Introduction
Ulcerative colitis and Crohn’s disease are chronic inflammatory bowel diseases of unknown aetiology which, although similar in various aspects, exhibit some contrasting features. A typical example is their relationship with cigarette smoking.
While smoking appears to exert deleterious effects in Crohn’s disease [1, 2], there is overwhelming epidemiological evidence that smoking protects against ulcerative colitis, the risk of developing the disease being significantly lower in smokers than in non-smokers or former smokers [2, 3]. The fact that patients with ulcerative colitis who resume or start smoking often experience clinical improvement [4] prompted attempts to verify the hypothesis that nicotine might be the active component of smoking responsible for the beneficial effects on the course of the disease.
Preliminary, uncontrolled observations employing nicotine gum, a pharmaceutical form generally poorly tolerated, yielded encouraging but inconclusive results [5]. The introduction of transdermal nicotine in the market made it possible to assess the potential therapeutic role of nicotine in a more extensive way.
Clinical studies with transdermal nicotine
In an open trial 16 patients with left-sided colitis receiving various types of therapies (mesalazine, sulphasalazine, steroids) were given in addition nicotine 30 mg daily in transdermal patches for 4 weeks. The majority of patients reported clinical, endoscopic and histological improvement during nicotine administration [6]. Further, anecdotal observations [7] supported that preliminary report.
A multicentre, double-blind, placebo-controlled trial carried out in the United Kingdom showed in patients with ulcerative colitis who had been applying transdermal nicotine (15–25 mg daily for 6 weeks) in addition to their ongoing therapy (oral mesalazine or corticosteroids) a significantly superior effect in terms of clinical and histological improvement [8]. Complete resolution of symptoms was observed in 48.6% of cases with nicotine and only in 24.3% of cases with placebo (P = 0.03). Similar results have been reported by a multicentre, controlled trial performed in the United States, where at 4 weeks 39% of patients who received transdermal nicotine reported clinical improvement—as assessed by a 13-point disease activity index that measured stool frequency, rectal bleeding, endoscopic findings and a clinical global evaluation—compared with 9% of patients who received placebo [9].
It should be noted that in both studies nicotine patches were added to standard treatment with either mesalazine or steroids, which were continued throughout the observation period. Apparently no attempt was made to perform a statistical power calculation before planning the studies and this may account for some inconsistencies in the endoscopic and histological results. The comparatively short period of treatment could also have hampered a proper evaluation of the effects (if any) of nicotine on histology in the US trial [9], whereas the reasons for histological but not sigmoidoscopic improvement [8] remain unclear (Table 1).
Table 1.
Controlled trials of transdermal nicotine in active ulcerative colitis.
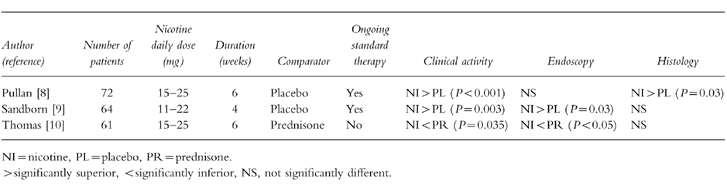
On the other hand, a trial comparing transdermal nicotine alone with oral prednisolone 15 mg daily reported beneficial effects in both treatment groups, with a clinical and endoscopic response which favoured the steroid [10].In subjects with mild to moderate left-sided ulcerative colitis unable or unwilling to receive corticosteroid treatment, a combination of mesalamine plus transdermal nicotine may represent an effective alternative in about 60% of cases [11].
In a controlled study, patients experiencing a clinical flare-up of ulcerative colitis during maintenance treatment with mesalazine were given an additional treatment with either transdermal nicotine 15 mg daily or prednisone for 5 weeks and then were followed up for 6 months while continuing mesalazine. Relapses of ulcerative colitis were observed in 20% of patients treated with nicotine and in 60% of patients in the prednisone group (P = 0.027). Relapses occurred earlier in subjects succesfully treated with steroids than in those treated with nicotine patches [12].
By contrast, in a double-blind trial where patients with ulcerative colitis in remission were treated for 6 months with either nicotine patches alone or placebo, the relapse rates in the two groups were similar [13].
