Abstract
Aims
The prevalence of hyperuricaemia and gout increases with age as does the incidence of adverse effects to allopurinol, the major uric acid lowering drug. The present study was performed to compare the disposition and effects of allopurinol and its active metabolite oxipurinol in elderly and young subjects without major health problems.
Methods
Ten elderly (age range 71–93 years) and nine young subjects (24–35 years) received an oral dose of 200 mg allopurinol in an open, single dose, cross sectional design. Four of these individuals were additionally dosed with 200 mg allopurinol intravenously. Plasma and urine concentrations of allopurinol, oxipurinol, hypoxanthine, xanthine, and uric acid were measured by h.p.l.c.
Results
Total clearance of allopurinol was not different in elderly (15.7±3.8 ml min−1 kg−1, mean±s.e. mean) and young subjects (15.7±2.1), whereas total clearance of oxipurinol was significantly reduced in the aged (0.24±0.03) compared with young controls (0.37±0.05) as was the distribution volume of oxipurinol (0.60±0.09 and 0.84±0.07 l kg−1, respectively). Oxipurinol was eliminated primarily by the kidneys, allopurinol by metabolism. Fractional peroral bioavailability of allopurinol was 0.81±0.16 (n = 4, two elderly and two young subjects). Although maximal plasma concentrations of oxipurinol were significantly higher in elderly (5.63±0.83 μg ml−1) than in young persons (3.75±0.25) as was the area under the oxipurinol plasma concentration-time curve, AUC (260±46 and 166±23 μg ml−1 h, respectively), the pharmacodynamic effect of oxipurinol was smaller in elderly than young subjects (time-dependent decrease of plasma uric acid 83±30 μg ml−1 h in elderly compared with 176±21 in young controls). Oxipurinol increased the renal clearance of xanthine, suggesting inhibition of tubular xanthine reabsorption by oxipurinol.
Conclusions
Although allopurinol elimination is not reduced in the aged, that of its active metabolite oxipurinol is because of an age-dependent decline in renal function. Xanthine oxidase inhibition by oxipurinol appears to be reduced in old age. In addition to its uricostatic action, oxipurinol has a xanthinuric effect which is also diminished in the elderly.
Keywords: allopurinol, elderly, oxipurinol, pharmacodynamics, pharmacokinetics, uric acid
Introduction
Allopurinol, a structural analogue of hypoxanthine, is used primarily in the treatment of hyperuricaemia and gout. Due to its inhibition of xanthine oxidase (E.C.1.2.3.2.), the enzyme that catalyses the production of xanthine from hypoxanthine and of uric acid from xanthine, both the plasma level of uric acid and its renal excretion are reduced. Allopurinol is not only a blocker but also a substrate of xanthine oxidase, the oxidized metabolite, oxipurinol or alloxanthine, is also a potent inhibitor of xanthine oxidase [1, 2]. Recently, a number of new indications for allopurinol have emerged, for instance prevention of tissue damage due to free radical formation, prevention of urinary stones, and treatment of protozoal diseases [3].
Gout mainly affects adult men and is usually first diagnosed in the fifth decade of life, in women gout is a disease of the postmenopausal period. On the whole, the prevalence of gout increases with age, possibly because of changes in body composition, renal excretory function, and treatment with diuretics [4]. In addition, risk factors for crystal deposition, for instance degenerative joint diseases and osteoarthritis, increase with advancing age [5]. But although gout is an increasingly recognized problem in the elderly [6], both the pharmacokinetics and pharmacodynamics of allopurinol, the most frequently used uric acid lowering drug, have not been examined in the aged. This shortcoming is all the more regrettable as drug treatment in the elderly poses special problems in general [7] and the toxicity to allopurinol in particular was reported to increase with age [6]. The present study addresses the fate and function of allopurinol in elderly in comparison to young individuals.
Methods
Subjects and study protocol
The elderly study participants (age 71–93 years) were patients of the Wiener Allgemeines Krankenhaus (Vienna General Hospital, University of Vienna Medical School) who were referred to the clinic for symptoms such as bronchitis, angina pectoris, or degenerative bone or joint disease. None had insufficiencies of the heart, lung, liver or kidneys, or signs of oedema, dehydration, or recent weight changes. Other exclusion criteria were chronic inflammatory bowel disease, diarrhoea, status post gastric or intestinal surgical procedures, disorders affecting micturation, primary or secondary hyperuricaemia, simultaneous therapy with uricosuric drugs, thiazides and loop diuretics, mercaptopurine, azathioprine, or adenine arabinoside.
The control group of young subjects (age 26–35 years) was recruited from the staff of the Wiener Allgemeines Krankenhaus (nurses, laboratory technicians, doctors). In addition to pregnancy and lactation the same exclusion criteria applied as in the elderly group.
