Abstract
Aims
To characterize milk/plasma (M/P) ratio and infant dose, for fluoxetine and norfluoxetine, in breast-feeding women taking fluoxetine for the treatment of depression, and to determine the plasma concentration of these drugs in their infants.
Methods
Fourteen women (mean age 32.2 years) taking fluoxetine (mean dose 0.51 mg kg−1 day−1) and their infants (mean age 3.4 months) were studied. Fluoxetine and norfluoxetine in plasma and milk were measured by high-performance liquid chromatography over a 24 h dose interval in four patients, and by single point data collection in 10 patients. Infant exposure was estimated as the product of estimated milk production, and average drug concentration in milk, normalized to body weight and expressed as a percentage of the weight-adjusted maternal dose.
Results
Mean M/P values of 0.68 (95% CI 0.52–0.84) and 0.56 (95% CI 0.35–0.77) were calculated for fluoxetine and norfluoxetine, respectively. Mean total infant exposure (fluoxetine equivalents) was estimated to be 6.81% (range 2.15–12%) of the weight-adjusted maternal dose of fluoxetine. Contributions from fluoxetine and norfluoxetine were approximately equal. Fluoxetine (range 20–252 μg l−1) was detected in five of the nine infants from whom samples were collected, and norfluoxetine (range 17–187 μg l−1) was detected in seven of the nine infants. The highest of these concentrations was about 70% of the maternal plasma concentrations.
Conclusions
The mean combined dose of fluoxetine and norfluoxetine transmitted to infants via breast milk is below the 10% notional level of concern. However, there was considerable interpatient variability in estimated infant dose and in some of the patients, the dose was >10%. Further, since adverse effects have been observed in breast-fed infants, careful monitoring of the infants is mandatory. Neonates exposed to these drugs in utero had higher concentrations of fluoxetine and norfluoxetine and are at greater risk of adverse effects.
Keywords: fluoxetine, human milk, in utero exposure, infant dose, norfluoxetine
Introduction
It is important to know the extent of drug transfer into human milk in order to assess the safety of breastfeeding during maternal ingestion of the drug. Depressive disorders are common, both during the antenatal period and in the early months after childbirth [1]. Fluoxetine, a selective serotonin reuptake inhibitor, is widely used to treat depression in this period. The drug is metabolized to norfluoxetine which has a much longer half-life and a similar potency as a serotonin reuptake inhibitor [2]. However, published data on the transfer of these drugs into human milk and their effects in breast-fed infants are limited to 18 cases described in six reports [3–8]. In the present study, the transfer of fluoxetine and norfluoxetine into milk has been quantified in 14 lactating women and related to adverse effects and plasma concentrations in their breast-fed infants.
Methods
Patients
Fourteen breast-feeding women (mean age 32 years, range 23–44 years; mean body weight 67.3 kg, range 44–85 kg) and their infants (7 M and 7 F; mean age 3.4 months, range 0.1–15 months; mean body weight 5.25 kg, range 2.8–10 kg) were enrolled in the study. The median dose of fluoxetine ingested by the women was 0.51 (range 0.24–0.94) mg kg−1 day−1, representing an absolute dose range of 20–80 mg daily. Therapy with fluoxetine had commenced a median of 70 (range 13–750) days prior to the study day, and all participants were considered to be at steady-state at the time of study.
Study protocol
The study design was approved by the Research and Ethics Committees of King Edward Memorial/Princess Margaret and Christchurch Hospitals, and written informed consent was obtained from all participants.
Data collection—limited sampling protocol
Ten mothers were enrolled in this arm of the study. A breast milk sample (15 ml) was collected by manual expression or by breast pump, immediately prior to a feed (prefeed) and also at the end (postfeed) of the same feed. A maternal blood sample (10 ml) was collected by venepuncture at the time of the postfeed milk sample. The mean time between the blood sample and last dose was 5.8 h (range 1.1–23.5). Six of these women gave consent for a venous blood sample (0.5–1 ml, heparinized) to be taken from their infant; this was taken immediately after the maternal blood sample.