It can be concluded from these data that transdermal nicotine alone has limited efficacy in active ulcerative colitis and is ineffective as maintenance treatment. On the other hand, if administered in combination with mesalazine, nicotine is superior to placebo in promoting clinical remission of ulcerative colitis of mild to moderate degree, may represent an efficacious alternative to steroids in selected cases and, when effective, seems to exert a longer-lasting therapeutic effect than prednisone.
Mechanisms of action of nicotine
In spite of extensive investigation, the exact mechanisms involved in the therapeutic effects of nicotine in ulcerative colitis remain elusive. It has been reported that nicotine increases the thickness of colonic mucus, thus enhancing the protection of the intestinal mucosa [14], but this remains to be confirmed. A reduction in intestinal blood flow by nicotine has also been described [15], but it is unlikely that this phenomenon may account for the favourable effects of nicotine in ulcerative colitis, since rectal blood supply in ulcerative colitis patients is already lower than normal [16].
It has been suggested that nicotine influences the cellular and the humoral immune system [17] and interferes with the inflammatory response, perhaps through stimulation of endogenous steroid release [18]. Indeed nicotine has been found to suppress in vivo Th2 cell function as measured by inhibition of interleukin-10 production [19], and to reduce the synthesis of interleukin-2 [20] and interleukin-8 [21] by mononuclear cells.
Nicotine can affect gut motility [22], but the possible relevance of this effect to its activity in ulcerative colitis is unknown. Smoking appears to decrease intestinal permeability [17, 23], but a similar effect by nicotine has not been demonstrated.
In short, the mechanism(s) responsible for the therapeutic benefit exerted by nicotine in ulcerative colitis are still to be determined, a fact which hampers the development of pharmacological agents mimicking the therapeutic activity of nicotine, but devoid of its adverse effects.
Tolerability of transdermal nicotine
Adverse effects during therapy with transdermal nicotine were significantly more frequent than with placebo in all three placebo-controlled trials [8, 9, 13]. The side-effects most commonly observed with nicotine were nausea, light-headedness, headache, sleep disturbances and skin irritation. The number of nicotine side-effects was significantly higher even compared with prednisolone (10) but it must be noted that the steroid was employed at a dose of only 15 mg daily. When administered in standard doses corticosteroids were no better tolerated than nicotine [12].
In general adverse reactions occurred much more frequently in lifelong non-smokers than in former smokers and tended to appear especially during the first 2 weeks of therapy [8–10]. Treatment withdrawals because of nicotine side-effects ranged from 5.7 to 13% [8–10, 13].
Although no clear correlation was found between nicotine plasma levels and incidence of adverse effects [8], it appears that daily doses of nicotine up to 15 mg are better tolerated [9, 11, 12].
On the whole transdermal nicotine treatment results in frequent side-effects, although most patients are able to complete the course of therapy. No withdrawal symptoms suggesting nicotine addiction have been reported either after 4–6 weeks of therapy in short-term studies, or after a period of up to 6 months in the only long-term study available [13]. Clearly, alternative nicotine formulations able to minimize the adverse effects of nicotine patches are of interest.
Other nicotine formulations for ulcerative colitis
Administering nicotine in high doses topically into the colon might achieve therapeutic results with only a modest rise in serum nicotine, thus limiting the side-effectsof the substance.
To this purpose nicotine enemas have been developed. A 100 ml liquid enema containing 6 mg nicotine complexed with a carbomer has been studied both in healthy volunteers and in ulcerative colitis patients [24] and administered for 4 weeks in an open-label fashion to 22 patients with active colitis, while continuing conventional therapy [25].
Encouraging results were observed in terms of clinical and sigmoidoscopic improvement. Similarly, a nicotine tartrate liquid enema has been administered in patients unresponsive to standard treatment with oral mesalazine or low-dose corticosteroids [26]. Clinical and endoscopic improvement has been observed in up to 70% of patients. The promising, preliminary findings from pilot trials need to be confirmed by placebo-controlled studies.
Systemic side-effects such as nausea, light-headedness and insomnia have been reported even though serum nicotine concentrations were very low. Moreover inability to retain nicotine liquid enemas has been observed in 30% of patients [26]. In order to further reduce and delay nicotine absorption, thereby improving tolerability, a new enema formulation containing trometamol as a buffering agent has been formulated [27] and awaits clinical evaluation.
