Abstract
Aims
Cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) are both expressed in the intestinal mucosa and present a barrier to oral drug delivery. CYP3A4 and P-gp share both overlapping tissue distribution and substrate specificity. Grapefruit juice interactions with CYP3A4 substrates are well documented and occur as a consequence of down regulation of intestinal CYP3A4. The aim of the present study was to screen grapefruit juice components against the CYP3A4-mediated metabolism and P-gp mediated transport of the HIV-1 protease inhibitor saquinavir.
Methods
Five grapefruit juice components: quercetin, naringin, naringenin, 6′,7′-dihydroxybergamottin and bergamottin were screened as potential inhibitors of the metabolism of saquinavir by human liver microsomes. The known CYP3A4 inhibitor ketoconazole was also screened for inhibitory potential. These compounds were also screened as modulators of P-gp activity by assessing the directional transport of saquinavir across Caco-2 cell monolayers which express P-gp. The effect of verapamil, a known modulator of P-gp function, was also determined in these cell lines.
Results
On preincubation, 6′,7′-dihydroxybergamottin and bergamottin inhibited the metabolism of saquinavir, with IC50 values of 0.33±0.23 μm and 0.74±0.13 μm, respectively (n = 3). Ketoconazole achieved an IC50 of 0.55±0.12 μm (n = 4). The other compounds studied failed to reach IC50 at concentrations of up to 100 μm. The transport of saquinavir in the basolateral→apical (BL→AP) direction exceeded that in the apical →basolateral direction (AP→BL), with apparent permeability coefficients of 199.2±15.8×10−7 cm s−1 and 8.00±1.13×10−7 cm s−1, respectively (n = 3) which is indicative of a polarized efflux mechanism. The ratio of BL→AP/AP→BL for saquinavir was 25, but in the presence of verapamil and ketoconazole this ratio was reduced to 3.6 and 4.0, respectively (n = 3), indicating extensive inhibition of P-gp mediated saquinavir efflux. Of the grapefruit juice components studied only naringin and 6′,7′-dihydroxybergamottin had any appreciable effect, reducing the ratio to 7.6 and 7.1, respectively (n = 3); but this was due solely to increased AP→BL transport.
Conclusions
Grapefruit juice components inhibit CYP3A4-mediated saquinavir metabolism and also modulate, to a limited extent, P-gp mediated saquinavir transport in Caco-2 cell monolayers. The in vivo effects of grapefruit juice coadministration are most likely the result of effects on CYP3A4 (inhibition and down regulation) and only to a minor extent on modulation of P-gp function.
Keywords: CYP3A4, grapefruit juice, P-glycoprotein
Introduction
Grapefruit juice increases the oral bioavailability of the CYP3A4 substrates felodipine [1, 2], cyclosporin A (CsA) [3, 4], nifedipine [1], midazolam [5] and terfenadine [6].
Many lines of evidence point to the intestine, rather than the liver as the major site of this interaction in vivo. Firstly, the metabolism of some of these compounds occurs in the intestine itself, for example that of midazolam [7] and CsA. [8, 9]. Secondly, CYP3A4 is abundantly expressed in small bowel enterocytes [10, 11]. Thirdly, grapefruit juice does not influence the clearance of drugs when they are administered intravenously. Ducharme et al. [4] observed no difference in pharmacokinetic parameters for CsA when coadministered intravenously with grapefruit juice. Finally, the major pharmacokinetic effect of grapefruit juice coadministration is to increase the peak plasma concentration of drugs (therefore increasing AUC), having little effect on the subsequent clearance (i. e. no change in half-life).
The interaction between grapefruit juice and felodipine was assessed in healthy volunteers [12]. At day 1, grapefruit juice caused a 73% increase in AUC and a 138% increase in maximum plasma concentrations of felodipine. These effects were sustained after 14 days of repeated ingestion, suggesting that the interaction is rapid and fully developed after the first glass. The effects of repeated grapefruit juice consumption was also investigated by Lown et al. [13]. The enterocyte level of CYP3A4 (normalized to villin expression) was assessed in small intestinal biopsy samples. Mean enterocyte CYP3A4 protein concentration decreased by 62%, while levels of CYP3A4 mRNA remained constant. Administration of grapefruit juice significantly increased AUC and maximal plasma concentrations of felodipine. The authors proposed that grapefruit juice caused selective down regulation of CYP3A4 protein in the small intestine. This was most likely the result of accelerated protein degradation induced by damage of the enzyme caused by suicide inactivation. A 42% decrease in the expression of enterocyte CYP3A4 protein 4 h after grapefruit juice ingestion has also been documented [14].
