. In an earlier survey, 25% of respondents ever reported an ADR to the national reporting centre compared with 43% in the present study [4]. Possibly, this increase is related to the introduction of the regionalized reporting centres, as the number of reports increased by approximately 75% after decentralization of the reporting scheme. However 43% is still rather low as one might expect that the vast majority of medical practitioners diagnose a serious ADR, a previously unknown ADR or an ADR to a new drug from time to time.
Over 35% of medical practitioners were of the opinion that reporting of ADRs takes too much time, and that it is too bureaucratic. This might explain why even serious reactions are underreported. This is worrying as the reporting procedure has been simplified as much as possible and the reporting of ADRs is widely regarded as a matter of good medical practice.
Other reasons for not reporting were due to a lack of knowledge: not knowing how to report, not knowing which ADR to report, and even unawareness of the existence of a reporting scheme. This is in spite of the fact that the report form, and instructions for its use, are included in the standard Dutch pharmacotherapy handbooks. This lack of knowledge was greatest among surgical specialists and might be overcome by a recently started information campaign which focuses on specialists. Knowledge about reporting has not increased in the last few years as a previous survey in 1993 showed that 13% of respondents did not know how to report an ADR vs 16% in 1997 [4].
Although the response rate was fairly high at 73%, nonresponse may have influenced the results of our survey. The actual percentage of medical doctors that ever reported an ADR might be somewhat lower, as nonresponders may be less prone to report ADRs than responders. Lack of knowledge regarding reporting procedures and criteria will probably be greater among nonresponders.
In conclusion, our survey suggests that even serious ADRs are underreported. This may partly be explained by lack of knowledge and misconceptions regarding the spontaneous reporting of adverse drug reactions. We believe that education on pharmacovigilance issues and the importance of reporting should be more extensively incorporated in medical training. In addition, regular communication to health care workers explaining reporting procedures and criteria may increase reporting rates of serious ADRs, unlabelled ADRs, and ADRs to new drugs.
Acknowledgments
This study was financially supported by grants from the European Commission (Biomed program, contract number BMH4/CT95/ 0467), the Dutch Medicines Evaluation Board and the Dutch Inspectorate for Health Care. Their support and the cooperation of the respondents is gratefully acknowledged.
References
- 1.Rogers AS, Israel E, Smith CR, et al. Physician knowledge, attitudes, and behavior related to reporting adverse drug events. Arch Intern Med. 1988;148:1596–1600. [PubMed] [Google Scholar]
- 2.Van der Klauw MM, Stricker BHCh, Herings RM, Cost WS, Valkenburg HA, Wilson JH. A population based case-cohort study of drug-induced anaphylaxis. Br J Clin Pharmacol. 1993;35:400–408. doi: 10.1111/j.1365-2125.1993.tb04157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stricker BHCh, de Groot RR, Wilson JH. Glafenine-associated anaphylaxis as a cause of hospital admission in The Netherlands. Eur J Clin Pharmacol. 1991;40:367–371. doi: 10.1007/BF00265845. [DOI] [PubMed] [Google Scholar]
- 4.Van Riemsdijk MM, Herings RM, Rawlins MD, Stricker BHCh. Redenen om wel of niet bijwerkingen van geneesmiddelen te melden bij het landelijke meldingssysteem in Nederland. Ned Tijdschr Geneeskd. 1995;139:2306–2308. [PubMed] [Google Scholar]
- 5.Verduijn MM, Höppener P, Pop P, Heeringa M. Meldgedrag in de eerste lijn. Pharm Weekbl. 1998;133:786–790. [Google Scholar]
- 6.Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. The European Pharmacovigilance Research Group. Eur J Clin Pharmacol. 1997;52:423–427. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- 7.Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39:223–226. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


