Abstract
Aims
To investigate whether an interaction between diltiazem and the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor simvastatin may enhance the cholesterol-lowering response to simvastatin in diltiazem-treated patients.
Methods
One hundred and thirty-five patients attending the Sheffield hypertension clinic who started consecutively on simvastatin for primary or secondary prevention of coronary heart disease (CHD) during the 2 years June, 1996—May 1998 were surveyed. From the clinic records we extracted and recorded absolute and percentage cholesterol responses to the starting dose of simvastatin and coprescription of diltiazem.
Results
The cholesterol reduction for the 19 patients on diltiazem was 33.3% compared with 24.7% in the remaining 116 patients (median difference 8.6%, 95% CI 1.1–12.2%, P < 0.02). The interindividual variability of cholesterol response to simvastatin was greater for patients not taking diltiazem than for those patients taking diltiazem. The ratio of the variances in response for the nondiltiazem group relative to the diltiazem group was 1.34 at 10 mg simvastatin daily (not significant, 95% CI 0.16–4.11), and 3.42 at 20 mg daily (P < 0.01, 95% CI 1.26–7.18). Concurrent diltiazem therapy (P < 0.04), age (P = 0.001) and starting dose of simvastatin (P = 0.002) were found to be significant independent predictors of percentage cholesterol response.
Conclusions
Patients who take both simvastatin and diltiazem may need lower doses of simvastatin to achieve the recommended reduction in cholesterol. The pharmacokinetic and pharmacodynamic aspects of this interaction need further study to confirm an enhanced effect on cholesterol reduction, and exclude an increased risk of adverse events.
Keywords: cholesterol reduction, CYP3A4, diltiazem, interaction, simvastatin
Introduction
Treatment with the HMG-CoA reductase inhibitor simvastatin to lower serum cholesterol has a major impact in the prevention of coronary heart disease (CHD) [1]. Simvastatin is an inactive lactone pro-drug that is hydrolysed in vivo to its active form simvastatin acid. In the liver this metabolite inhibits the conversion of HMG-CoA to mevalonate, the rate-limiting step in cholesterol biosynthesis. Simvastatin undergoes extensive first pass metabolism, and cytochrome P4503A4 (CYP3A4) is the main enzyme involved in this process [2]. Many patients will need statin treatment for secondary or primary prevention of CHD [3] and many will have hypertension as an associated risk factor. These patients, and those with angina, may require the coprescription of the calcium channel blocker diltiazem, which is a relatively potent inhibitor of CYP3A4 [4]. Co-administration of diltiazem with lovastatin causes a four-fold increase in plasma concentrations of lovastatin [5]. Lovastatin and simvastatin have very similar pharmacokinetic characteristics, and a comparable interaction may be anticipated with simvastatin [6]. The main aim of this study was to investigate whether an interaction between diltiazem and simvastatin may enhance the cholesterol-lowering response to simvastatin in diltiazem-treated patients. A second aim was to examine the predictors of response to simvastatin in ordinary medical practice.
Methods
A cohort of hypertensive patients started consecutively on simvastatin during the two years June 1996–May 1998 was identified by retrospective review of the standard treatment records maintained for all patients attending the Sheffield hypertension clinic. Policy in the clinic was to prescribe simvastatin aiming for a serum total cholesterol of ≤5.0 mmol l−1 or a 25% reduction in cholesterol in those with initial values below 6.6 mmol l−1, in accordance with Standing Medical Advisory Committee guidance [7]. From the clinic records we extracted and recorded weight, sex, age, reported smoking habit and whether the indication for simvastatin therapy was primary or secondary prevention of CHD. Also extracted from the records were: two pretreatment cholesterol measurements; the starting date and initial dose of simvastatin; the first cholesterol measurement on simvastatin; all concurrent medication; reported adverse events including muscle symptoms and abnormal values for serum transaminases and creatinine phosphokinase; and reasons for discontinuation of simvastatin.