Increased emphasis is now placed on the role of p-glycoprotein (P-gp) mediated drug efflux in the low and variable oral bioavailability of many drugs, for example CsA. 75% of interpatient variability in CsA clearance has been attributed to a combination of hepatic CYP3A4 and enterocyte P-gp expression [15]. Intestinal CYP3A4 did not correlate with CsA clearance indicating a lack of involvement [15]. A progressive decrease in AUC and Cmax and an increase in tmax values was observed when CsA was instilled into either the stomach, jejunum/ileum or the colon [16], while P-gp mRNA is known to increase progressively from the stomach to the colon [17]. The AUC for CsA after administration to distinct regions of the gastrointestinal tract was shown to correlate significantly with P-gp expression, suggesting that CsA absorption in man is modulated by P-gp [16].
A striking overlap in the tissue distribution and the substrate specificity of CYP3A4 and P-gp has been observed, covering a wide variety of therapeutic agents including antiarrhythmic and cancer chemotherapeutic agents [18]. Due to observations such as these, it has been suggested that CYP3A4 and P-gp may play complementary roles in drug disposition, biotransformation and antitransport. This is especially important in the villi of the small intestine, where CYP3A4 and P-gp act synergystically as an oral drug delivery barrier.
The Caco-2 cell line is the most common in vitro model for investigating the gastrointestinal absorption of drugs [19]. This cell line resembles mature enterocytes of the human small intestinal epithelium, both structurally and functionally [20]. For transport studies Caco-2 cells are grown on Transwell polycarbonate membranes and form highly differentiated, polarized monolayers which are joined by tight junctions, preventing the paracellular diffusion of solutes [21]. In keeping with their origin Caco-2 cells express high levels of P-gp which is localized to the apical brush border [22], consistent with the site of expression of P-gp on the apical surface of enterocytes [23].
Saquinavir (SQV) has been identified as a substrate for P-gp by its ability to modulate the cytotoxicity of chemotherapeutic agents in P-gp positive cell lines [24]. More recently, studies have described the polarized transport of SQV (and other protease inhibitors) across Caco-2 cell monolayers [25]. Other evidence, including the ability of protease inhibitors to stimulate P-gp specific ATPase activity, inhibit competitively the concentration dependent binding of [125I]-iodoarylazidoprazosin to P-gp and increasing the accumulation of fluorescent substrates of P-gp in P-gp positive cell lines has also been presented [26].
As with other drugs that interact with grapefruit juice, SQV is metabolized by CYP3A4 in the intestine [27] and indeed these authors have highlighted an extensive role for intestinal CYP3A4 in the poor oral bioavailability of SQV. In vivo, grapefruit juice has been shown to cause a two-fold increase in the AUC and Cmax of orally administered SQV, an effect which was absent following intravenous administration [28].
The aims of the present study were to screen several grapefruit juice components: naringin, naringenin, quercetin, 6′,7′-dihydroxybergamottin and bergamottin for inhibition of the CYP3A4-mediated metabolism of SQV and the directional transport of SQV in Caco-2 cell monolayers cultured on Transwell polycarbonate filters.
Methods
Chemicals
Quercetin, naringin, naringenin, verapamil hydrochloride, β-NADPH (reduced form), penicillin (5000 U ml−1)/streptomycin (5000 μg ml−1), PBS tablets (×10) and trypsin EDTA (0.25%) were purchased from the Sigma Chemical company (Poole, Dorset, UK). Dulbecco’s modified Eagle’s medium (DMEM), Hank’s balanced salt solution (HBSS), HEPES, and foetal bovine serum were purchased from Gibco (Gibco Life Sciences Ltd, Paisley, UK). Bergamottin and 6′,7′-dihydroxybergamottin were a kind gift from Dr David Bailey (Ontario, Canada). Ketoconazole was a gift from Janssen (Beerse, Belgium). Saquinavir (SQV) and [14C]-saquinavir were provided by Roche Products Ltd (Welwyn Garden City, UK). H.p.l.c. grade for u.v. acetonitrile (AcN) and methanol (MeOH) were purchased from Fisons Plc (Loughborough, UK). Ultima Gold liquid scintillation cocktail was obtained from Packard (Groningen, Netherlands). All other reagents were of the highest grade possible.
Human liver samples
Histologically normal livers were obtained from kidney transplant donors. Consent for their removal was obtained from the donor’s relatives and Ethics Committee approval was granted for their use in this study. Liver tissue (10–20 g portions) was frozen in liquid nitrogen and stored at −80° C until required. Washed microsomes (105 000 g pellets) were prepared by differential centrifugation. Microsomal protein yield was determined by the method of Lowry et al. [29].
Caco-2 cell lines
Caco-2 cells (passage number 106) were generously provided by Dr Alex Dodoo (Roche Products Ltd, Welwyn Garden City, UK). Cells were grown in tissue-culture treated flasks (162 cm2; Costar, High Wycombe, Bucks., UK) at 37° C in a 10% CO2 incubator. Cells were maintained in DMEM supplemented with foetal bovine serum (10%; v/v), penicillin (50 U ml−1 and streptomycin (50 μg ml−1). Medium was changed every 2 days until confluence of the cell monolayer was achieved.