The effects of age on the pharmacokinetics and pharmacodynamics of allopurinol were studied in an open, single dose design. 12 h before and throughout the study period the test persons consumed no methylxanthine-containing or alcoholic beverages. During the first day of the study all participants were on the hospital diet. After a standard breakfast, 200 mg allopurinol (2 Zyloric® 100 mg tablets, Glaxo-Wellcome) were administered orally with 250 ml water. This amount of allopurinol is in the dose range for treating gout. Blood samples, withdrawn from a catheter in an antebrachial vein, and urine were collected before (blank) and in periodic intervals after dosing of allopurinol. To avoid possible contamination of plasma with hypoxanthine generated by blood cells, plasma was immediately separated from blood cells and, together with urine, stored at −20° C until assay. The study participants were instructed to adjust their fluid intake to produce a urine volume of at least 2 l per day.
In addition to peroral administration, 200 mg allopurinol sodium salt were also injected intravenously in four subjects to permit calculation of the fractional peroral bioavailability of the drug. A period of at least 2 weeks was allowed between the peroral and intravenous administrations for drug washout.
The study was approved by the ethics committee of the hospital. Each of the participants gave informed written consent.
Assay procedure
Allopurinol, oxipurinol, hypoxanthine, xanthine, and uric acid in plasma and urine were determined by high performance liquid chromatography (h.p.l.c.) with ultraviolet spectrophotometric detection (at 254 nm), similar to the procedure described by Wung & Howell [8]. Plasma (250 μl) was deproteinized with 1/10 volume of 4.4 m perchloric acid and buffered to pH 2 using potassium acetate. After centrifugation to remove particular matter, 50 μl were injected into the analytical columns (Spherisorb ODS II RP18, 2504.6 mm and 1504.6 mm, Waters Co.) of the h.p.l.c. system (HP 1050 Chem-Station, Hewlett Packard Co.) with 50 mm KH2HPO4 as the mobile phase (1.5 ml min−1) at 28° C. 5-Fluorouracil was used as internal standard. Allopurinol and oxipurinol eluted with retention times of 11.5 and 9.9 min, respectively, the corresponding values for hypoxanthine, xanthine, uric acid, and 5-fluoro-uracil were 7.4, 8.4, 6.3, and 5.8 min. After each run the columns were cleaned with acetonitrile.
Urine samples (40 μl) were diluted 1:1 with water and made alkaline with 1/8 volume 1 m Na2CO3. After acidifying with three volumes of 0.2 m H3PO4 to pH 3, 50 μl were applied to the h.p.l.c. system with 10 mm KH2PO4 as the mobile phase at 50° C.
Four point standard calibration curves were generated for allopurinol, oxipurinol, hypoxanthine, xanthine, and uric acid on each assay day. The lower detection limit for these compounds was 0.1 μg ml−1, the absorption—concentration relation was linear to 50 μg ml−1 for allopurinol and oxipurinol, to 10 μg ml−1 for hypoxanthine and xanthine, and to 100 μg ml−1 for uric acid. The calibration curves passed through the origin (±0.03 μg ml−1). The standards, which were purchased from Sigma Chemical Co., were dissolved in 7% bovine serum albumin for plasma calibration curves and in 1 m H3PO4 for urine calibration curves.
Data analysis
The area under the plasma concentration—time curve (AUC) was obtained by the trapezoidal method with extrapolation to infinity according to Clast/ke, where Clast is the last measured plasma concentration of the compound in question and ke is the apparent elimination rate constant. ke was derived from linear least-square regression analysis of the falling portion of the semilogarithmic plasma concentration–time curves.
Total clearance, CL, was calculated from
| 1 |
in which D is the dose administered. In the case of oxipurinol, the dose taken for the calculation of CL was obtained from
| 2 |
The apparent volume of distribution, V, was set equal to CL/ke. Renal clearance, CLR, was calculated from
| 3 |
in which Cu and Cp are the urine and plasma concentrations, and Vu represents the urine volume per time unit. It follows that the nonrenal clearance, CLNR, is CL–CLR.
The absorption rate constant, ka, the maximum plasma concentration, Cmax, and the time of Cmax after peroral drug administration, tmax, of allopurinol and oxipurinol were obtained by approximating the parameters of the equation (the Bateman function)
| 4 |
to the measured values of the plasma concentration—time curves using nonlinear regression analysis (iterative curve—fitting procedure). Cmax was calculated by setting the first derivative of the Bateman function equal to zero, and tmax by solving for t [9]. F is the fractional peroral bioavailability.