Data collection—intensive sampling protocol
Four women in this arm of the study were admitted to the research ward at 07.30 h, and had a venous catheter inserted into a forearm vein immediately prior to the morning dose of fluoxetine at 08.00 h. Venous blood samples (8 ml, heparinized) were collected from the catheter at 0, 1, 2, 3, 4, 6 and 8 h postdose, and also by venepuncture at 12 and 24 h. At the same time intervals, both breasts were emptied via an electric or manual breast pump. Samples of milk (15 ml) were retained for drug assay and the remainder made available to bottle feed their infants. The women were discharged from hospital after 8 h and milk and plasma samples at 12 and 24 h postdose were collected at the patient’s home. Three of these women gave consent for a venous blood sample (0.5–1 ml, heparinized) to be taken from their infants.
For all studies, infant health and wellbeing were evaluated by enquiry of the mother and/or paediatrician and by checking the infant’s achievement of normal developmental milestones.
Materials
Fluoxetine hydrochloride and norfluoxetine maleate standards were donated by Eli Lilly Australia Pty Ltd, and amitriptyline hydrochloride by Merck Sharp and Dohme (Australia) Pty Ltd. All solvents and other chemicals were of analytical or h.p.l.c. grade.
High performance liquid chromatography (h.p.l.c.)
Following the addition of amitriptyline (200 ng) as an internal standard, 1 ml aliquots of plasma were made alkaline with 0.2 ml of 1 m NaOH and the analytes were extracted into 10 ml 1% isoamylalcohol in hexane by shaking vigorously for 5 min. After centrifugation (2000 g for 5 min), the organic phase (9 ml) was back-extracted into 0.2 ml 0.05 m HCl by vortex mixing for 1 min, and 0.05–0.1 ml aliquots of the acid phase were injected into the h.p.l.c. Milk samples were extracted in a similar manner except that the method of addition was used to enable correction for differing recoveries that may arise because of variable matrix composition. Rac-fluoxetine and rac-norfluoxetine concentrations in milk were determined by taking four equal aliquots of each milk sample (1 ml) and spiking three of these aliquots with increasing concentrations of authentic fluoxetine and norfluoxetine. The samples were then extracted and analysed as for plasma. The h.p.l.c. system consisted of a Beckman Ultrasphere C8 column (250×4.6 mm) and a solvent of 40% acetonitrile containing 0.09% sodium 1-octanesulphonic acid and 0.01% NNNN-tetramethylethylene diamine (final pH adjusted to 2.5 using H3PO4). Eluting compounds were detected by their u.v. absorbance at 230 nm. Plasma fluoxetine and norfluoxetine concentrations were interpolated from a standard curve (peak height ratio analyte: amitriptyline vs analyte concentration (r2 > 0.995) run with each batch of samples. For each individual milk sample, a standard curve (peak height ratio analyte: amitriptyline vs added analyte concentration (r2 > 0.995) was constructed and drug concentrations were determined from the negative x-axis intercept. The intraday coefficients of variation (CV; n = 5) for the plasma assay were 3.3 and 1.5% at 25 and 500 μg l−1, respectively, for fluoxetine, and 4.8 and 1.9% at 25 and 500 μg l−1, respectively, for norfluoxetine. The interday coefficients of variation (n = 15) for the plasma assay were 3.8 and 2.0% at 25 and 500 μg l−1, respectively, for fluoxetine, and 7.7 and 4.1% at 25 and 500 μg l−1, respectively, for norfluoxetine. For the milk assay intraday CVs (n = 4) were 3.4 and 5.0% at 25 and 500 μg l−1, respectively, for fluoxetine, and 5.0 and 5.1% at 25 and 500 μg l−1, respectively, for norfluoxetine. For the milk assay interday CVs (n = 12) were 4.1 and 5.0% at 25 and 500 μg l−1, respectively, for fluoxetine, and 5.4 and 5.6% at 25 and 500 μg l−1, respectively, for norfluoxetine. The limit of quantification for both analytes in milk and plasma was 15 μg l−1.