The response of serum cholesterol to the starting dose of simvastatin was calculated as the difference of the first measurement on treatment from the mean of two pretreatment values. The primary endpoint was the difference in cholesterol response between patients taking diltiazem and those not taking this drug, using the Mann–Whitney test. The difference in variability of response between the two groups was tested for significance using the F-test. Multiple regression analysis was also performed to examine the percentage and absolute cholesterol responses with concurrent diltiazem therapy, initial dose of simvastatin, age, sex, weight, smoking status and baseline cholesterol as predictors.
Results
Patient characteristics
Of 162 patients who consecutively started simvastatin treatment during the 2 year study period, 135 had complete data and were included in the full analysis. Five patients discontinued simvastatin before a cholesterol response was measured and four patients had no cholesterol measurement available after starting simvastatin. Medical records were not available for 18 patients.
Of 135 patients with complete data 86 (64%) were male; mean values were for age 61.5 years, weight 82.0 kg and pretreatment cholesterol 7.0 mmol l−1; 55 (41%) currently smoked cigarettes; and 57 (42%) were treated for primary prevention of CHD, 78 (58%) for secondary prevention. The initial dose of simvastatin was 10 mg daily in 59 patients, 20 mg daily in 75 patients and 40 mg daily in one patient. All patients were taking concomitant antihypertensive medication when simvastatin was started. Diltiazem was coprescribed in 19 cases (14%). Other calcium channel blockers used were amlodipine (16%), nifedipine (14%) and verapamil (4%). The other drugs used most commonly were aspirin (61%), diuretics (55%), atenolol (54%) and enalapril (32%).
Diltiazem and simvastatin response
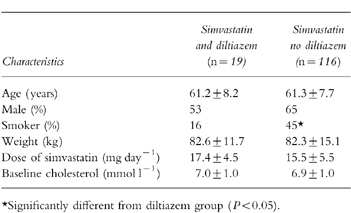
Demographic and baseline data for 19 patients taking diltiazem at a mean daily dose of 226 mg and 116 patients not taking diltiazem are shown in Table 1. The number of smokers was significantly higher in the nondiltiazem group (45%vs 16%, P < 0.05). The mean dose of simvastatin was slightly but not significantly higher in the diltiazem group (17.4 vs 15.5 mg daily).
Table 1.
Mean (±s.d.) characteristics of patients taking simvastatin and diltiazem and of patients taking simvastatin but not diltiazem.
The median percentage cholesterol responses from pretreatment values after 8.3 weeks of simvastatin treatment for the diltiazem group (range 4–18 weeks) and 8.7 weeks of treatment for the nondiltiazem group (range 4–24 weeks) are shown in Table 2. The cholesterol reduction for the 19 patients on diltiazem was 33.3% compared with 24.7% in the remaining 116 patients (median difference 8.6%, 95% CI 1.1–12.2%, P < 0.02). At both doses of simvastatin, 10 and 20 mg daily, cholesterol reduction was greater in the diltiazem group than the nondiltiazem group, but the differences did not attain statistical significance (Table 2; Figure 1). The interindividual variability in cholesterol response to simvastatin was greater for patients not taking diltiazem than for patients taking diltiazem (Figure 1). The ratio of the variances in response for the nondiltiazem group relative to the diltiazem group was 1.34 at 10 mg simvastatin daily (not significant, 95% CI 0.16–4.11), and 3.42 at 20 mg daily (P < 0.01, 95% CI 1.26–7.18).
Table 2.
Cholesterol reduction from pretreatment values after 8 weeks of simvastatin treatment. Median percentage cholesterol reduction (and range).
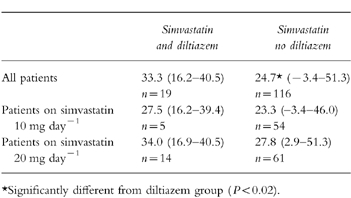
Figure 1.
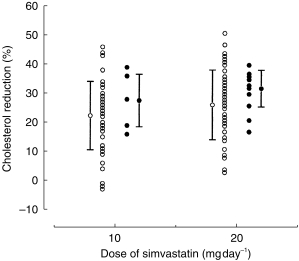
% cholesterol response to simvastatin 10 and 20 mg daily for individual patients taking diltiazem (•) and those not taking diltiazem (○). The mean±s.d. is also illustrated for each group.