Urinary excretion rates were calculated from the product of the urine concentration and the urine volume per unit time. Fractional renal excretion, FE, was obtained from
| 5 |
in which the superscript Cr denotes the concentration of creatinine in plasma and urine, respectively. The parameter FE, which is expressed in percentage of the creatinine clearance, has the advantage that it is independent of urine volume, hence it is not affected by inaccuracies in collection of urine.
Statistics
Results are given as means±s.e. mean based on n, the number of test persons. The statistical significance of a difference between means was calculated using the unpaired t-test. A two tailed P value smaller than 0.05 is considered significant.
Results
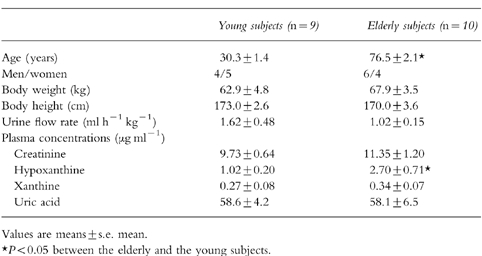
Characteristics of the elderly and young subjects in which the study was performed are given in Table 1. There was no difference in mean body weight and height between the two age groups. Urine flow rate was somewhat lower in the elderly. Hypoxanthine plasma levels were significantly higher in the old compared with the young group, whereas xanthine, uric acid, and creatinine plasma concentrations were not significantly different.
Table 1.
Demographic parameters, urine flow rate, and plasma concentrations of creatinine, hypoxanthine, xanthine, and uric acid of the elderly and young subjects in which the study was performed.
Pharmacokinetics
Following oral administration of 200 mg allopurinol, maximum plasma concentrations of oxipurinol, the active metabolite of allopurinol, were higher in subjects older than 70 years than in young adults aged 24–35 years. In contrast, maximum allopurinol plasma levels were slightly lower in elderly than in young individuals (Figure 1). The pharmacokinetic parameters calculated from the plasma concentration—time curves of allopurinol and oxipurinol are given in Table 2.
Figure 1.
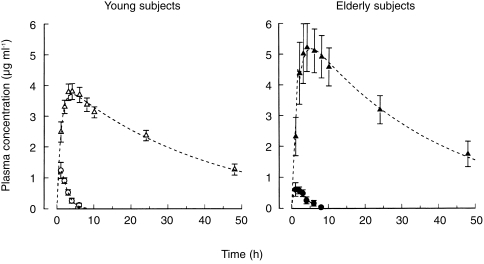
Plasma concentrations of allopurinol (○, •) and oxipurinol (▵, ▴) after oral administration of 200 mg allopurinol to elderly (solid symbols) and young subjects (open symbols). The dashed curves were calculated by nonlinear regression analysis underlying Equation (4). Values are means±s.e. mean.
Table 2.
Pharmacokinetic parameters of allopurinol (200 mg orally) and oxipurinol in young and elderly subjects. The values for clearance and distribution volume are uncorrected for the fractional bioavailability, hence represent CL/F and V/F, respectively.
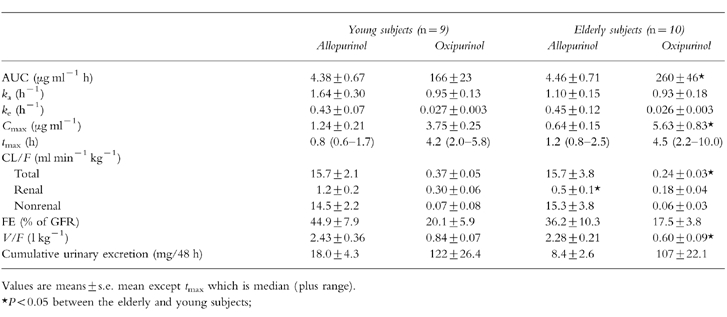
The absorption rate constant, ka, of allopurinol was lower in the elderly than in the young individuals (95% confidence interval on mean difference, CI: 1.20; −0.11), the maximum plasma concentration, Cmax, was decreased in this age group (95% CI: 1.14; 0.04), and more time was required to reach Cmax (95% CI: 0.06; −0.94). Although these changes did not reach statistical significance at least in the t-test, together they suggest retarded intestinal absorption in the aged.
The ka value of oxipurinol, on the other hand, not only reflects the rate of absorption but also the rate of conversion of allopurinol to oxipurinol. ka for oxipurinol was not different in the elderly and young subjects (Table 2), indicating that oxidation of allopurinol to oxipurinol is unchanged in old age. This notion is also supported by the unchanged elimination rate constant, ke, of allopurinol and total and nonrenal clearance of allopurinol in elderly compared with young individuals.