Metabolism of saquinavir
A 500 μl incubation mixture containing 0.1 mg microsomal protein was incubated with [14C]-SQV (3 μm; 0.03 μCi) in the presence of MgCl2 (10 mm), NADPH (1 mm) in phosphate buffer (0.067 m; pH 7.4). Incubations were terminated by the addition of MeOH (500 μl) to precipitate microsomal protein, followed by centrifugation to remove the precipitated protein. Supernatants were evaporated to dryness prior to reconstitution in mobile phase (120 μl). SQV metabolism was assessed by h.p.l.c. analysis of an aliquot (90 μl) with on-line radiometric detection. SQV and metabolites were resolved on a Prodigy column (15 cm×4 mm: Phenomenex, Macclesfield, UK) using a gradient mobile phase system, flow rate 1.5 mlmin−1. Mobile phase comprised ammonium acetate (75 mm; pH 6.8) and AcN (far UV). Initial run conditions were: 72% ammonium acetate: 28% AcN. Between 25 and 36 min there was a linear increase to 40% AcN. At 42 min there was a further linear increase to 50% AcN over 8 min, which remained constant for 5 min. This was followed by a return to initial run conditions over 5 min and a 5 min re-equilibration period.
LC/MS analysis of saquinavir metabolites
For LC/MS analysis larger quantities of SQV metabolites were prepared from an incubation containing 10 sequential additions of [14C]-SQV (3 μm; 0.03 μCi) in the presence of 0.2 mg microsomal protein and MgCl2 (10 mm), NADPH (1 mm) in phosphate buffer (0.067 m; pH 7.4). Additions of SQV were made at 20 min intervals, termination and extraction of samples were as above. LC/MS analysis was performed on a Quattro II ‘triple quadrupole’ mass spectrometer (Micromass Ltd, Manchester, UK) by electronspray ionization. An eluate flow to LC/MS interface of 50 μl min−1 was employed with an interface temperature of 60° C, capillary voltage of 3.85 kV and a cone voltage of 30 V. SQV and metabolites were resolved using the above h.p.l.c. conditions. Detection was by on-line radiometric detection and selected ion monitoring for mono-and di-hydroxylated products along with parent compound. Analytes were detected as protonated molecules.
Inhibition studies
The inhibitory potential of grapefruit juice constituents: quercetin (QTN), naringin (NAR), naringenin (NGN), 6′7′-dihydroxybergamottin (DHB) and bergamottin (BEG) was investigated using the standard assay conditions. Ketoconazole (KET) was also studied. Incubations were performed in the presence of a range of inhibitor concentrations (0–100 μm). Inhibitors were prepared as stock solutions in methanol, with an appropriate volume (to achieve the required concentration in the incubation) dried down prior to reconstitution in the incubation volume containing protein and phosphate buffer. There was no visible evidence of precipitation of any of these compounds over the concentration range studied.
IC50 values (concentration of inhibitor to cause 50% inhibition of original enzyme activity) were determined by GraFit (Version 3.01) where appropriate using the following equation:
where V0 is uninhibited velocity, v is observed velocity, s is slope factor and I is inhibitor concentration. In addition to coincubations, preincubations (30 min) were performed in the absence of substrate, to determine if any of the grapefruit juice constituents studied caused mechanism based inhibition of CYP3A4-mediated saquinavir metabolism.
Transport studies
Viable cells (passage number 110), as determined by trypan blue exclusion, were seeded onto polycarbonate filters (4.7 cm2; 24 mm diameter; 0.4 μ pore size; Costar, UK) at an initial density of 0.5×106 cells/cm2 and maintained at 37° C in a 10% CO2 atmosphere in ‘Transwell’ 6 well culture plates (Costar, High Wycombe, Bucks., UK). The medium was changed (above and below filters) every other day. Confluent monolayers were utilized for transport studies on 15 days post seeding. The transepithelial electrical resistance (TEER; calculated from [(resistance of filter+cells)−(resistance of filter alone)]×effective growth area; expressed as Ω/cm2) was measured using a Millicell electrical resistance system. Measurement of TEER gave a quantitative indication of monolayer confluence and integrity.
Prior to transport studies the monolayer was washed with transport medium, Hanks balanced salt solution (HBSS; ×2) containing HEPES (10 mm; pH 7.4). Apical and basolateral chambers of the Transwells were then filled with HBSS (1.5 ml and 2.5 ml, respectively) and equilibrated for 1 h (37° C; 10% CO2 incubator), after which TEER was re-assessed.
To study the apical to basolateral (AP→BL) and basolateral to apical (BL→AP) transport of saquinavir, medium in either the apical or basolateral chamber was removed, respectively, and replaced with an equal volume of HBSS containing [14C]-saquinavir (1 μm; 0.02 μCi). At predetermined times of 30, 60, 90, 120 and 150 min an aliquot (500 μl) was removed from the receiver chamber and replaced with an equal volume of HBSS. Scintillation cocktail (4 ml; Ultima Gold) was added to each sample and SQV transport was assessed by liquid scintillation counting. The inhibition of saquinavir transport was assessed by the simultaneous addition of inhibitor to the same chamber as saquinavir.