Predictors of response
In all 135 patients the mean dose of 15.8 mg simvastatin daily reduced the serum cholesterol by 26.9% (range −3.4% to 61.6%) and by 1.8 mmol l−1 (range −0.2– 5.3 mmol l−1). Concurrent diltiazem therapy (P < 0.04), age (P = 0.001) and starting dose of simvastatin (P = 0.002) were found to be significant independent predictors of percentage cholesterol change accounting for 3%, 7% and 8%, respectively, of the total variance. The use of diltiazem, increasing age and higher starting dose correlated with greater reductions of cholesterol. Similar results were obtained using absolute cholesterol change as the response (coprescription of diltiazem P = 0.01, 3% of variance; age P < 0.001, 8%; starting dose of simvastatin P = 0.001, 10%). Smoking status, weight, sex, baseline cholesterol and coprescription of the other calcium channel blockers did not have a significant effect on the response to simvastatin.
The association with age was examined further by tertiles of age. At mean ages of 52.6 (group A), 61.9 (group B) and 69.9 (group C), median cholesterol responses were 23.0, 24.7 and 29.7%, respectively (A vs C, P = 0.004, 95% CI 2.3–12.3%; A vs B, not significant; B vs C, P = 0.04, 95% CI 0.3–11.0%).
Tolerability
Of 144 patients with evaluable data for tolerability, 10 (7%) discontinued simvastatin treatment, 8 (6%) of them because of clinical or laboratory adverse events that resolved on withdrawal of the drug and the remaining 2 changed their minds and declined treatment for primary prevention of CHD. Of the 10 discontinuations, 9 (7%) occurred in the nondiltiazem group. In the diltiazem group, 1 patient (5%) discontinued simvastatin due to abnormal liver function tests. Further investigation revealed that this was not drug-related and therefore simvastatin was re-introduced with no adverse effect.
Discussion
Diltiazem is an inhibitor and substrate of the human liver microsomal enzyme CYP3A4. Simvastatin is metabolized extensively in the liver by CYP3A4, and it was postulated that a pharmacokinetic interaction between the statin and diltiazem might enhance the pharmacodynamic effect of simvastatin. The findings in this cohort study support this as patients taking simvastatin and diltiazem achieve significantly larger cholesterol responses than those not coprescribed diltiazem, with median responses of 33.3% and 24.7%, respectively. Doubling the dose of simvastatin reduces serum cholesterol by an average of 5% [8], and coprescription of diltiazem with simvastatin appears approximately equivalent to doubling the dose of simvastatin. The best estimate of the relative potency of simvastatin taken with diltiazem, compared with no diltiazem treatment, determined by parallel line bioassay [9], was 2.6 (95% CI 0.6–11.1) (Figure 2). Some clinicians routinely start patients on simvastatin at 10 mg daily, the lowest available dose, but others at 20 mg daily as used in the 4S study [1]. The results of this study suggest that patients who take both simvastatin and diltiazem may need lower doses of simvastatin to achieve the recommended reduction in cholesterol [10].
Figure 2.
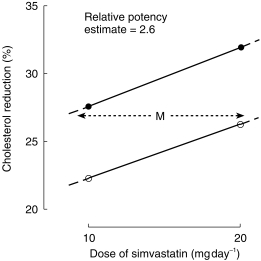
Estimate of relative potency (M) of simvastatin taken with diltiazem (•) compared with no diltiazem treatment (○). The best estimate of the relative potency was 2.6 (95% CI 0.6–11.1).
The idea of using diltiazem as a drug-sparing agent is not new. Diltiazem inhibits the CYP3A4-mediated metabolism of cyclosporin, the main immunosuppressive drug used in organ transplantation [11]. The costs of maintaining grafts with cyclosporin are considerable, and the interaction with diltiazem has been exploited clinically to facilitate lower cyclosporin dosages while sustaining therapeutic plasma concentrations of the immunosuppressant [12]. In addition, the calcium channel blocker appears to have vasoprotective properties as indicated by a lower incidence of graft rejection in diltiazem-treated renal transplant patients [13, 14]. For these reasons, diltiazem is widely prescribed in Australia as a cyclosporin-sparing agent [15].
