Abstract
Aims
Ulcerative colitis is predominantly a disease of nonsmokers and transdermal nicotine has therapeutic value in active disease; however side-effects are troublesome. The aim of this study was to develop an oral formulation of nicotine which would be slowly released in the colon over 6 h, and to examine its pharmacokinetic profile in 12 healthy volunteers, with measurements of serum nicotine and cotinine, its principal metabolite.
Methods
Nicotine was combined with a polyacrylic carbomer, Carbopol 974P which was incorporated into 13 different vehicles and their release profiles examined in vitro. The polyglycolized glyceride, Gelucire 50/13, was chosen for subsequent kinetic studies because it consistently produced a suitable release pattern which was linear. Capsules containing 3 mg nicotine, combined with carbomer in Gelucire 50/13, were coated with an acrylic resin Eudragit L; this ensured the capsule would remain intact until the ileum. On 2 separate days, 6 and 15 mg nicotine, contained in 2 and 5 capsules, respectively, were administered to 12 subjects, all nonsmokers, mean age 28 years. Serial blood measurements were taken for 36 h, serum nicotine and cotinine concentrations were measured by gas liquid chromatography.
Results
There was considerable intersubject variability in the nicotine and cotinine values. Plasma nicotine levels began to rise about 4 h after ingestion of the capsules, corresponding with the oro-caecal transit time. Cmax nicotine values were 2.2 and 5 ng ml−1, obtained 7 h after the ingestion of 6 and 15 mg, respectively, of the formulation. The corresponding Cmax values for cotinine were 37 and 94.4 ng ml−1, occurring after 9–10 h. The mean for elimination half-lives in the 24 studies, including the 6 and 15 mg doses, for nicotine were 4.3±2.7 h and for cotinine 16.8±7.5 h. With 6 mg nicotine-carbomer, only 1 of 12 volunteers had possible side-effects, but with the 15 mg dose 11 out of the 12 reported adverse effects which were systemic or gastrointestinal in nature—their timing corresponded with peak serum concentrations of nicotine.
Conclusions
An oral formulation of nicotine has been developed; in the ileum and colon, this becomes available for slow linear release over 6 h and delivers high concentrations of nicotine for topical effect on the colon. 6 mg Nicotine was well tolerated, whilst 15 mg gave both systemic and gastrointestinal side-effects. High concentrations of topical nicotine in the colon are achieved with relatively low systemic bioavailablity—reflected by the Cmax and AUC values for nicotine. This, or comparable formulations, may be of therapeutic value in ulcerative colitis.
Keywords: carbomers, drug formulation, nicotine, ulcerative colitis
Introduction
Whilst ulcerative colitis (UC) is predominantly a disease of nonsmokers [1–5], current smokers have relatively inactive disease and intermittent smokers find that their symptoms improve whilst smoking [6, 7]. Controlled trials have shown that transdermal nicotine added to conventional therapy has a beneficial effect in active UC [8, 9]. Since side-effects with transdermal nicotine occur in up to two thirds of patients, particularly in lifelong nonsmokers, we have developed an oral nicotine preparation in which nicotine was combined with a polyacrylic carbomer [10]. We have previously reported observations on an enema formulation of nicotine carbomer containing 6 mg nicotine [11, 12]. The carbomer adheres to colonic mucosa [13, 14] and slows the rate of nicotine absorption, giving a high sustained concentration of nicotine at the disease site with relatively low systemic levels, due to rapid conversion to its major metabolite cotinine on first pass through the liver [15]. The enema was well tolerated in a pharmacokinetic study [11], and appeared beneficial in 22 patients with active UC when given for a month in addition to conventional therapy [12].
The oral formulation of nicotine carbomer has been designed to give sustained release of nicotine in the distal ileum and colon. The release pattern was first established by in vitro dissolution tests, followed by pharmacokinetic studies of 6 mg and 15 mg nicotine given to 12 healthy volunteers with serial measurements of serum nicotine and cotinine.