From the clearance values of allopurinol given in Table 2 it is obvious that this compound is eliminated primarily by metabolism, renal clearance is much smaller than nonrenal clearance of allopurinol. The fractional renal excretion of allopurinol amounts to 45% of the glomerular filtration rate in young adults, hence net allopurinol reabsorption is only half of the filtered allopurinol. In the elderly, renal clearance of allopurinol was significantly smaller than in the young subjects. The age-dependent decrease of renal allopurinol clearance was of similar magnitude to the fall in creatinine clearance (Table 3). Hence, fractional renal allopurinol excretion was not significantly decreased in the elderly, in accordance with the notion that in the ageing kidney nephrons are lost but the remaining nephrons operate normally [10].
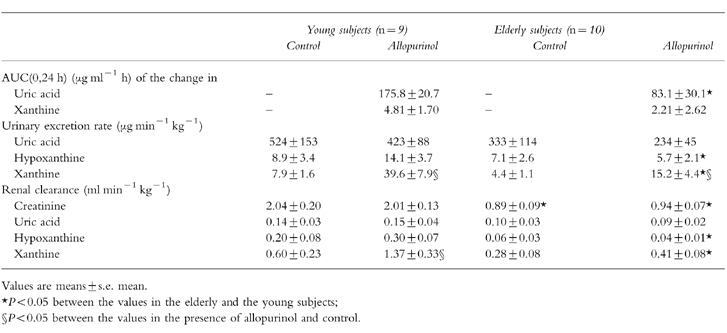
Table 3.
Effects of allopurinol administration (200 mg orally) in young subjects and elderly. The values for urinary excretion rates and renal clearances in the presence of allopurinol represent the means from 2 to 8 h after allopurinol administration, the control values for these parameters were obtained from urinary and blood samples taken before allopurinol dosing.
Oxipurinol, in contrast to allopurinol, is eliminated primarily by the kidneys, metabolic (nonrenal) elimination is relatively small. But the absolute value of renal oxipurinol clearance is lower than that of allopurinol (Table 2), indicating that net tubular reabsorption of oxipurinol is more extensive than that of allopurinol. In old age, both total and renal clearance of oxipurinol are decreased compared with young subjects.
The distribution volume of oxipurinol is smaller than that of allopurinol (Table 2). This difference is in agreement with the higher hydrophilicity of oxipurinol. The octanol/water (pH 7.4) distribution coefficient of oxipurinol was measured to be 0.2, the corresponding value for allopurinol was 1.0. In the elderly, the distribution volume of oxipurinol was reduced compared with young subjects, whereas that of allopurinol was not changed.
The pharmacokinetic parameters for allopurinol and oxipurinol were not statistically different between men and women in the elderly and young group, respectively (data not shown).
F, the fractional oral bioavailability, was derived from a comparison of the allopurinol AUC after oral and intravenous allopurinol administration in a total of four test persons, two young and two elderly. The mean value of F for these four individuals was 0.81±0.16. As was pointed out above, allopurinol is eliminated predominately by metabolism which is not changed by age. Hence it appears justified to pool the bioavailability data from the young and elderly individuals.
Although plasma concentrations of oxipurinol were higher in the elderly than the young subjects, the concentrations of this compound in urine were lower in this age group (Figure 2). The values for the cumulative urinary excretion of allopurinol and oxipurinol are given in Table 2.
Figure 2.
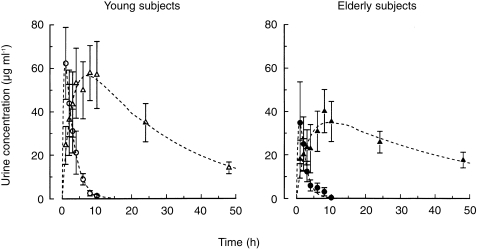
Urine concentrations of allopurinol (○, •) and oxipurinol (▵, ▴) after oral administration of 200 mg allopurinol to elderly (solid symbols) and young subjects (open symbols). Values are means±s.e. mean.
Pharmacodynamics
Plasma uric acid concentrations decreased in response to allopurinol administration both in elderly and young subjects (Figure 3), but the time-integrated effect on plasma uric acid (AUC of the decrease in plasma uric acid with time) was significantly smaller in the old age group than the young (Table 3) although the oxipurinol plasma levels were higher in the elderly (Figure 1). Both hypoxanthine and xanthine plasma levels increased, the relative changes being more pronounced with xanthine (Figure 3). The AUC of the change in plasma xanthine in response to allopurinol dosing was, on average, again half as high in the aged than in the young group (Table 3). The findings of smaller time-integrated effects of oxipurinol on plasma uric acid and xanthine levels suggest that oxipurinol inhibits xanthine oxidase to a lesser degree in elderly than in young individuals.