In in vitro kinetic studies the N-desmethyl and N,N,didesmethyl metabolites of diltiazem are, respectively, 11 and 200 times more potent than the parent drug, as competitive inhibitors of CYP3A4 [4]. During chronic therapy these metabolites accumulate in plasma and can reduce the clearance of the parent drug [16, 17]. The N-demethylated metabolites of diltiazem may possibly contribute to the inhibition of the CYP3A4-mediated metabolism of simvastatin in vivo.This has important clinical implications for the cholesterol-lowering response in individuals. In the absence of diltiazem, patients with high baseline hepatic CYP3A4 activity may have reduced responses to simvastatin, whereas patients with low CYP3A4 activity may have larger responses. Diltiazem treatment will substantially enhance the response to simvastatin in the former group but cause only a modest increase in response to simvastatin in the latter group. The net effect of coprescribing diltiazem will therefore be a reduction of the interindividual variability in the response to simvastatin. The findings in this study suggest that this is indeed the case (Figure 1).
Like diltiazem, the calcium channel blockers verapamil and nifedipine increase the plasma concentrations of the prototype CYP3A4 substrates midazolam and cyclosporin in vivo [18, 19]. In the present study coprescription of verapamil or nifedipine had no significant effect on the response to simvastatin. However, only 4% of patients were coprescribed verapamil and the power to detect any effect was low. Nifedipine was coprescribed with simvastatin as often as diltiazem, each in 14% of patients. The significant effect of diltiazem but not nifedipine, on the response to simvastatin may be due to accumulation of N-desmethyl-and N,N-didesmethyl-diltiazem during chronic therapy, as discussed above.
The response to simvastatin was significantly related to age, with median cholesterol reductions of 23.5% and 29.7%, respectively, at mean ages of 53 and 70 years. Age also significantly predicts the response to cerivastatin [20]. Cholesterol reductions with 0.1 mg cerivastatin twice daily, were 22.2%, 25.6% and 30.5% at ages <45 years, 45–60 years, and >60 years, respectively [20]. Larger cholesterol responses with increasing age may reflect reduced clearance of these statins in older patients. They may require lower doses of simvastatin to achieve the recommended reduction in cholesterol.
The overall contribution of concurrent diltiazem therapy, age and dose of simvastatin to the total variance in response to simvastatin was small, approximately 18%. There are several factors that may have contributed to the high interindividual variability in this study. The results of this study were based on one cholesterol measurement on simvastatin therapy, taken over a range of 4–24 weeks after initiation of treatment. Other determinants that may contribute to the variability in response to simvastatin and have not been considered in this study, are compliance, endogenous synthesis of cholesterol and baseline intestinal cholesterol absorption [21].
Simvastatin is generally very well tolerated and causes few subjective side-effects during chronic treatment. Acute rhabdomyolysis is a rare but serious adverse reaction that may follow the addition of drugs such as itraconazole [22] and mibefradil [23] to the stable regimen of patients taking simvastatin. The onset of rhabdomyolysis has been associated with dramatic increases in plasma concentrations of simvastatin, due to inhibition of the CYP3A4-mediated metabolism by the interacting drugs. Co-administration of diltiazem causes a four-fold increase in plasma concentrations of lovastatin, a drug that has similar pharmacokinetic properties to simvastatin [4]. The magnitude of this interaction was smaller than those observed with the CYP3A4 inhibitors that have been reported to induce rhabdomyolysis. The pharmacokinetic study was conducted in relatively young healthy subjects, and may underestimate the magnitude of the interaction in older patients taking lovastatin or simvastatin who are on chronic treatment with diltiazem. However, to date there has been one report of rhabdomyolysis possibly induced by a simvastatin–diltiazem interaction—the one patient who developed rhabdomyolysis during the 4S study [24]. The findings in the present study suggest that side-effects and withdrawals from simvastatin treatment may not be more common with diltiazem, at least in the short term.