Methods
Nicotine carbomer
A freeze dried powder was prepared which contained 3 mg of l-nicotine combined with 40 mg of Carbopol 974P (Goodrich UK). Ten grams of carbomer were slowly sieved into 450 ml of rapidly stirred deionized water to produce a uniform colloidal dispersion, to which 750 mg nicotine was added. This was then stirred for 45 min before adjusting the volume to 500 ml. The gel which formed was deep-frozen and then freeze dried for 24 h to produce a material which was homogenized into a fine powder and passed through a 250 micron sieve. Recovery of nicotine from the two batches, used in the study, was 93% and 96% by spectrophotometry.
Formulation of the capsules
Nicotine carbomer has a molecular weight of about 3 million, is hydrophilic and its release pattern may be controlled by its incorporation into sustained release vehicles. Maximum loading of these vehicles with carbomer should not exceed 40% since this irreversibly alters the vehicles thermodynamic character. Size 2 hard gelatin capsules were used which hold about 400 mg, ±7.5% uniformity of weight, of the vehicle with 43 mg nicotine-carbomer. Capsules were later coated with an acrylic resin, Eudragit L.
A series of 13 different oral formulations (Table 1) were prepared and their in vitro dissolution profiles determined. Most of them were polyglycolized glycerides (Gelucire–Alpha Chemicals, UK) with different hydrophilic/lipophilic properties and melting points. The vehicle was melted in a water bath, and nicotine-carbomer powder incorporated. The molten mixture was then dispensed into capsules using an oral syringe, allowed to cool, and stored in a cool place. This was done with each formulation except the lactose/starch vehicle; capsules were filled manually. Five capsules of each formulation were made initially and a total of 13 nonsink dissolution tests performed. The Erweka Flow Through Cell Tester Model DF21 (Copley, UK) was used for the first two tests, but was then replaced by the Erweka Tablet Dissolution tester Model DT80 (Copley, UK) for the remaining 11; this was because the small cell volume of Model DF21 with its fine gauze and glass bead filtration mechanism became blocked with gel produced by the released nicotine-carbomer. Model DT80 depends on continuous sampling for measurement of nicotine released into a container of 500 ml of fluid which was stirred constantly, conforming to the standard British Pharmacopoeal method [16].
Table 1.
Physical properties of 13 possible vehicles for formulations of nicotine examined for their dissolution profile at pH 6 8. HLB values refer to the hydrophilic/lipophilic balance of the vehicle—the extremes of 14 and 1 are, respectively, very hydrophilic and lipophilic.

The ultraviolet spectrum for nicotine gave peaks at 259 nm, aqueous acid, and 261 nm, aqueous alkali. Serial dilutions were prepared and a standard calibration curve produced as part of a software package for the dissolution programme (Hewlett Packard HP89550 A for the HP8452 A Diode Array Spectrophometer). This package controlled the sampling, measurements, data processing, and documentation of reports for the dissolution tests. Capsule sinkers were used and the pH of the dissolution media was initially varied between 2 and 7.2. Each dissolution run was 6 h.
The carbomer and Gelucire products interfered with the nicotine spectrum during capsule dissolution. Because of this control capsules of carbomer and Gelucire were included in the later runs to subtract their background absorption values from the nicotine-carbomer dissolution record. Standard apparatus for the dissolution of tablets and capsules [16] was used in two sets of studies; capsules containing the nicotine carbomer in gelucire were compared with carbomer and gelucire alone and no significant difference was identified in 10 paired observations. The chosen formulation should ideally provide a linear release of nicotine over at least 6 h. Initial studies focused on release profiles of the nicotine-carbomer from the vehicle and those without a linear release were not pursued; these included the lactose/starch, hard fat–Witepsol, white beeswax, and Gelucires 46/07, 54/02, 62/05 and 64/02. Subsequent studies compared PEG 4000, and the Gelucires 48/09, 44/14, 42/12, 50/13 and 53/10 against controls. These experiments were all performed at pH 6.8 since earlier studies had shown no significant difference in dissolution profiles at other pHs.