Figure 3.
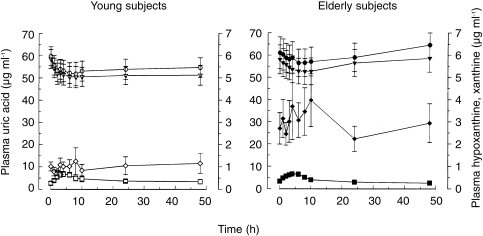
Plasma concentrations of uric acid (▿, ▾), hypoxanthine (◊, ♦), xanthine (□, ▪), and the sum of uric acid, hypoxanthine, and xanthine (( ), (
), ( )), after oral administration of 200 mg allopurinol to elderly (solid symbols) and young subjects (open symbols). Note that the scale for hypoxanthine and xanthine (right y-axis) is 10-fold expanded compared with that for uric acid. Values are means±s.e. mean.
)), after oral administration of 200 mg allopurinol to elderly (solid symbols) and young subjects (open symbols). Note that the scale for hypoxanthine and xanthine (right y-axis) is 10-fold expanded compared with that for uric acid. Values are means±s.e. mean.
The sum of uric acid, hypoxanthine, and xanthine concentrations in plasma was decreased after allopurinol administration in both age groups (Figure 3). This finding indicates inhibition of de novo purine synthesis in response to allopurinol dosing [2, 11].
Parallel to the changes in plasma levels, allopurinol administration reduced urinary excretion of uric acid but increased that of hypoxanthine and xanthine at least in the young test persons (Table 3). The rise in hypoxanthine and xanthine excretion in response to allopurinol administration was smaller or nonexisting in the aged despite higher plasma levels particularly in the case of hypoxanthine.
The renal clearance of uric acid, which amounted to 6–10% of creatinine clearance, was not altered by allopurinol dosing. In contrast, renal xanthine clearance was markedly increased in young individuals (Figure 4), indicating that the renal handling of xanthine was altered by allopurinol and oxipurinol. The fractional renal excretion of xanthine rose from 25% before allopurinol to a peak of 85% after allopurinol dosing in young persons. In elderly subjects the increase in xanthine clearance in response to allopurinol administration was much less pronounced than in the young.
Figure 4.
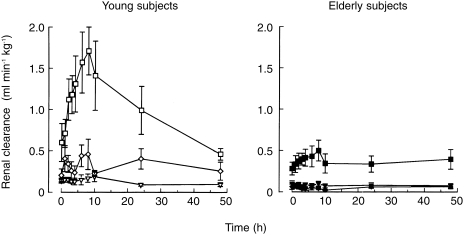
Renal clearance of xanthine (□, ▪), hypoxanthine (◊, ♦), and uric acid (▿, ▾) after oral administration of 200 mg allopurinol to elderly (solid symbols) and young subjects (open symbols). Values are means ± s.e. mean.
Discussion
In the present study the disposition and actions of allopurinol are compared in a group of elderly persons without major health problems with the corresponding values in a group of healthy young subjects. Hence it is assumed that the observed differences in the pharmacokinetics and pharmacodynamics of this uricostatic agent between the two age groups are a result of physiological ageing and not of age-related diseases [12].
Pharmacokinetics
In old age, renal clearances of allopurinol and oxipurinol are decreased compared with young individuals. These changes can be attributed to the age-related reduction in renal function [10, 13–15]. Metabolism of allopurinol, the major elimination mechanism of this agent, is not altered in the elderly as shown by the unchanged elimination rate constant and nonrenal clearance. Oxipurinol, on the other hand, is predominately eliminated via the kidneys. A linear relationship between renal oxipurinol clearance and creatinine clearance was reported previously for patients with renal insufficiency [16].
The distribution volume of allopurinol is considerably larger than that of oxipurinol (Table 2). Differences in plasma protein binding are not responsible for the higher distribution volume of allopurinol, as both compounds are only negligibly bound to plasma proteins [17, 18]. Therefore, the values for the distribution volume indicate more extensive cellular uptake of allopurinol than of oxipurinol, which can be accounted for by the higher lipid solubility (octanol/water distribution coefficient) of allopurinol in comparison with oxipurinol. The distribution volume of oxipurinol only slightly exceeds the fractional water content of the body. The decrease of the distribution volume of oxipurinol in old age is in agreement with the reduction in water content in the ageing body [7, 14, 15].
The plasma concentrations of oxipurinol were found previously to increase linearly with the dose of allopurinol in the dose range 50–600 mg [19], suggesting linear pharmacokinetics. The pharmacokinetic values given in Table 2 may therefore be assumed to be valid also for doses other than 200 mg.