Statistical evaluation of data
Data have been summarized as mean (95% CI) unless otherwise specified. Differences between means were assessed using Student’s t-test for paired or independent data groups. Area under the plasma and milk concentration-time profiles (AUC(0,24h)) was calculated using the log trapezoidal rule.
Calculation of infant dose
The absolute infant dose of fluoxetine or norfluoxetine calculated as fluoxetine equivalents was determined by two different methods, both assuming an oral availability of 100% and an average infant milk intake of 0.15 l kg−1 day−1 [9]. For the limited sampling protocol, the milk concentration was taken as the mean of the pre- and post-feed milk concentrations, while for the intensive sampling protocol the average milk concentration (AUCmilk/24 h dose interval) was used. This value was multiplied by the average milk intake of 0.15 l kg−1 day−1 [9] to give a weight-adjusted estimate of daily infant dose. For both methods, the infant dose was then expressed as a percentage of the maternal weight-normalized dose.
Results
Plasma and milk concentration-time profiles for fluoxetine and norfluoxetine in the four patients where the intensive sampling protocol was used are shown in Figure 1. Milk and plasma concentration data and milk to plasma ratio (M/P) for all 14 patients are summarized in Table 1. For the limited sampling group, mean M/P was 0.74 (0.53–0.95) for fluoxetine and 0.62 (0.42–0.82) for norfluoxetine. Corresponding M/Ps for the intensive sampling group were 0.52 (0.32–0.72) and 0.41 (0.18–0.64), respectively. Although the mean M/P was higher with the limited sampling approach, the difference was not statistically different, albeit with a suboptimal power of study. For all patients combined, mean M/P ratio was 0.68 (0.52–0.84) for fluoxetine and 0.56 (0.35–0.77) for norfluoxetine.
Figure 1.
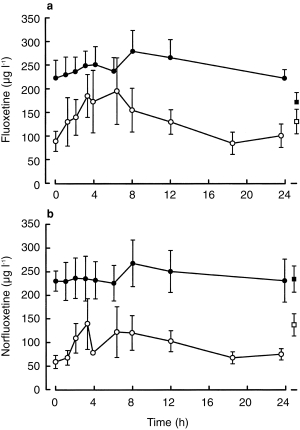
Plasma (•) and milk (○) concentration-time profiles (intensive sampling protocol) for fluoxetine (a) and norfluoxetine (b) in four volunteers over a 24 h dose interval (commencing 08.00 h) at steady-state. Also shown are plasma (▪) and milk (□) fluoxetine (a) and norfluoxetine (b) data for 10 other volunteers (limited sampling protocol). All data are dose corrected to the median daily dose of 40 mg fluoxetine and are presented as mean±s.e.mean; for clarity, some error bars are shown in one direction only.
Table 1.
Plasma and milk concentrations and M/P ratios for fluoxetine and norfluoxetine.