Verapamil (VER; 500 μm), KET (500 μm), QTN (250 μm), NAR (250 μm), NGN (250 μm), DHB (200 μm) and BEG (100 μm) were screened as potential P-gp modulators.
Monolayer TEER was re-assessed following sampling at the last time point.
Apparent permeability coefficients (Papp) were determined from the cumulative transport data, as follows:
where dQ/dt=pmol transported min−1, A=area of filter, C0=initial concentration of saquinavir.
Results
Inhibition of saquinavir hydroxylation
Saquinavir (3 μm; 0.03 μCi) was metabolized to a number of products by human liver microsomal preparations. A representative radiometric h.p.l.c. chromatogram is shown in Figure 1. LC/MS analysis with single ion monitoring at the appropriate pseudomolecular mass (for protonated molecules) indicated that the major metabolites of saquinavir were M2−di-hydroxylated (m/z=703), M4 and M10−mono-hydroxylated (m/z=687). The other metabolites of saquinavir were a mixture of mono-and di-hydroxylated products.
Figure 1.
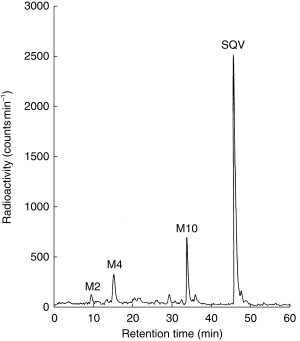
Representative radio-chromatogram of the metabolism of saquinavir (3 μm; 0.03 μCi) by human liver microsomes (0.1 mg; 10 min incubation).
Upon coincubation, BEG and DHB inhibited saquinavir metabolism with IC50 values of 3.07±0.61 μm and 1.68±0.38 μm (n = 3), respectively (Figure 2a and b). Pre-incubation of BEG and DHB with microsomal protein and NADPH caused greater inhibition with IC50 values of 0.74±0.13 μm and 0.33±0.23 μm, respectively (n = 3) (Figure 2a and b). In comparison the known CYP3A4 inhibitor, KET, achieved an IC50 of 0.55±0.12 μm (n = 4) (Figure 2c). Quercetin (preor coincubation) elicited around 40% inhibition of saquinavir hydroxylation at 100 μm (Figure 2d). NAR and NGN failed to inhibit either upon preincubation or coincubation (Figure 2e and f, respectively).
Figure 2.
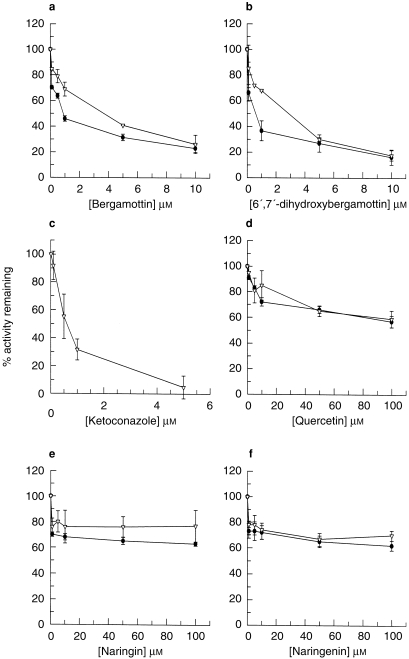
Inhibition of saquinavir metabolism (3 μm; 0.03 μCi) by: (a) bergamottin (b) 6′,7′-dihydroxybergamottin (c) ketoconazole (d) quercetin, (e) naringin and (f) naringenin. Data represent the mean±s.d. of determinations in three individual human liver microsomal preparations, except (c), where n = 4. ▿ coincubations; • preincubations.
Inhibition of saquinavir transport
TEER readings at the start and finish (at 150 min) remained constant at 610±65 and 747±180 Ω cm−2, respectively, in the presence of SQV alone and all P-gp modulators examined. The transport of SQV (1 μm) in the BL→AP direction was approximately 25-fold that in the opposite direction (Table 1, Figures 3a and 4). VER (500 μm) increased the AP→BL transport of SQV (1 μm), demonstrating an increase in Papp from 8.00±1.13–40.53±1.64×10−7 cm s−1 and also decreased the BL→AP transport of SQV (1 μm) from 199.2±15.84×10−7 cm s−1 to 146.9±4.55×10−7 cm s−1 (Table 1, Figures 3b and 4). VER reduced the ratio of BL→AP/AP→BL transport from around 25-fold to around 3.6-fold (Table 1, Figure 5). KET (500 μm) also increased the permeability of SQV in the AP→BL direction and decreased the BL→AP transport (Table 1 and Figures 3c and 4). The presence of KET reduced the ratio of BL→AP/AP→BL transport to around 4 (Table 1, Figure 5).
Table 1.