The earlier studies on the pharmacokinetics of allopurinol [18, 20–22] were all performed in young persons (age 22–38 years). The total clearance of allopurinol in these reports ranges from 9.6 to 13.0 ml min−1 kg−1, no values were given for the total clearance of oxipurinol. The distribution volume of allopurinol reported by Appelbaum and coworkers [20] and Walter-Sack et al. [22] is 1.6 and 1.5 l kg−1, respectively, Breithaupt & Tittel [18] give a lower value of 0.6 l kg−1. No value for the distribution volume of oxipurinol was available previously.
The fractional oral bioavailability is given by Breithaupt & Tittel [18] as 0.90 and by Appelbaum et al. [20] as 0.67 compared with 0.81 in the present study, suggesting near complete intestinal absorption and/or only a small first-pass effect of allopurinol.
Pharmacodynamics
In the present study the time-integrated effect of allopurinol and oxipurinol on plasma urate concentration (AUC of the change in plasma urate) was significantly lower in elderly than in young subjects (Table 3) although plasma oxipurinol levels were higher in the elderly (Figure 1). These findings suggest a reduced inhibitory effect of this uricostatic agent on xanthine oxidase in old age. The observation that the AUC of the increase in plasma xanthine in response to allopurinol administration was also reduced in the elderly additionally supports the notion of diminished xanthine oxidase inhibition in the aged. It is known that the structure of many proteins is altered in senescence by post-translational events, the accumulation of abnormal enzymes is one of the characteristics of ageing [23, 24]. A structurally modified xanthine oxidase may have a different sensitivity towards allopurinol and oxipurinol. A decrease in both the rates of synthesis and degradation of xanthine oxidase at high age compared with maturity was reported by Haining and coworkers [25] for rats. Slower protein turnover is one of the factors assumed to be responsible for the formation of altered proteins in old age [23].
However, as discussed above, the rate of conversion of allopurinol to oxipurinol, a reaction also catalysed by xanthine oxidase, was not different in the two age groups. The discrepancy of unchanged conversion of allopurinol to oxipurinol in old age whereas the metabolism of the endogenous xanthine oxidase substrates appears to be diminished may be related to the fact that the oxidation of allopurinol to oxipurinol is inhibited only by much higher concentrations of allopurinol and oxipurinol when compared with the oxidation of the endogenous substrates hypoxanthine and xanthine. It should also be kept in mind that allopurinol may be additionally converted to oxipurinol by aldehyde oxidase (see [11]).
Allopurinol and oxipurinol do not affect glomerular filtration rate as reflected by unchanged creatinine clearance, but renal xanthine clearance is markedly increased by these uricostatic agents especially in young subjects, hence the fractional renal excretion of xanthine is augmented. As is clear from a comparison of Figures 2 and 4, the changes in renal xanthine clearance after allopurinol dosing are very similar to the time course of urinary oxipurinol concentrations. Hence, tubular xanthine reabsorption appears to be inhibited by oxipurinol, most likely as a result of competition between these two oxipurines for carrier-mediated tubular transport. Mediated transport systems for nucleobases were demonstrated in epithelial cell lines from proximal tubules of pig and opossum [26, 27] and other cells [28, 29]. Inhibition of nucleobase transport by allopurinol and oxipurinol has been shown for human erythrocytes [30]. Oxipurinol therefore acts not only as a uricostatic agent, it also has ‘xanthinuric’ properties.
As is clear from the fractional renal excretion of oxipurinol (Table 2), net tubular reabsorption of this compound is higher than that of allopurinol. Oxipurinol is more hydrophilic than allopurinol, hence passive reabsorption of oxipurinol is expected to be lower than that of allopurinol. It therefore appears, that oxipurinol is not only an inhibitor but also a substrate for the renal nucleobase transport system. The mediated reabsorption mechanism for oxipurinol in the tubules appears to be absent in cases of hereditary renal hypouricaemia. These patients do not respond to allopurinol because of renal wasting of oxipurinol, resulting in extremely low plasma concentrations of oxipurinol [31].
The renal clearance of uric acid, in contrast to that of xanthine, was not changed by allopurinol administration, indicating that uric acid is not a substrate for the tubular purine base transporter. Hence oxipurines on the one hand and uric acid on the other appear to be reabsorbed by different tubular transport systems, the nucleobase transport system and the organic acid transport system, respectively. This notion is in contrast with earlier reports that oxipurinol and uric acid share the same renal transport mechanism [32, 33].