The drug interaction with diltiazem is likely to extend to other HMGCoA reductase inhibitors including atorvastatin, which is metabolized primarily by CYP3A4. Atorvastatin differs from lovastatin and simvastatin in that it has a long elimination half-life and the magnitude of its interactions with potent inhibitors of CYP3A4, such as erythromycin, appear to be lower than those observed with simvastatin and lovastatin [25, 26]. Fluvastatin and cerivastatin are metabolized primarily by CYP2C8 and CYP2C9, respectively, and therefore it is unlikely that either of them will have a clinically significant interaction with diltiazem [27]. The effect of coadministration of diltiazem on the pharmacokinetics of pravastatin has been investigated in healthy volunteers [5]. The lack of effect was predictable since metabolism is a minor route of pravastatin elimination, and CYP3A4 does not contribute significantly to the hepatic clearance of the drug.
According to current SMAC guidance at least 8% of the population will need statin treatment [3], and if the Joint British Societies guidelines [10] are implemented this figure could increase to about 20% [3]. The coprescription of simvastatin and diltiazem is likely to occur commonly. Patients who take simvastatin with diltiazem may need lower doses of simvastatin to achieve the recommended reduction in cholesterol. The pharmacokinetic and pharmacodynamic aspects of this interaction need further study to confirm an enhanced effect on cholesterol reduction, and exclude any increase in risk of serious adverse effects such as rhabdomyolysis.
References
- 1.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Prueksaritanont T, Gorham LM, Bennett MA, et al. In vitro metabolism of simvastatin in humans—identification of metabolising enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997;25:1191–1199. [PubMed] [Google Scholar]
- 3.Haq IUL, Ramsay LE, Pickin DM, Yeo WW, Jackson PR, Payne JN. Lipid-lowering for prevention of coronary heart disease: what policy now? Clin Sci. 1996;91:399–413. doi: 10.1042/cs0910399. [DOI] [PubMed] [Google Scholar]
- 4.Sutton D, Butler AM, Nadin L, Murray M. Role of CYP3A4 in human hepatic diltiazem N-demethylation: inhibition of CYP3A4 activity by oxidised diltiazem metabolites. J Pharmacol Exp Ther. 1997;282:294–300. [PubMed] [Google Scholar]
- 5.Azie NE, Brater DC, Becker PA, Jones DR, Hall SD. The interaction of diltiazem with lovastatin and pravastatin. Clin Pharmacol Ther. 1998;64:369–377. doi: 10.1016/S0009-9236(98)90067-4. [DOI] [PubMed] [Google Scholar]
- 6.Desager JP, Horsmans Y. Clinical pharmacokinetics of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors. Clin Pharmacokin. 1996;31:348–371. doi: 10.2165/00003088-199631050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Standing Medical Advisory Committee on use of statins. NHS Executive. London: Department of Health; May 1997. [Google Scholar]
- 8.Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80:106. [PubMed] [Google Scholar]
- 9.Armitage P, Berry G. 2. Oxford: Blackwell Scientific Publications; 1987. Statistical methods in medical research; pp. 484–498. [Google Scholar]
- 10.Wood D, Durrington P, Poulter N, McInnes G, Rees A, Wray R. on behalf of the Societies. Joint British recommendations on prevention of coronary heart disease in Clinical Practice. Heart. 1998;80(supplement 2):S1, S29. [Google Scholar]
- 11.Brockmoller J, Neumayer HH, Wagner K, Weber W, Heinemeyer G, Kewitz H, Roots I. Pharmacokinetic interaction between cyclosporin and diltiazem. Eur J Clin Pharmacol. 1990;38:237–242. doi: 10.1007/BF00315023. [DOI] [PubMed] [Google Scholar]