Permeability coefficients (Papp) for the transport of saquinavir (1 μm) in the apical→basolateral and basolateral→apical directions: the effect of verapamil, ketoconazole and grapefruit juice constituents on permeability in both directions.
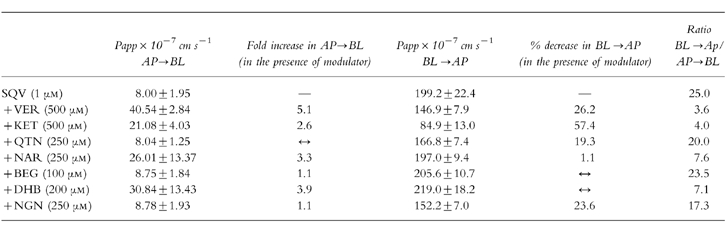 |
Figure 3.
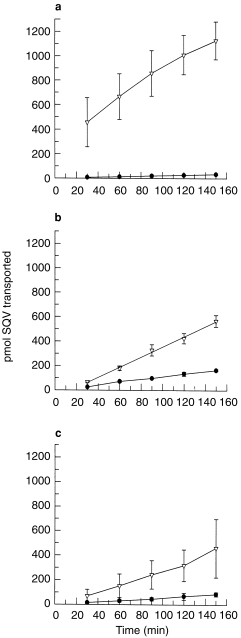
The cumulative transport of saquinavir (1 μm; 0.02 μCi) across Caco-2 cell monolayers in the basolateral→apical and apical→basolateral directions: (a) saquinavir alone (b) in the presence of verapamil (500 μm) and (c) in the presence of ketoconazole (500 μm). Data represent the mean±s.d. of three individual determinations. ▿ basolateral→apical direction; • apical→basolateral direction.
Figure 4.
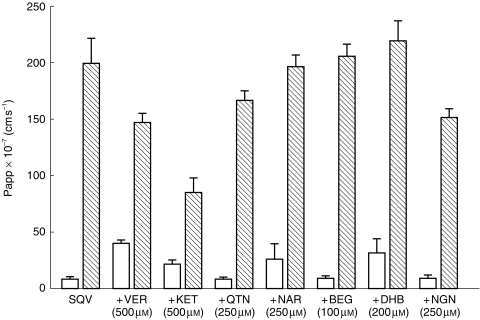
The effect of verapamil, ketoconazole and various grapefruit juice components on the directional transport of saquinavir (1 μm; 0.02 μCi) across Caco-2 cell monolayers. Data represent the mean±s.d. of three individual determinations. □ AP→BL transport,  BL→AP transport.
BL→AP transport.
Figure 5.
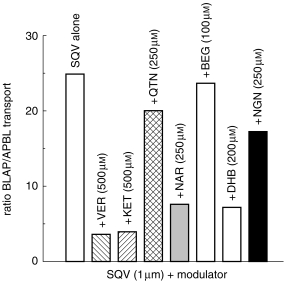
The effects of verapamil, ketoconazole and grapefruit juice components on the ratio of basolateral→apical/apical→basolateral transport of saquinavir (1 μm; 0.02 μCi) in Caco-2 cell monolayers.
Of the other compounds screened NAR (250 μm) and DHB (100 μm) increased the AP→BL transport of SQV (1 μm), increasing Papp in this direction by factors of 3.3 and 3.9 receptively (Table 1, Figure 4). Although these compounds caused minimal inhibition of transport in the opposite direction (BL→AP) there was a reduction in the ratio of BL→AP/AP→BL from around 25-fold to around 7-fold (Table 1, Figure 4 & 5).
QTN (250 μm), BEG (200 μm) and NGN (250 μm) caused minimal changes in the transport of SQV (1 μm), with Papp remaining essentially unchanged in the AP→BL direction. Although QTN and NGN reduced transport in the BL→AP direction (20% and 23%, respectively; Table 1), when the ratio of BL→AP/ AP→BL transport was calculated there was no net effect on the flux of saquinavir (Table 1, Figures 4 and 5).
Discussion
Both DHB and BEG are potent in vitro inhibitors of the CYP3A4-mediated metabolism of saquinavir. This inhibition appeared to occur by a mechanism–based interaction, since both compounds caused greater inhibition following preincubation with microsomal protein and NADPH prior to the addition of either substrate (Figure 1a and b).
DHB has been previously shown to inhibit CYP3A4 activity (assessed by the 6′-hydroxylation of testosterone) with an IC50 value of 25 μm [30]. Further studies demonstrated that DHB inhibited CYP3A4 in an NADPH and time dependent manner [31]. When DHB was added to the apical side of Caco-2 cells, expressing CYP3A4, grown on polycarbonate filters a 40% reduction in CYP3A4 protein expression was observed [14]. In addition to mechanism-based inhibition, it has been proposed that DHB has the ability to competitively inhibit CYP3A4 because it is metabolized to multiple hydroxylated products by this isoform [14]. This would indicate that DHB is a substrate of CYP3A4 which is in keeping with the concept of mechanism-based or ‘suicide’ inhibition. These in vitro findings support the hypothesis that in vivo grapefruit juice consumption causes a down regulation of intestinal CYP3A4 expression by mechanism-based inactivation of the enzyme causing its rapid intracellular degradation [14].