Figure 1 shows the different release profiles over 6 h for nicotine in Gelucire 50/13, 44/14 and 53/10; Gelucire 50/13 was chosen for subsequent kinetic studies because it most consistently met the linear release pattern required. The percentage dissolution values were calculated by subtracting control from the active capsule values to obtain the rate and duration of nicotine release from the matrix. Nicotine carbomer is released from the vehicle, but measurements were made of free nicotine released from the carbomer complex. After an initial lag period of 60–90 min, the vehicle released significant quantities of nicotine carbomer (Figure 1). The outer capsule coating of Eudragit L with an optimum pH dissolution profile of 6.8, was chosen to ensure it would remain intact until the ileum, where it would begin to disintegrate, exposing the inner matrix.
Figure 1.

The in vitro dissolution profile, over 6 h, of nicotine carbomer in three different vehicles—Gelucire 50/13, 44/14 and 53/10, showing differing patterns of release.
The manual system used to fill capsules for each experimental formulation was also used to produce a batch of capsules using Gelucire 50/13, for the pharmacokinetic study. Sixty-six capsules were prepared and the dissolution profile confirmed (Figure 2). The results conform to the 1998 British Pharmacopoeia standard for the uniformity of content in capsules [17].
Figure 2.

The in vitro dissolution of nicotine carbomer in Gelucire 50/13 from four separate capsules and their mean values at time points over 6 h.
A modified air suspension technique was used to coat the capsules [18]; they were placed in a glass dome and swirled around the wall by a jet of compressed air injected at the base of the bench scale apparatus. The volatile coating solution was injected through a second port and vaporized by the air flow to coat capsules evenly. Dummy capsules were also coated for disintegration tests. Eudragit L, 3% solution, also containing 0.75% w/w of diethyl phthalate as a plasticiser, in a volatile solvent was used to coat the 66 capsules.
The dummy capsules passed the British Pharmacopoeia 1998 standard enteric coated tablet disintegration test which requires the formulation to remain intact at pH 2.0 for 2 h, and then disintegrate within 30 min at pH 6.8 [19]. The nicotine content of capsules was verified by dissolution of the contents of two capsules in dilute hydrochloric acid overnight—assays of the two solutions [20] gave estimates for nicotine content of 3.01 and 2.75 mg.
Pharmacokinetic study
Subjects
Twelve normal healthy volunteers, all nonsmokers—3 ex-smokers and 9 lifelong nonsmokers took part; 7 were male, mean age 28, range 21–33 years, and average body weight was 73 kg, range 57–96, (Table 2). All gave written informed consent and the study was approved by the ethics committee of Bro Taf Health Authority. The Medicines Control Agency also approved the product manufacture and the study design. They all had a satisfactory physical examination with normal biochemical and haematological profiles. Exclusion criteria for the study were—a history or evidence of significant medical or psychiatric disease, previous gastrointestinal resection, known sensitivity to nicotine, pregnancy or lactation, current participation in other studies and subjects who had been prescribed medication within the last month.
Table 2.
Characteristics of the 12 subjects who participated in the study. Nature, severity and time of side-effects that occurred after the 15 mg oral dose. Period during which side-effects were present and the number of bowel movements are given in hours after ingestion of nicotine.

Drug administration and blood sampling protocol
The initial dose was 6 mg nicotine contained in two capsules; the dose was chosen because it was well tolerated in the enema formulation. When these results had been reviewed and at least a month later the study was repeated in the same group with 15 mg—5 capsules.