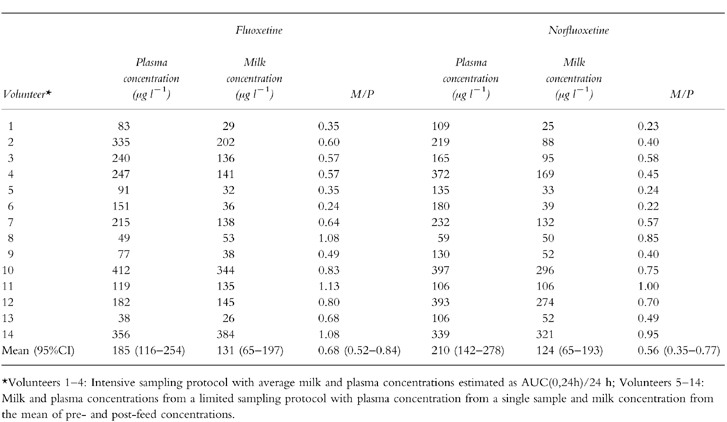
The limited sampling protocol also afforded an opportunity to assess differences in drug concentrations between the pre- and post-feed milk samples. Both fluoxetine (pre-feed 98 (15–181) μg l−1, post-feed 164 (48–279) μg l−1) and norfluoxetine (pre-feed 88 (19–157) μg l−1, post-feed 164 (68–260) μg l−1) mean concentrations were significantly (paired t-test, P < 0.05) higher in the post-feed than in the pre-feed milk.
Table 2 summarizes the maternal daily dose of fluoxetine and duration of therapy, the calculated infant doses of fluoxetine and norfluoxetine (as fluoxetine equivalents) together with the age of the infant at the time of the study. Calculated infant doses were 3.36 (2.38–4.34)% for fluoxetine and 3.45 (2.46–4.44)% for norfluoxetine giving a mean total infant exposure of 6.81% of the weight-adjusted maternal dose.
Table 2.
Maternal dose, duration of therapy, infant age, plasma concentration of fluoxetine and norfluoxetine and estimated dose.
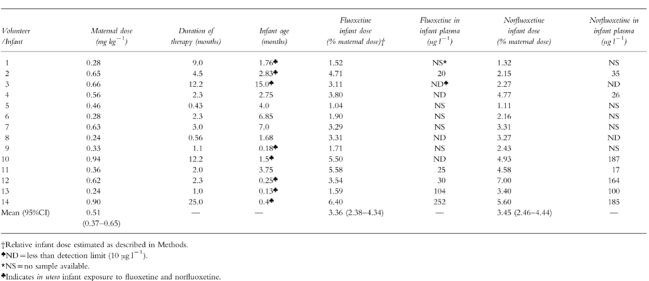
Blood samples were collected from nine infants in the study. Fluoxetine was detected in plasma from five infants and norfluoxetine in seven infants (Table 2). Norfluoxetine concentration generally was highest in infants aged 1.5 months or lower (infants 10,12,13,14) compared with older infants (infants 2,4,11). However, it is important to note that all of these infants (infants 10,12,13,14) were exposed to fluoxetine in utero. The long half-lives of both fluoxetine and norfluoxetine would suggest that a large contribution to these concentrations derived from the in utero exposure.
A detailed history of infant wellbeing and progress was obtained for the four infants in the intensive sampling arm of the study. Two of the four had no symptoms that might be considered fluoxetine-related, and had achieved normal developmental milestones. Infant 1 was described as having colic by her mother. Infant 3 had colic at the age of 2–4 months but was well at the time of study (15 months). She was also reported as always having been hyperactive. The infants enrolled in the limited sampling collection arm of the study were also assessed for achievement of weight-for-age milestones and by interviewing the mother and/or her paediatrician. Infants 10 and 14 were referred for study because of ‘withdrawal symptoms’ (uncontrollable crying, irritability and poor feeding) that were consistent with high plasma concentrations of norfluoxetine and/or fluoxetine, and their recent in utero exposure to the drug and its metabolite (Table 2). However, for infant 14 maternal methadone use may have been a contributing factor. These two cases also had high values for infant dose via breast milk and this may have contributed to the symptoms observed. No adverse effects were reported for the other eight infants in this arm of the study. All of these infants had body weights within the normal range.