Enemas would only be useful in subjects with distal colitis, however, hence other formulations must be devoloped. A new oral formulation consisting of a nicotine tartrate capsule with an Eudragit S100 coating for ileo-colonic release [28] warrants clinical investigation.
Conclusions
Ulcerative colitis is a disease more commonly seen in non-smokers or former smokers. In cases of mild to moderate degree, addition of transdermal nicotine to conventional therapy (usually mesalazine) for 4–6 weeks results in clinical improvement and may also represent a therapeutic alternative when corticosteroids cannot be employed. On the whole, the therapeutic effect of nicotine is consistent with the concept that, except for a few negative reports, smoking has a favourable influence on the course of active ulcerative colitis [2, 4, 29]. Since smoking exerts a protective effect on the development of pouchitis [30], nicotine might find a place also in the treatment of this condition, but this still needs to be investigated.
Recent data [12] suggest that flare-up of ulcerative colitis seems to be more frequent and occur earlier in steroid-treated patients than after treatment with nicotine patches. The reason for this phenomenon, which needs confirmation by further studies, remains unknown, and the mechanism of action of nicotine in ulcerative colitis is still to be determined. Stimulation of colonic mucus and inhibition of inflammatory cytokines may play a role.
The results of the only available maintenance study [13] indicate that nicotine is ineffective in preventing clinical relapses of ulcerative colitis. This appears to contrast with epidemiological evidence that smoking prevents development of ulcerative colitis in healthy individuals [2, 3], but secondary prevention in patients with already established disease may involve different considerations. Moreover serum nicotine levels in the studies employing transdermal nicotine were lower than in smokers and less than expected in relation to the administered dose [2].
Side-effects of transdermal nicotine (nausea, light-headedness, sleep disturbances, skin reactions) can be at least in theory reduced by giving the substance by a different route. In the attempt to increase the systemic tolerability of nicotine, various types of enemas and a delayed-release oral formulation have been developed and are currently under clinical investigation.
Hopefully the future will provide us with safer nicotine preparations for more extensive clinical use. However only the full understanding of the exact modes of action of nicotine in ulcerative colitis will lead to the discovery and development of drugs with the same therapeutic characteristics, but devoid of the drawbacks of nicotine administration.
References
- 1.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology. 1994;106:643–648. doi: 10.1016/0016-5085(94)90697-1. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GO, Rhodes J, Green JT. Inflammatory bowel disease and smoking—a review. Am J Gastroenterol. 1998;93:144–149. doi: 10.1111/j.1572-0241.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 3.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34:1841–1854. doi: 10.1007/BF01536701. [DOI] [PubMed] [Google Scholar]
- 4.Fraga XF, Vergara M, Medina C, et al. Effects of smoking on the presentation and clinical course of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:683–687. doi: 10.1097/00042737-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lashner BA, Hanauer SB, Silverstein MD. Testing nicotine gum for ulcerative colitis patients. Experience with single-patient trials. Dig Dis Sci. 1990;35:827–832. doi: 10.1007/BF01536795. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava ED, Russell MAH, Feyerabend C, Williams GT, Masterton JG, Rhodes J. Transdermal nicotine in active ulcerative colitis. Eur J Gastroenterol Hepatol. 1991;3:875–878. [Google Scholar]
- 7.Guslandi M, Tittobello A. Steroid-sparing effect of transdermal nicotine in ulcerative colitis. J Clin Gastroenterol. 1994;18:347–348. doi: 10.1097/00004836-199406000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Tremaine WJ, Offord KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Thomas GO, Rhodes J, Ragunath K, et al. Transdermal nicotine compared with oral prednisolone therapy for active ulcerative colitis. Eur J Gastroenterol Hepatol. 1996;8:769–776. [PubMed] [Google Scholar]