The capsules were taken after a 10 h overnight fast and venous blood sampled from an indwelling cannula at 0, 3, 4, 5, 6, 7, 8, 9, 10, 12, 18, 24 and 36 h. Serum was obtained by centrifugation for 20 min and stored at −20° C until analysis of cotinine and nicotine levels by gas liquid chromatography [20]. During the study subjects were allowed to mobilize and encouraged to eat their normal diet. Bowel habit was recorded throughout the study.
Pharmacokinetic analysis
Time concentration curves were generated from the data, peak plasma concentration (Cmax) and concentration peak times (tmax) were derived directly from the original variables. The area under the concentration time curves, ng.hr ml−1, from 0 to 36 h (AUC(0,36h)) was calculated by the trapezoidal method. For the nicotine data, the area under the curve from zero to infinity (AUC(0,∞)) was calculated also by the trapezoidal rule and extension of the linear terminal slope. The area under the curve was also calculated using a one compartmental model (AUC(1co)). Half-lives were determined by extending the terminal log linear decline phase. The pharmacokinetic analysis was performed by the Siphar, Simed France, computer programme.
Side-effects
The nature, severity and time of any side-effects experienced by the subjects were recorded.
Results
Pharmacokinetics
Concentration-time curves of serum nicotine and cotinine levels are plotted for each individual (Figure 3) with mean values (Figure 4) for both the 6 mg and 15 mg doses of nicotine carbomer. The calculated pharmacokinetic parameters are in Table 2.
Figure 3.
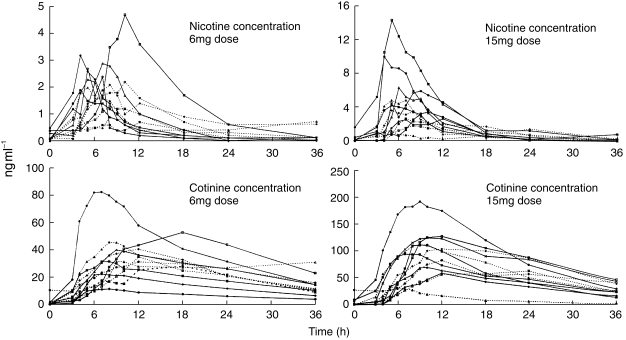
The time-concentration curves for nicotine and cotinine in 12 healthy volunteers over 36 h after the ingestion of 6 mg and 15 mg nicotine carbomer. There is a wide intersubject variation in concentrations, in all four graphs the same symbol is used for each subject, for example subject 3 is represented by —▴—.
Figure 4.

Mean time-concentration curves for nicotine and cotinine calculated from the individual values over 36 h, in the 12 healthy volunteers.
Mean maximum concentrations of nicotine 2.2 and 5 ng ml−1, were obtained about 7 h after the ingestion of 6 and 15 mg, respectively, of oral nicotine carbomer. The corresponding Cmax values for cotinine, the principal metabolite of nicotine were 37 and 94.4 ng ml−1 which occurred after 9–10 h. The means for elimination half lives in the 24 studies, including the 6 and 15 mg doses, for nicotine were 4.3±2.7 h and cotinine 16.8±7.5 h. The mean AUCs when extrapolated to infinity and fitted to a one compartmental model with the 6 mg dose were somewhat distorted by one subject who had higher nicotine concentrations at 36 compared with 24 h (Figure 3).
There was considerable intersubject variability in the nicotine and cotinine values, reflected in the standard deviations seen in Table 3. Two volunteers, subjects 3 and 5 (Table 2) had very low levels of both nicotine and cotinine after the 15 mg dose suggesting little release from the capsules. After further questioning, subject 5 later admitted to a viral infection with diarrhoeal symptoms at the time of the study. It was decided to repeat the study with the 15 mg dose in these two subjects. The resulting nicotine and cotinine values were almost identical for subject 3 and her original data were used in the final analysis. Subject 5 had higher values on the second occasion, suggesting that the initial poor absorption was due to a rapid gastrointestinal transit time.