Discussion
Measurement of M/P and infant exposure to drugs in human milk is generally most robust when an intensive AUC sampling protocol is utilized [10]. In the present study M/P for the limited sampling protocol was higher and more variable than for the intensive sampling approach. Nevertheless, variability with the limited sampling protocol may have been minimized by the long half-lives of fluoxetine (1–4 days) and norfluoxetine (7–15 days) [2]. Drug concentrations measured in milk from the limited sampling showed significantly greater concentrations in postfeed compared with prefeed milk. The difference is likely to be due to the increase in lipid concentration that occurs during a feed [11] together with the relatively high lipid solubility of fluoxetine (log P octanol:buffer pH 7.4 = 1.988) and norfluoxetine (log P octanol:buffer pH 7.4 = 1.82). The finding also suggests that protocols using only a single milk sample for lipid soluble drugs will be associated with increased variability in M/P and infant dose.
Mean M/P for fluoxetine and norfluoxetine were 0.68 and 0.56, respectively, suggesting a modest potential for penetration into breast milk. However, the resultant mean concentrations of fluoxetine and norfluoxetine in milk were 131 and 210 μg l−1, respectively, translating to a mean infant dose exposure of 3.36% for fluoxetine and 3.45% for norfluoxetine. In a previous study, Taddio et al. [6] estimated infant exposure (fluoxetine plus norfluoxetine) as a mean of 10.8% of the maternal dose (infant daily milk intake assumed to be 1 l). However, recalculation of this exposure using the widely accepted milk intake of 0.15 l kg−1 day−1 [9] gave a value of infant exposure of 6.4% [12]. This compares well with our mean dose exposure of 6.8% for fluoxetine plus norfluoxetine and with the range of 3–10% reported in four patients by Yoshida et al. [8].
The question of adverse effects in infants due to the ingestion of fluoxetine via breast milk is controversial. Both Taddio et al. [6] and Yoshida et al. [8] reported no adverse effects in their series of 10 and 4 patients, respectively. Burch & Wells [4] also reported a single case with no adverse effects and milk fluoxetine concentrations of 17–67 μg l−1and norfluoxetine concentrations of 13–52 μg l−1. However, Isenberg described irritability in a breast-fed 5 month old infant [3]. Milk concentrations of fluoxetine and norfluoxetine were 29 μg l−1and 42 μg l−1, respectively. Colic has been reported in an infant exposed to similar amounts of fluoxetine via breast milk [5]. Milk drug concentrations were not measured, but analysis of this infant’s plasma showed 340 μg l−1 of fluoxetine and 208 μg l−1 of norfluoxetine. Brent & Wisner described a single case of seizure-like episodes in a breast-fed infant whose mother had taken fluoxetine throughout pregnancy [7]. At 3 weeks of age, milk concentration of fluoxetine was 38 μg l−1 and norfluoxetine 28 μg l−1. Corresponding concentrations in the infant’s plasma were 61 μg l−1 for fluoxetine and 57 μg l−1 for norfluoxetine. However, the relationship with fluoxetine is tenuous as the seizures continued well beyond the cessation of breast feeding. Hyperglycaemia and glycosuria have also been reported in a 5 month old infant exposed to fluoxetine via breast milk, both of which resolved on cessation of the drug [13]. In the present study, ‘withdrawal symptoms’ were associated with two out of 14 cases. Moreover, these infants were 1.5 and 0.4 month-old, they had substantial concentrations of norfluoxetine and/or fluoxetine in their plasma and their mothers had taken fluoxetine during pregnancy. Two other infants described as having ‘colic’ also were exposed to fluoxetine in utero. Thus, our data strongly suggest that adverse effects from fluoxetine therapy are probably attributable to in utero exposure to the drug and its metabolite. In utero exposure is also strongly supported by our measurements of norfluoxetine in the infant’s plasma. This metabolite was detected in plasma samples from seven of the nine infants sampled. Concentrations were high in four infants whose age was 1.5 months or less. Of these four infants, fluoxetine concentrations also were correspondingly high in two of them. Since drug clearance may range from approximately 10% of maternal clearance for a very premature neonate to 33% at term, and 100% at about 6 months of age [14], the potential for adverse effects from fluoxetine therapy either during pregnancy and/or during breast-feeding is expected to be greatest in preterm neonates as well as during the first 1–2 weeks of life for full-term infants.