Evidence to support the fact that DHB is not the sole component responsible for the effects of grapefruit juice was proposed by Bailey et al. [32], who studied supernatant and reconstituted particulate matter from centrifuged grapefruit juice on the oral pharmacokinetics of felodipine. Although there was a greater concentration of DHB in the supernatant fraction, the effects of the reconstituted particulate matter more closely resembled those of grapefruit juice itself. This would indicate the existence of at least one additional substance in grapefruit juice that contributes to the observed in vivo effect. In the present study BEG also caused potent, apparently mechanism-based inhibition of saquinavir metabolism. BEG has previously been shown to cause mechanism-based inhibition of CYP3A4, with a Ki value of 7.7 μm [33]. Therefore BEG may also contribute to the observed in vivo effects of grapefruit juice consumption. The data on inhibition by flavonoids is equivocal with 100 μm NGN reported to cause marked inhibition [34] or no inhibition [35] of nifedipine metabolism.
Transport of SQV (1 μm) by the Caco-2 cell monolayer in the BL→AP direction far exceeded that in the opposite direction by a factor of around 25-fold, indicating the presence of an active efflux mechanism (Table 1; Figure 2a). Previous studies have demonstrated an average 25-fold greater apparent permeability of SQV across Caco-2 cell monolayers in the BL→AP direction [36]. Evidence to support the involvement of P-gp in this directional transport is provided by the fact that in the presence of VER the apparent permeability of SQV in the BL→AP direction was only three fold that in the AP→BL direction, which would indicate extensive inhibition of P-gp-mediated SQV transport.
KET (albeit at a high concentration, 500 μm) also inhibited the BL→AP translocation of SQV (1 μm) and increased transport in the opposite direction. In the presence of KET the ratio of BL→AP/AP→BL transport fell from around 25 fold to 4 fold, indicating that this concentration of KET was as effective as VER (500 μm) (Table 1). In vivo coadministration of KET with SQV causes a 3 fold increase in SQV AUC [37].
The grapefruit juice constituents had variable effects on the directional transport of saquinavir with NAR and DHB causing the most obvious effects, decreasing the ratio of BL→AP/AP→BL transport from around 25-fold to 8-and 7-fold, respectively. However, this was solely based on an increase in AP→BL flux. Also, the concentration of DHB used (200 μm) is in excess of that found in concentrated grapefruit juice (30 μm [30, 38]); and is higher than that used (50 μm) in the recent study of Edwards et al. [39] which showed only a 9% increase in calcein fluorescence in L-MDR1 cells. The concentration of NAR in grapefruit juice is much higher (>1000 μm [38]). QTN had essentially no effect on the transport of saquinavir in either direction, although it has previously been indicated as a substrate for an efflux mechanism in Caco-2 cell monolayers [40] and has been shown to potentiate the activity of adriamycin in MCF-7 adriamycin resistant human breast carcinoma cells [41]. In a study on vinblastine transport in Caco-2 cells, NAR and NGN increased uptake from the apical side, although the observed effects were stated to be insufficient to account for the effect of grapefruit juice [42].
In vivo the coadministration of grapefruit juice and SQV resulted in a two fold increase in SQV AUC and bioavailability [28]. This effect was absent with intravenously administered saquinavir and the authors proposed that the main effect was inhibition of intestinal CYP3A4. However these observations do not rule out the involvement of P-gp inhibition.
The effects of grapefruit juice on the intestinal expression of P-gp have been investigated and, unlike CYP3A4 immunoreactive protein, there was no difference in P-gp levels pre- and post-exposure [13]. Therefore if any grapefruit juice constituents are capable of modulating P-gp function this would be via direct competition for the efflux pump rather than down regulation of protein expression.
The finding that saquinavir is a substrate of P-gp (demonstrated here and in previous studies) may limit its access to various sites of action. This has implications when considering the penetration of SQV through the blood brain barrier, as P-gp is expressed at this site [43]. HIV is known to reside in the brain, as evidenced by the presence of unintegrated viral DNA in brain tissue of HIV-infected individuals [44]. Therefore the presence of P-gp in the blood brain barrier could prevent therapeutic concentrations of protease inhibitors being achieved in the central nervous system.
P-gp has also been detected on haematopoietic stem cells [45] and lymphoid cells [46–48], although its function at this site is poorly understood. The fact that a large proportion of human lymphocytes express functional P-gp which is capable of excluding drugs from the cellular interior could be implicit in the failure of protease inhibitors to inhibit viral replication in some lymphocyte populations. This could be a reason for the observed reservoirs of viable, replication competent, nonmutated HIV-1 which survive in T-cells [49–51].