Table 3.
Pharmacokinetic variables after administration of a single oral dose of 6 mg and 15 emsp14;mg nicotine carbomer to the same 12 healthy normal volunteers on 2 separate occasions.

Side-effects
After the 6 mg dose, only 1 of the 12 volunteers reported any possible side-effects, with mild lower abdominal discomfort which began 8 h after ingestion and lasted 45 min, subject 1 (Table 2). With the 15 mg dose, 11 of the 12 reported adverse effects (Table 3); the remaining subject—number 3, had persistently low levels of nicotine and cotinine on two occasions. These side-effects were mainly gastrointestinal, consisting of nausea (5), lower abdominal pain (7), bloating (4), urgency (5) and frequency of defaecation with loose bowel motions (2); three felt light-headed. Symptoms occurred between 2 and 12 h after administration but in most subjects were after 4 h, corresponding with the release of nicotine in the colon. The duration of any symptom was 3 h or less. Five subjects had 4 or more loose stools during the study period; the timing of defecation in most subjects corresponded with the recorded occurrence of side-effects.
Discussion
This novel oral formulation was designed to produce sustained release of nicotine in the ileum and colon. It was made possible by a combination of features; Eudragit L was used as a capsule coating for the contents which consisted of a polyglycolized glyceride vehicle containing the polyacrylic carbomer combined with nicotine. The Eudragit coating delays exposure of the capsule contents until the ileum is reached. The vehicle then releases the drug in a sustained manner over at least 6 h, whilst the carbomer produces mucoadhesion [13] with release of nicotine at the epithelial surface. The combination has proved useful for the delivery to the colon of other drugs, such as lignocaine [21]. In vitro tests with the capsule contents show a consistent release pattern over 6 h when the nicotine-carbomer is contained in Gelicure 50/13. The considerable variation in the time-concentration curves of nicotine and its major metabolite cotinine, is probably due to a combination of differences in gastric emptying, small bowel and colonic transit times and in rates of nicotine metabolism. Plasma nicotine levels began to rise about 4 h after oral ingestion of the capsules, corresponding with the time taken to reach the terminal ileum; the release then continues over the subsequent 8 h. Maximum concentrations of cotinine are achieved after 7–10 h after administration with appreciable levels still present at 36 h. With the 6 mg dose of nicotine, only 1 of 12 volunteers possibly had side-effects, but with the 15 mg dosage 11 out of the 12 reported adverse effects which were gastrointestinal or systemic. The timing of these side-effects corresponded with the peak serum concentrations of nicotine.
The design of the study was simply to examine two doses of a formulation which released nicotine slowly avoiding troublesome side-effects associated with more rapid release; previous work with oral nicotine [22, 23] has shown a high incidence of intolerance. The findings are probably valid since frequent blood measurements were taken for 36 h and the same 12 subjects were used to study the levels with both doses; this minimizes the effect of intersubject variation which is known to occur with nicotine metabolism [13]. The regular measurement of cotinine concentrations is also an important feature of any pharmacokinetic study of nicotine; it is the major metabolite with a much longer half life than nicotine −18 h compared with a mean of 2 h for nicotine [24, 25]. It therefore gives a better representation of overall nicotine exposure. The formulation was stable at both room and body temperature and all measurements of nicotine and cotinine were performed at an internationally recognized laboratory—the average coefficient of variation for these measurements over the range 1–100 ng ml−1 nicotine and 1–1000 ng ml−1 cotinine is 3.9% and 2.1% respectivley [20].