- 11.Guslandi M, Tittobello A. A pilot trial of nicotine patches as an alternative to corticosteroids in ulcerative colitis. J Gastroenterol. 1996;31:627–629. doi: 10.1007/BF02355071. [DOI] [PubMed] [Google Scholar]
- 12.Guslandi M, Tittobello A. Outcome of ulcerative colitis after treatment with transdermal nicotine. Eur J Gastroenterol Hepatol. 1998;10:513–515. doi: 10.1097/00042737-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Thomas GO, Rhodes J, Mani V, et al. Transdermal nicotine as maintenance therapy for ulcerative colitis. N Engl J Med. 1995;332:988–992. doi: 10.1056/NEJM199504133321503. [DOI] [PubMed] [Google Scholar]
- 14.Zijistra FJ, Srivastava ED, Rhodes M, et al. Effect of nicotine on rectal mucosa and mucosal eicosanoids. Gut. 1994;35:247–251. doi: 10.1136/gut.35.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava ED, Russel MA, Feyerabend C, Rhodes J. Effect of ulcerative colitis and smoking on rectal blood flow. Gut. 1990;31:1021–1024. doi: 10.1136/gut.31.9.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guslandi M, Polli D, Sorghi M, Tittobello A. Rectal blood flow in ulcerative colitis. Am J Gastroenterol. 1995;90:579–580. [PubMed] [Google Scholar]
- 17.Cohen RD, Hanauer SB. Nicotine in ulcerative colitis—how does it work and how can we use it. Clin Immunotherap. 1996;5:169–174. [Google Scholar]
- 18.Kershbaum A, Pappajohn DJ, Bellet S, et al. Effect of smoking and nicotine on adrenocortical secretion. JAMA. 1968;203:275–278. [PubMed] [Google Scholar]
- 19.Madretsma S, Wolters LMM, van Dijk JPM, et al. In-vivo effect of nicotine on cytokine production by human non-adherent mononuclear cells. Eur J Gastrenterol Hepatol. 1996;8:1017–1020. doi: 10.1097/00042737-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk APM, Meijssen MAC, Brouwer AJB, et al. Transdermal nicotine inhibits interleukin 2 synthesis by mononuclear cells derived from healthy volunteers. Eur J Clin Invest. 1998;28:664–671. doi: 10.1046/j.1365-2362.1998.00344.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti MA, Hodgson I. Inflammatory bowel disease IL-8 production and tissue expression is modulated by nicotine and crude tobacco extract. Gut. 1997;40(Suppl 1):A74. [Google Scholar]
- 22.Scott AM, Kellow JE, Eckerseley GM, et al. Cigarette smoking and nicotine delay postprandial mouth-cecum transit time. Dig Dis Sci. 1992;37:1544–1547. doi: 10.1007/BF01296500. [DOI] [PubMed] [Google Scholar]
- 23.Prytz H, Benoni C, Tagesson C. Does smoking tighten the gut? Scand J Gastroenterol. 1989;24:1084–1088. doi: 10.3109/00365528909089259. [DOI] [PubMed] [Google Scholar]
- 24.Green JT, Thoms GO, Rhodes J, et al. Pharmacokinetics of nicotine carbomer enemas; a new treatment modality for ulcerative colitis. Clin Pharmacol Ther. 1997;61:340–348. doi: 10.1016/S0009-9236(97)90167-3. [DOI] [PubMed] [Google Scholar]
- 25.Green JT, Thomas GAO, Rhodes J, et al. Nicotine enemas for active ulcerative colitis—a pilot study. Aliment Pharmacol Ther. 1997;11:859–863. doi: 10.1046/j.1365-2036.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Tremaine WJ, Leighton JA, et al. Nicotine tartrate liquid enemas for mildly to moderately active left-sided ulcerative colitis unresponsive to first-line therapy: a pilot study. Aliment Pharmacol Ther. 1997;11:661–671. doi: 10.1046/j.1365-2036.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- 27.Green JT, Rhodes J, Thomas GO, et al. Nivotine carbomer enemas-pharmacokinetcs of revised formulation. Ital J Gastroenterol Hepatol. 1998;30:260–265. [PubMed] [Google Scholar]
- 28.Compton RF, Sandborn WJ, Lawson GM, et al. A dose-ranging pharmacokinetic study of nicotine tartrate following single-dose delayed-release oral and intravenous administration. Aliment Pharmacol Ther. 1997;11:865–874. doi: 10.1046/j.1365-2036.1997.00236.x. [DOI] [PubMed] [Google Scholar]
- 29.Green JT, Rhodes J, Ragunath K, et al. Clinical status of ulcerative colitis in patients who smoke. Am J Gastroenterol. 1998;93:1463–1467. doi: 10.1111/j.1572-0241.1998.00464.x. [DOI] [PubMed] [Google Scholar]
- 30.Merrett MN, Mortensen N, Kettlewell M, Jewell D. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38:362–364. doi: 10.1136/gut.38.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]