In breast-fed infants, the calculated mean total dose of fluoxetine and its metabolite norfluoxetine of 6.8% of the weight-adjusted maternal dose is less than the 10% level of concern proposed by Begg et al. [14]. It should be noted that there was considerable interpatient variability in the estimated dose with five of our infant doses being in the range 8.6–12%. Our study, and results from others [6, 8] indicate that many infants will tolerate these levels of exposure through breast milk. However, because of the variability in infant dose, and because the drug and its metabolite were detected in infant plasma (especially high concentrations in young infants), caution should be exercised at all times, and particularly during the early neonatal period. Special consideration should be given to infants who are additionally exposed to fluoxetine in utero. The long-term effects of fluoxetine/norfluoxetine exposure via breast milk have not been studied. However, studies of infants exposed to fluoxetine during pregnancy, have shown that neurodevelopment was normal [15, 16].
Acknowledgments
We acknowledge funding from the Women and Infants Research Foundation, referral of patients from Drs A. Groves, J. Kemp, J. Forward, J. Rampono and P. M. Graham and we thank Ruth Barrett-Lennard for expert assistance with data collection.
References
- 1.Buist A. Psychiatric disorders associated with childbirth. A guide to management. Sydney: McGraw-Hill; 1991. [Google Scholar]
- 2.Altamura AC, Moro AR, Percudani M. Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Isenberg KE. Excretion of fluoxetine in human breast milk. J Clin Psychiat. 1990;52:169. [PubMed] [Google Scholar]
- 4.Burch KJ, Wells BG. Fluoxetine/norfluoxetine concentrations in human milk. Paediatrics. 1992;89:676–677. [PubMed] [Google Scholar]
- 5.Lester BM, Cucca J, Andreozzi L, Flanagan P, Oh W. Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Adolesc Psychiat. 1993;32:1253–1255. doi: 10.1097/00004583-199311000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Taddio A, Ito S, Koren G. Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol. 1996;36:42–47. doi: 10.1002/j.1552-4604.1996.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 7.Brent NB, Wisner KL. Fluoxetine and carbamazepine concentrations in a nursing mother/infant pair. Clin Pediatr. 1998;37:41–44. doi: 10.1177/000992289803700107. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Smith B, Craggs M, Channi Kumar R. Fluoxetine in breast milk and developmental outcome of breast-fed infants. Br J Psychiat. 1998;172:175–179. doi: 10.1192/bjp.172.2.175. [DOI] [PubMed] [Google Scholar]
- 9.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and human lactation. 2. Amsterdam: Elsevier; 1996. pp. 67–74. [Google Scholar]
- 10.Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151–167. doi: 10.2165/00003088-199018020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Neville MC, Keller RP, Seacat J, Casey CE, Allen JC, Archer P. Studies on human lactation.1. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutrit. 1984;40:645–646. doi: 10.1093/ajcn/40.3.635. [DOI] [PubMed] [Google Scholar]
- 12.Duffull SB, Begg EJ, Ilett KF. Fluoxetine distribution in human milk (letter) Br J Clin Pharmacol. 1996;36:1078. doi: 10.1177/009127009603601112. [DOI] [PubMed] [Google Scholar]
- 13.Australian Adverse Drug Reactions Bulletin. 1997. p. 14.
- 14.Begg EJ, Atkinson HC, Duffull SB. Prospective evaluation of a model for the prediction of milk: plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol. 1992;33:501–505. doi: 10.1111/j.1365-2125.1992.tb04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- 16.Nulman I, Koren G. The safety of fluoxetine during pregnancy and lactation. Teratology. 1996;53:304–308. doi: 10.1002/(SICI)1096-9926(199605)53:5<304::AID-TERA4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]