Pharmacokinetic studies have been performed with nicotine in chewing gum, transdermal patches, tablets and enemas. After smoking a cigarette, serum levels of nicotine may increase from between 5 and 30 ng ml−1, depending on how it is smoked, with peak levels achieved soon after cessation [25]. We found that 51 current smokers with ulcerative colitis had well controlled disease and a median cotinine level of 180 ng ml−1, range 20–453 ng ml−1 [7], consistent with smoking a mean of 15 cigarettes daily. In the trials of transdermal nicotine in UC, where significant clinical benefit was observed, mean serum nicotine levels achieved were 8±7 ng ml−1 and 11±8 ng ml−1; cotinine values were 120±98 ng ml−1 and 192±95 ng ml−1 with patch doses of 25 and 22 mg nicotine, respectively [8, 9]. Nicotine gum (4 mg) gives peak nicotine levels about 9 ng ml−1 after 30 min [25]. Nicotine bitartrate, 4 mg, gives peak serum nicotine concentrations of 7.5 ng ml−1 1 h after ingestion [22], whilst 3 mg and 6 mg nicotine tartrate coated with Eudragit, delayed release formulation, gave mean Cmax nicotine of 7.3 and 10.1, respectively, ±3.5 ng ml−1 with a tmax of about 5 h [23]. Side-effects of nausea, vomiting, light-headedness tremor or diaphoresis were experienced by 3 of 10 subjects with 3 mg and 7 of 10 for the 6 mg dose but no reference was made to the occurrence of colonic symptoms.
We have previously developed an enema formulation combining 6 mg nicotine with 400 mg carbomer in a 100 ml liquid enema; the pharmacokinetic profile for this enema was similar in healthy volunteers and patients with active UC [11]. Mean peak serum concentrations of nicotine and cotinine in the whole group were 8 ng ml−1 and 60 ng ml−1, respectively, achieved after a median of 1 and 4 h. Higher levels, and therefore more side-effects, were seen in four females of low body weight who were lifelong nonsmokers. In a separate study, 24 healthy volunteers had liquid enemas of nicotine tartrate, 45 μg kg−1 body weight of nicotine tartrate, approximately 3 mg; the Cmax nicotine was between 2 and 3 ng ml−1 and 19 had side-effects [26]. Both the nicotine carbomer and nicotine tartrate enemas appeared to have clinical benefit in open studies [12, 27].
In the clinical context it is important that any formulation should be well tolerated. One would expect the bioavailability of nicotine absorbed in the distal small bowel and colon to be low since most will be converted to cotinine on first pass through the liver [15]; mean Cmax for nicotine after a 15 mg dose was only 5±3.8 ng ml−1. Repeated measurements of cotinine are particularly valuable in such studies. In total, 10 of the 12 subjects had side-effects with 15 mg and although some of their symptoms were similar to those noted with nicotine administered in other forms, most of our subjects also developed gastro-intestinal effects suggesting a local irritant effect. Cigarette smoking is known to affect oesophageal and gastric motility [28, 29], and whilst the mouth–caecum time appears to be delayed with acute cigarette smoking [30], habitual smoking has no chronic effect [31]. Nicotine taken as a cigarette or as nicotine gum, initially stimulates, and then inhibits colonic motility [32]. Colonic-transit time appears to be significantly shorter in nonsmoking males compared with smokers, although this relationship was not observed in females, perhaps because the phase in the menstrual cycle was important [33]. Low serum concentrations and AUCs for both nicotine and cotinine were noted in two subjects after the 15 mg dose. This could be due to rapid intestinal transit, delayed gastric emptying or small bowel transit; subject 5 had reported loose stools the day prior to the day he had the medication. The serum levels after the second administration were still low for subject 3. Wide intersubject variation in serum nicotine and cotinine levels is well recognized; in this study differences in transit times would only be part of the reason since the fraction metabolized from nicotine to cotinine varies in healthy volunteers between 55% and 92%, mean 72% [34].