Clinical implications
Because of reduced renal excretion and a diminished distribution volume, plasma levels of oxipurinol are higher in elderly after allopurinol administration that in young subjects. This increase in oxipurinol levels may be responsible for the higher allopurinol toxicity in old age [6], as the incidence and severity of adverse effects of allopurinol have been attributed to oxipurinol accumulation [16, 34, 35]. These adverse effects include hyper-sensitivity reactions (maculopapular rash to exfoliative dermatitis, malaise, fever, eosinophilia, liver function abnormalities, renal impairment), gastrointestinal problems (nausea, diarrhoea), and neurological symptoms (headache, drowsiness) [35, 36]. It is well established (and also clear from the present results) that kidney function declines with age, hence elderly have to be treated like renally insufficient patients. Dosage modifications of allopurinol according to the creatinine clearance have been given by Hande and coworkers [16] to reduce the life-threatening toxicity of allopurinol. These dosage guidelines should also be adhered to in elderly. If therapeutic drug monitoring is available, doses should be adjusted to result in steady-state target plasma concentrations of 5–8 μg ml−1 oxipurinol (40–60 μm). In this context it should be noted that levels far higher than required to inhibit xanthine oxidase activity are found on average when plasma oxipurinol concentrations are measured in patients on routine allopurinol therapy [19]. Hence patients appear to be given excess doses of allopurinol in general.
Acknowledgments
This study was supported by the Ludwig Boltzmann Institute for Ageing Research, Vienna, Austria.
References
- 1.Rundles RW. The development of allopurinol. Arch Intern Med. 1985;145:1492–1503. [PubMed] [Google Scholar]
- 2.Elion GB. The purine path to chemotherapy. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 3.Day RO, Birkett DJ, Hicks M, Miners JO, Graham GG, Brooks PM. New uses for allopurinol. Drugs. 1994;48:339–344. doi: 10.2165/00003495-199448030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Stewart R, Yost R, Hale W, Marks R. Epidemiology of hyperuricemia in an ambulatory elderly population. J Am Geriatr Soc. 1979;27:552–554. doi: 10.1111/j.1532-5415.1979.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 5.Doherty M, Dieppe P. Crystal deposition disease in the elderly. Clin Rheumatol. 1986;12:97–116. [PubMed] [Google Scholar]
- 6.Fam AG. Gout in the elderly. Clinical presentation and treatment. Drugs Aging. 1998;13:229–243. doi: 10.2165/00002512-199813030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Turnheim K. Drug dosage in the elderly: Is it rational? Drugs Aging. 1998;13:357–379. doi: 10.2165/00002512-199813050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wung WE, Howell SB. Simultaneous liquid chromatography of 5-fluorouracil, uridine, hypoxanthine, xanthine, uric acid, allopurinol, and oxipurinol in plasma. Clin Chem. 1980;26:1704–1708. [PubMed] [Google Scholar]
- 9.Dost FH. Grundlagen der Pharmakokinetik. Stuttgart: Georg Thieme-Verlag; 1968. 2. Aufl. [Google Scholar]
- 10.McLachlan MSF. The aging kidney. Lancet. 1978;ii:143–146. [Google Scholar]
- 11.Elion GB. Allopurinol and other inhibitors of urate synthesis. In: Kelley WN, Weiner IM, editors. Handbook of Experimental Pharmacology, Uric Acid. Vol. 51. Berlin Heidelberg New York: Springer-Verlag; 1978. pp. 485–514. [Google Scholar]
- 12.Rowe JW. Clinical research on aging, strategies and directions. N Engl J Med. 1977;297:1332–1336. doi: 10.1056/NEJM197712152972406. [DOI] [PubMed] [Google Scholar]
- 13.Lindeman RD. Renal and urinary tract. In: Masoro EJ, editor. Handbook of Physiology, Sect 11: Aging. New York Oxford: Oxford University Press; 1995. pp. 485–503. [Google Scholar]
- 14.Tumer N, Scarpace PJ, Lowenthat DT. Geriatric pharmacology: Basic and clinical considerations. Annu Rev Pharmacol Toxicol. 1992;32:271–302. doi: 10.1146/annurev.pa.32.040192.001415. [DOI] [PubMed] [Google Scholar]
- 15.Mayersohn MB. Special pharmacokinetic considerations in the elderly. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics. 3. Vancouver, WA: Applied Therapeutics, Inc; 1992. pp. 9.1–9.43. [Google Scholar]