- 12.Valentine H, Koegh A, McIntosh N, Hunt S, Oyer P, Schroeder J. Cost containment: coadministration of diltiazem with cyclosporin after heart transplantation. J Heart Lung Transplant. 1992;11:1–8. [PubMed] [Google Scholar]
- 13.Al Edreesi M, Caliile G, Dupuis C, Theoret Y, Paradis K. Safety, tolerability, and pharmacokinetic actions of diltiazem in pediatric liver transplant recipients on cyclosporin. Liver Transplantation Surgery. 1995;1:383–388. doi: 10.1002/lt.500010609. [DOI] [PubMed] [Google Scholar]
- 14.Chrysostomou A, Walker RG, Russ GR, d’Apice AJ, Kincaid-Smith P, Matthew TH. Diltiazem in renal allograft recipients receiving cyclosporin. Transplantation. 1993;55:300–304. doi: 10.1097/00007890-199302000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Jones TE. Survey of cyclosporin sparing agent use in Australasian transplant centres. Aust NZ J Med. 1996;26:772–776. doi: 10.1111/j.1445-5994.1996.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 16.Abernethy DR, Montamat SC. Acute and chronic studies of diltiazem in elderly versus young hypertensive patients. Am J Cardiol. 1987;60:1161–1201. doi: 10.1016/0002-9149(87)90471-1. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Sudo T, Sakamoto K, Tateishi T, Fujimara Y, Ebihara A. The influence of pretreatment periods with diltiazem on nifedipine kinetics. J Clin Pharmacol. 1993;33:222–225. doi: 10.1002/j.1552-4604.1993.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 18.Backman JT, Olkkola KT, Aranko K, Himberg JJ, Neuvonen PJ. Dose of midazolam should be reduced during diltiazem and verapamil treatments. Br J Clin Pharmacol. 1994;37:221–225. doi: 10.1111/j.1365-2125.1994.tb04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crocker JF, Renton KW, Le Vatte TL, McLellan DH. The interaction of the calcium channel blockers verapamil and nifedipine with cyclosporin A in pediatric renal transplant patients. Hypertension. 1996;28:109–114. doi: 10.1007/BF00856514. [DOI] [PubMed] [Google Scholar]
- 20.Stein E, Sprecher D, Allenby KS, Tosiello MS, Whalen E, Ripa SR. Cerivastatin, a new potent syntheic HMG-CoA Reductase inhibitor: effect of 0.2 mg daily in subjects with primary hypercholesterolaemia. J Cardiovasc Pharmacol Ther. 1997;2:7–16. doi: 10.1177/107424849700200102. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen TA, Gylling H, Strandberg T, Sarna S. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Br Med J. 1998;316:1127–1130. doi: 10.1136/bmj.316.7138.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segaert MF, De Soete C, Vandewiele I, Verbanck J. Drug-interaction-induced rhabdomyolysis. Nephrol Dial Transplant. 1996;11:1846–1847. [PubMed] [Google Scholar]
- 23.Schmassmann-Suhijar D, Bullingham R, Gasser R, Schmutz J, Haefeli WE. Rhabdomyolysis due to interaction of simvastatin with mibefradil. Lancet. 1998;351:1929. doi: 10.1016/S0140-6736(05)78613-X. [DOI] [PubMed] [Google Scholar]
- 24.Neuvonen PJ, Kantola T, Kivisto KT. Calcium channel blocker–simvastatin interaction. Clin Pharmacol Ther. 1999;65:583. [PubMed] [Google Scholar]
- 25.Kantola T, Kivisto KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–182. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 26.Yang BB, Siedlek PH, Sedman AJ, Stern RH. Atorvastatin pharmacokinetic interactions with other CYP3A4 substrates: erythromycin and ethinyl estradiol. Pharmacol Res. 1996;13(Suppl 9):S437. [Google Scholar]
- 27.Wierzbicki AS, Reynolds TM, Crook MA, Tatler J, Peters BS. Lipid lowering therapy in patients with HIV infection. Lancet. 1998;352:1782. doi: 10.1016/S0140-6736(05)79858-5. [DOI] [PubMed] [Google Scholar]