Ileo-colonic release of nicotine brings high doses to the disease site in ulcerative colitis and reduces systemic exposure to nicotine. There is however, a possible problem due to local colonic irritation with higher concentrations of 15 mg nicotine carbomer. The intersubject variability in levels of nicotine, together with the more rapid colonic transit in active ulcerative colitis require this group to be studied separately, probably with doses of 6 mg. If the therapeutic effect of nicotine in UC is due to a local effect in the colon, the formulation may be of clinical value in doses which do not produce local colonic irritation or systemic side-effects because of the relatively high concentrations of nicotine released in the colon. Topical preparations of nicotine will need to be evaluated by clinical trial to establish their value in patients with inflammatory bowel disease.
Acknowledgments
This project, with the salary for Dr John Green, was funded through the Gastro-Intestinal Foundation Trust.
We gratefully acknowledge statistical help from Dr Robert Newcombe, Department of Medical Computing and Statistics, University of Wales College of Medicine, Cardiff and the assistance of Janice Sharp from the Department of Media Resources, University Hospital of Wales, Cardiff. Andrew Mitchell from Alpha Chemicals Ltd, Bracknell, Berkshire supplied and gave advice on the use of Gelucire products. We are also grateful to Professor Philip Routledge, Department of Clinical Pharmacology and Therapeutics, University Hospital of Wales, Cardiff for his advice.
Through a collaborative agreement, the work is linked with the Mayo Clinic, Rochester, Minnesota where Dr W.J. Sandborn of the Department of Gastroenterology is the principal collaborator.
References
- 1.Harries AD, Baird A, Rhodes J. Non smoking: a feature of ulcerative colitis. Br Med J. 1982;284:706. doi: 10.1136/bmj.284.6317.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jick H, Walker AM. Cigarette smoking and ulcerative colitis. N Engl J Med. 1983;308:261–263. doi: 10.1056/NEJM198302033080507. [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Koepsell TD, Perera DR, Inui TS. Risk of ulcerative colitis among former and current cigarette smokers. N Engl J Med. 1987;316:707–710. doi: 10.1056/NEJM198703193161202. [DOI] [PubMed] [Google Scholar]
- 4.Logan RFA, Edmond M, Somerville KW, Langman MJ. Smoking and ulcerative colitis. Br Med J. 1984;288:751–753. doi: 10.1136/bmj.288.6419.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34:1841–1854. doi: 10.1007/BF01536701. [DOI] [PubMed] [Google Scholar]
- 6.Rudra T, Motley R, Rhodes J. Does smoking improve colitis? Scand J Gastroenterol. 1989;170(Suppl):61–63. doi: 10.3109/00365528909091354. [DOI] [PubMed] [Google Scholar]
- 7.Green JT, Rhodes J, Ragunath K, et al. Clinical status of ulcerative colitis in patients who smoke. Am J Gastroenterol. 1998;93:1463–1467. doi: 10.1111/j.1572-0241.1998.00464.x. [DOI] [PubMed] [Google Scholar]
- 8.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Tremaine W, Offord KP, et al. Transdermal nicotine for mildly to moderatley active ulcerative colitis, a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Carbomer Monograph in The British Pharmacopoeia published by HMSO London on behalf of The British Pharmacopoeial Commission. 1993;1:112–113. [Google Scholar]
- 11.Green JT, Thomas GAO, Rhodes J, et al. Pharmacokinetics of nicotine carbomer enemas: a new treatment modality for ulcerative colitis. Clin Pharmacol Ther. 1997;61:340–348. doi: 10.1016/S0009-9236(97)90167-3. [DOI] [PubMed] [Google Scholar]
- 12.Green JT, Thomas GAO, Rhodes J, et al. Nicotine enemas for active ulcerative colitis—a pilot study. Aliment Pharmacol Ther. 1997;11:859–863. doi: 10.1046/j.1365-2036.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutton DA, Pearson JP, Allen A, Foster SNE. Mucolysis of the colonic mucus barrier by fecal proteinases: Inhibition by interacting polyacrylate. Clin Sci. 1990;78:265–271. doi: 10.1042/cs0780265. [DOI] [PubMed] [Google Scholar]