In conclusion we have demonstrated that both DHB and BEG cause mechanism-based inhibition of CYP3A4-mediated metabolism. This is in keeping with in vivo studies [13, 14] and in studies with Caco-2 cells expressing CYP3A4 [14]. From the literature it is still unclear which component is responsible for the observed grapefruit juice interaction in vivo, if in fact it is one component alone which is responsible rather than a combination of the inhibitory effects of multiple components.
Although none of the grapefruit juice constituents tested appears to markedly inhibit in vitro P-gp activity, it is still possible that unidentified components of grapefruit juice will have greater inhibitory effects on P-gp function which may contribute to improved oral bioavailability of some compounds. Inhibition of P-gp function by grapefruit juice could lead to higher concentrations of substrate reaching, and therefore saturating, intestinal CYP3A4 which may have already been down regulated by repeated exposure to grapefruit juice.
Acknowledgments
We are grateful to Dr J. L. Maggs for the LC/MS analysis of saquinavir metabolites, and the Medical Research Council and Roche Products Ltd who provided financial support for these studies.
References
- 1.Bailey DG, Spence JD, Munoz C, Arnold JMO. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DG, Arnold JR, Bend LT, et al. Grapefruit juice–felodipine interaction: reproducibility and characterization with the extended release formulation. Br J Clin Pharmacol. 1995;40:135–140. [PMC free article] [PubMed] [Google Scholar]
- 3.Ducharme MP, Provenzano R, Dehoorne-Smith M, Edwards DJ. Trough concentrations of cyclosporine in blood following administration with grapefruit juice. Br J Clin Pharmacol. 1993;36:457–459. doi: 10.1111/j.1365-2125.1993.tb00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducharme MP, Warbasse LH, Edwards DJ. Disposition of oral and intravenous cyclosporin after administration with grapefruit juice. Clin Pharmacol Ther. 1995;57:485–491. doi: 10.1016/0009-9236(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 5.Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;50:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 6.Benton RE, Hoing PK, Zamani LR, et al. Grapefruit juice alters terfenadine pharmacokinetics, resulting in prolonged repolarization on the electrocardiogram. Clin Pharmacol Ther. 1996;59:383–388. doi: 10.1016/S0009-9236(96)90105-8. [DOI] [PubMed] [Google Scholar]
- 7.Paine MF, Shen DD, Kunze KL, et al. First pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 8.Kolars JC, Awni WM, Merion RM, Watkins PB. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338:1488–1490. doi: 10.1016/0140-6736(91)92302-i. [DOI] [PubMed] [Google Scholar]
- 9.Wu CY, Benet LZ, Herbert SK, et al. Differentiation of absorption and first pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther. 1995;58:492–497. doi: 10.1016/0009-9236(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 10.Watkins PB, Wrighton SA, Schuetz EG, et al. Identification of glucocorticoid-inducible cytochromes P450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolars JC, Schmiedlin-Ren P, Schuetz JD, et al. Identification of Rifampicin-inducible P4503A4 in human small bowel enterocytes. J Clin Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundahl JUE, Regardth CG, Edgar B, Johnsson G. The interaction effect of grapefruit juice is maximal after the first glass. Eur J Clin Pharmacol. 1998;54:75–81. doi: 10.1007/s002280050424. [DOI] [PubMed] [Google Scholar]
- 13.Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmeidlin-Ren P, Edwards DJ, Fitzsimmons ME, et al. Mechanisms of enhanced oral bioavailability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25:1228–1233. [PubMed] [Google Scholar]
- 15.Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- 16.Fricker G, Huwyler DJ, Gutmann H, Beglinger C. Relevance of P-glycoprotein for the enteral absorption of cyclosporin A. in vitro-in vivo correlation. Br J Pharmacol. 1996;118:1841–1847. doi: 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fojo AT, Ueda K, Slamon DJ, et al. Expression of a multidrug resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wacher VJ, Wu C-Y, Benet LZ. Overlapping substrate specificities and tissue distribution of Cytochrome P450, 3A and P–glycoprotein: implications for drug delivery in cancer chemotherapy. Molec Carcin. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 19.Stewart BH, Chan OH, Lu RH, et al. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relation to absorption in humans. Pharm Res. 1995;12:693–699. doi: 10.1023/a:1016207525186. [DOI] [PubMed] [Google Scholar]
- 20.Pinto M, Robine-Leon S, Appay M-D, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Bio Cell. 1983;47:323–330. [Google Scholar]
- 21.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 22.Hunter J, Jepson MA, Tsuruo T, et al. Functional expression of P-glycoprotein in apical membrane of human intestinal Caco-2 cells. J Biol Chem. 1993;268:14991–14997. [PubMed] [Google Scholar]
- 23.Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washigton CB, Duran GE, Silkic BI, et al. Saquinavir is a high affinity substrate for the multidrug transporter, P-glycoprotein. Clin Pharmacol Ther. 1997;63 OII-A-3. [Google Scholar]