- 16.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 17.Elion GB, Kovensky A, Hitchings GH, Metz E, Rundles RW. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966;15:863–880. doi: 10.1016/0006-2952(66)90163-8. [DOI] [PubMed] [Google Scholar]
- 18.Breithaupt H, Tittel M. Kinetics of allopurinol after single intravenous and oral dose. Noninteraction with benzbromarone and hydrochlorothiazide. Eur J Clin Pharmacol. 1982;22:77–84. doi: 10.1007/BF00606429. [DOI] [PubMed] [Google Scholar]
- 19.Graham S, Day RO, Wong H, et al. Pharmacodynamics of oxipurinol after administration of allopurinol to healthy subjects. Br J Clin Pharmacol. 1996;41:299–304. doi: 10.1046/j.1365-2125.1996.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appelbaum SJ, Mayersohn M, Dorr RT, Perrier D. Allopurinol kinetics and bioavailability. Intravenous, oral and rectal administration. Cancer Chemother Pharmacol. 1982;8:93–98. doi: 10.1007/BF00292878. [DOI] [PubMed] [Google Scholar]
- 21.Colin JN, Farinotti R, Fredj G, Clavel JP, Vignon E, Dietlin F. Kinetics of allopurinol and oxipurinol after chronic oral administration. Interactions with benzbromarone. Eur J Clin Pharmacol. 1986;31:53–58. doi: 10.1007/BF00870986. [DOI] [PubMed] [Google Scholar]
- 22.Walter-Sack I, de Vries JX, Kutschker C, Ittensohn A, Voss A. Disposition and uric acid lowering effect of oxipurinol: comparison of different oxipurinol formulations and allopurinol in healthy individuals. Eur J Clin Pharmacol. 1995;49:215–220. doi: 10.1007/BF00192382. [DOI] [PubMed] [Google Scholar]
- 23.Van Remmen H, Ward WF, Sabia RV, Richardson A. Gene expression and protein degradation. In: Masoro EJ, editor. Handbook of Physiology, Section 11: Aging. New York Oxford: Oxford University Press; 1995. pp. 171–234. [Google Scholar]
- 24.Gafni A. Structural modifications of proteins during aging. J Am Geriatr Soc. 1997;45:871–880. doi: 10.1111/j.1532-5415.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 25.Haining JL, Legan JS, Lovell WJ. Synthesis and degradation of rat liver xanthine oxidase as a function of age and protein deprivation. J Gerontol. 1970;25:205–209. doi: 10.1093/geronj/25.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Griffith DA, Jarvis SM. High affinity sodium-dependent nucleobase transport in cultured renal epithelial cells (LLC-PK1) J Biol Chem. 1993;268:20085–20090. [PubMed] [Google Scholar]
- 27.Akerman R, Jarvis SM. Nucleobase transport in cultured renal epithelial cells. Biochem Soc Trans. 1995;23:29S. doi: 10.1042/bst023029s. [DOI] [PubMed] [Google Scholar]
- 28.Plagemann PGW, Wohlhueter RM, Wolffendin C. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988;947:405–443. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 29.Kraupp M, Marz R. Membrane transport of nucleobases: Interactions with inhibitors. Gen Pharmacol. 1995;26:1185–1190. doi: 10.1016/0306-3623(95)00005-l. [DOI] [PubMed] [Google Scholar]
- 30.Razavi M, Kraupp M, Marz R. Allopurinol transport in human erythrocytes. Biochem Pharmacol. 1993;45:893–897. doi: 10.1016/0006-2952(93)90174-u. [DOI] [PubMed] [Google Scholar]
- 31.Löffler W, Seibke W, Seibke E, et al. Non-responsiveness to allopurinol in renal hypouricaemia. Klin Wochenschr. 1989;67:47. doi: 10.1007/BF01736535. [DOI] [PubMed] [Google Scholar]
- 32.Elion GB, Yü TF, Gutman AB, Hitchings GH. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med. 1968;45:69–77. doi: 10.1016/0002-9343(68)90008-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Yamakita J, Higashino K. Difference in the plasma concentration and urinary excretion of allopurinol, oxypurinol, and purine bases between dietary intake and fasting. Int J Clin Pharmacol Ther. 1996;34:157–162. [PubMed] [Google Scholar]
- 34.Cameron JS, Simmonds HA. Use and abuse of allopurinol. Br Med J. 1987;294:1504–1505. doi: 10.1136/bmj.294.6586.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson GM, Boyle RR, Francis HW, et al. Dosage prescribing and plasma oxipurinol levels in patients receiving allopurinol therapy. Eur J Clin Pharmacol. 1990;39:419–421. doi: 10.1007/BF00315424. [DOI] [PubMed] [Google Scholar]
- 36.Conaghan PG, Day RO. Risks and benefits of drugs used in the management and prevention of gout. Drug Safety. 1994;11:252–258. doi: 10.2165/00002018-199411040-00004. [DOI] [PubMed] [Google Scholar]