- 14.Ch’ng Seng H, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery II. synthesis and evaluation of some swelling, water insoluble bio-adhesive polymers. J Pharmacol Sci. 1985;94:398–406. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL, Jacob III, Jones RT, Rosenborg J. Individual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221:368–372. [PubMed] [Google Scholar]
- 16.British Pharmacopoeia; 1998. Dissolution test for tablets and capsules (Dissolution test for solid dosage forms) p. 2. Appendix XII D A189. [Google Scholar]
- 17.British Pharmacoepia: 1998. Uniformity of content for capsules; p. 2. Appendix XII H. [Google Scholar]
- 18.Ekberg L, Kallstrand G. Magsaftresistant dragering av operkulatkapslar i recepturskala. Svenk Farm Tidskr. 1972;74:375–378. [Google Scholar]
- 19.British Pharmacopoeia; 1998. Disintigration test for enteric coated tablets; 2 pp. Appendix XII B A187 1998. [Google Scholar]
- 20.Feyerabend C, Russell MAH. A rapid gas-liquid chromatographic method for the determination of nicotine and cotinine in biological fluids. J Pharm Pharmacol. 1990;42:450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans BK, Ranshaw C, Rhodes P, Green JT, Fuller G, Rhodes J. A new presentation of lignocaine for bowel disease. Eur Hosp Pharm. 1998;4 [Google Scholar]
- 22.Benowitz NL, Kuyt F, Jacob P, III, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Jacob P, Denard C, Jenkins R. Stable isotope studies of nicotine kinetics and bioavailability. Clin Pharmacol Ther. 1991;49:270–277. doi: 10.1038/clpt.1991.28. [DOI] [PubMed] [Google Scholar]
- 24.Compton RF, Sandborn WJ, Lawson GM, et al. A dose ranging study of nicotine tartrate following single dose delayed-release oral and intravenous administration. Aliment Pharmacol Ther. 1997;11:865–884. doi: 10.1046/j.1365-2036.1997.00236.x. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and chewing gum. Clin Pharmacol Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 26.Zins BJ, Sandborn WJ, Mays D, et al. Pharmacokinetics of nicotine tartrate after single dose liquid enema, oral and intravenous administration. J Clin Pharmacol. 1997;37:426–436. doi: 10.1002/j.1552-4604.1997.tb04320.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Tremaine WJ, Leighton JA, et al. Nicotine tartrate enemas for mildly to moderately active left-sided ulcerative colitis unresponsive to first line therapy: a pilot study. Aliment Pharmacol Ther. 1997;11:663–671. doi: 10.1046/j.1365-2036.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- 28.Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4–10. doi: 10.1136/gut.31.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott Am Kellow JE, Shuter B, Prescott B, Nolan JM, Hoschi R. How does cigarrette smoking delay solid and liquid gastric emptying? Aust NZ J Med. 1990;20:371–376. [Google Scholar]
- 30.Scott AM, Kellow JE, Eckersley GM, Nolan JM, Jones MP. Cigarette smoking and nicotine delay post prandial mouth-cecum transit time. Dig Dis Sci. 1992;10:1544–1547. doi: 10.1007/BF01296500. [DOI] [PubMed] [Google Scholar]
- 31.Scott AM, Kellow JE, Shuter B, Nolan JM, Hoschl R, Jones MP. Effects of chronic smoking on upper gastrointestinal motor function. J Nucl Med. 1990;31:801. [Google Scholar]
- 32.Jameson JS, Rogers J, Raimundo AH, Feyerabend C, Henry MM, Misiewicz JJ. The effect of nicotine and its metabolites on the distal colon. Gastroenterology. 1993;104:527. [Google Scholar]
- 33.Meier R, Beglinger C, Dederding JP, et al. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motility. 1995;7:235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz NL, Jacob P, III, Jones RT, Rosenborg J. Individual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221:368–372. [PubMed] [Google Scholar]