- 25.Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1988;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CGL, Gottesman MM, Cardarelli CO, et al. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 27.Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small intestinal cytochrome P450. Drug Metab Dispos. 1997;25:256–267. [PubMed] [Google Scholar]
- 28.Kupferschmidt HHT, Fattinger KE, Ha HR, et al. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br J Clin Pharmacol. 1998;45:355–359. doi: 10.1046/j.1365-2125.1998.t01-1-00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein determination with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Edwards DJ, Bellevue FH, Woster PM. Identification of 6′,7′-dihydroxybergamottin, a cytochrome-P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–1290. [PubMed] [Google Scholar]
- 31.Bellevue FH, Woster PM, Edwards DJ, et al. Synthesis and biological evaluation of 6′,7′-dihydroxybergamottin (6,7-DHB), a naturally occurring inhibitor of cytochrome P450 3A4. Bioorg Med Chem Lett. 1997;7:2593–2598. [Google Scholar]
- 32.Bailey DG, Monoz C, Kreeft JH, et al. Grapefruit juice–felodipine interaction: amount and effect of active ingredients in juice fraction in man. Clin Pharmacol Ther. 1998;63 doi: 10.1016/S0009-9236(98)90173-4. [(Abstract) PI–99] [DOI] [PubMed] [Google Scholar]
- 33.He K, Iyer KR, Hayes RN, et al. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–259. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 34.Guengerich FP, Kim D-H. In vitro inhibition of dihydropyridine oxidation and aflatoxin B activation in human liver microsomes by naringenin and other flavinoids. Carcinogenesis. 1990;11:2275–2279. doi: 10.1093/carcin/11.12.2275. [DOI] [PubMed] [Google Scholar]
- 35.Miniscalco A, Lundahl J, Regardh CG, et al. Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice. J Pharmacol Exp Therapeut. 1992;261:1195–1199. [PubMed] [Google Scholar]
- 36.Alsenz J, Steffen H, Alex R. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharmaceut Res. 1998;15:423–428. doi: 10.1023/a:1011924314899. [DOI] [PubMed] [Google Scholar]
- 37.Noble S, Faulds D. Saquinavir. A review of its pharmacology and clinical potential in the management of HIV infection. Drugs. 1996;52:93–112. doi: 10.2165/00003495-199652010-00007. [DOI] [PubMed] [Google Scholar]
- 38.Bailey DG, Kreeft JH, Munoz C, et al. Grapefruit juice–felodipine interaction: effect of naringin and 6′,7′-dihydroxybergamottin in humans. Clin Pharmacol Ther. 1998;64:248–256. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 39.Edwards DJ, Fitzsimmons ME, Schwatz EG, et al. 6′,7′-dihydroxybergamottin in grapefruit juice and Seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4 and p-glycoprotein. Clin Pharmacol Ther. 1999;65:237–244. doi: 10.1016/S0009-9236(99)70102-5. [DOI] [PubMed] [Google Scholar]
- 40.Walgren RA, Walle UK, Walle T. Transepithelial absorption of quercetin and quercetin glucosides across the Caco-2 human intestinal cell line. Clin Pharmacol Ther. 1998;63 doi: 10.1016/s0006-2952(98)00048-3. [(Abstract) PIII–20] [DOI] [PubMed] [Google Scholar]
- 41.Scambia G, Ranelletti FO, Panici PB, et al. Quercetin potentiates the effect of adriamycin in a multidrug resistant MCF-7 human breast cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994;34:459–464. doi: 10.1007/BF00685655. [DOI] [PubMed] [Google Scholar]
- 42.Takanaga H, Ohnishi A, Matsuo H, Sawada Y. Inhibition of vinblastine efflux mediated by p-glycoprotein by grapefruit juice components in Caco-2 cells. Biol Pharm Bull. 1998;21:1062–1066. doi: 10.1248/bpb.21.1062. [DOI] [PubMed] [Google Scholar]
- 43.Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug resistance gene (P-glycoprotein) is expressed by endothelial cells at blood brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang S, Koyangi Y, Miles S, et al. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990;34:85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human haematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Kim CH, Tsuruo T, et al. Preferential expression and activity of multidrug resistance gene 1 product (P-glycoprotein) a functionally active efflux pump, in human CD8+ T cells: a role in cytotoxic effector function. J Clin Immunol. 1993;12:451–458. doi: 10.1007/BF00918857. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhary PM, Mechetner EB, Roninson IB, et al. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 48.Pilarski LM, Paine D, McElhaney JE, et al. Multidrug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes: modulation with differentiation stage and during ageing. Am J Hematol. 1995;49:323–325. doi: 10.1002/ajh.2830490411. [DOI] [PubMed] [Google Scholar]
- 49.Wong JK, Hezareh HF, Gunthard DV, et al. Recovery of replication competent HIV despite prolonged suppression of plasma viraemia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 50.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 replication in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 51.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193, 13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]


